Identifying Ecological Corridors of the Bush Cricket Saga pedo in Fragmented Landscapes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
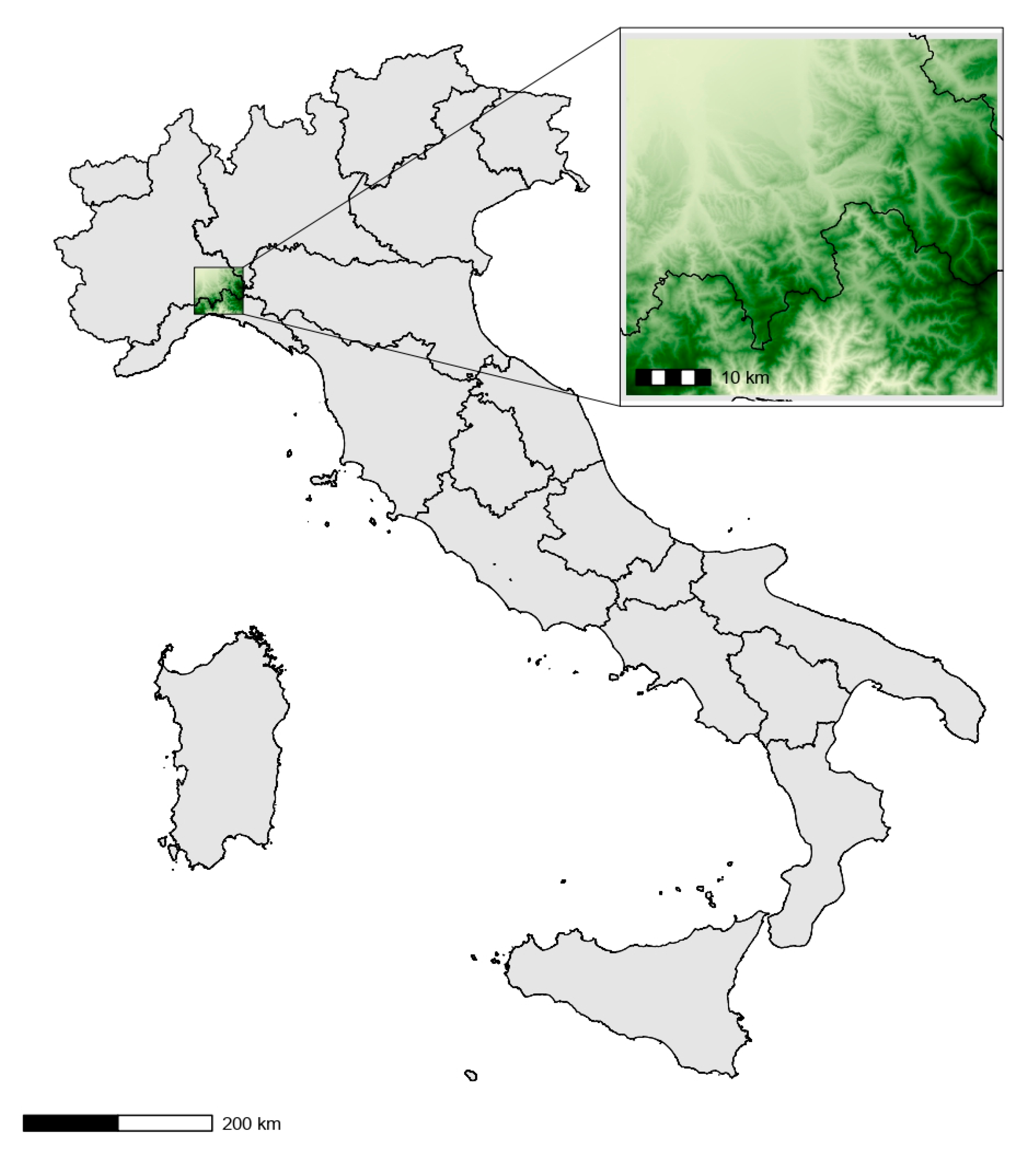
2.1. Study Area
2.2. Study Species and Data
2.3. Predictor Variables
2.4. Data Analysis
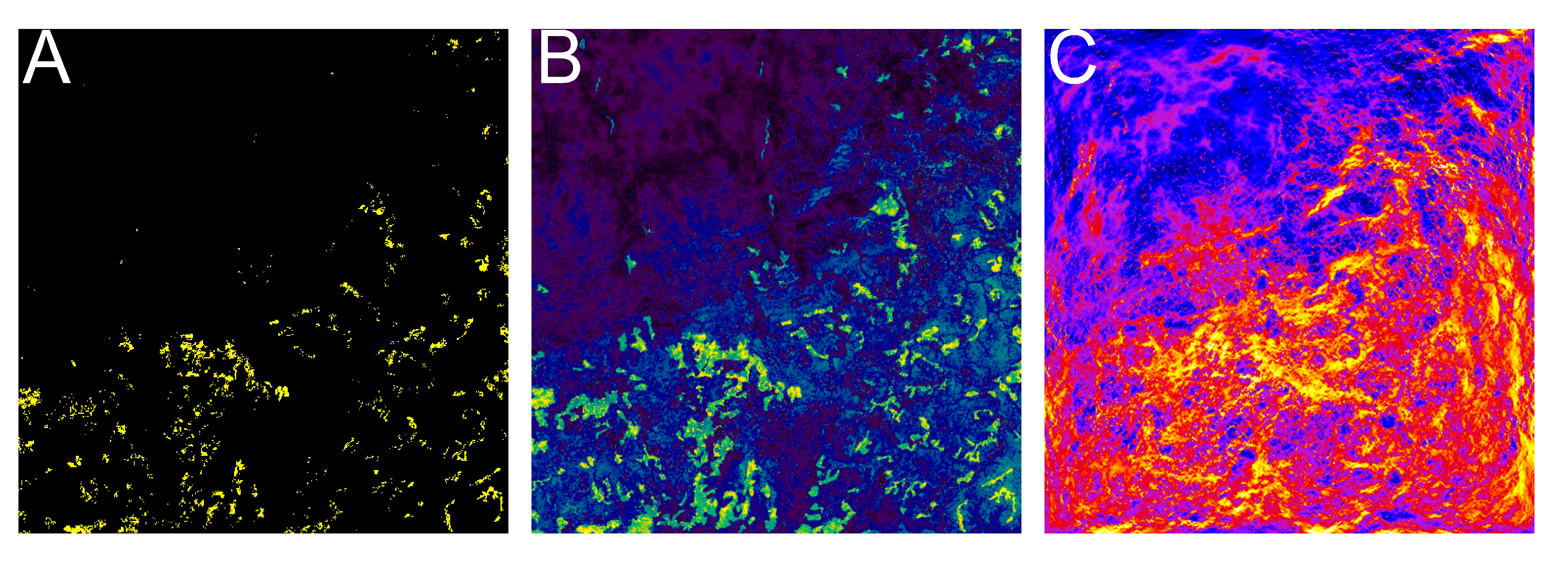
2.5. Landscape Connectivity Analysis
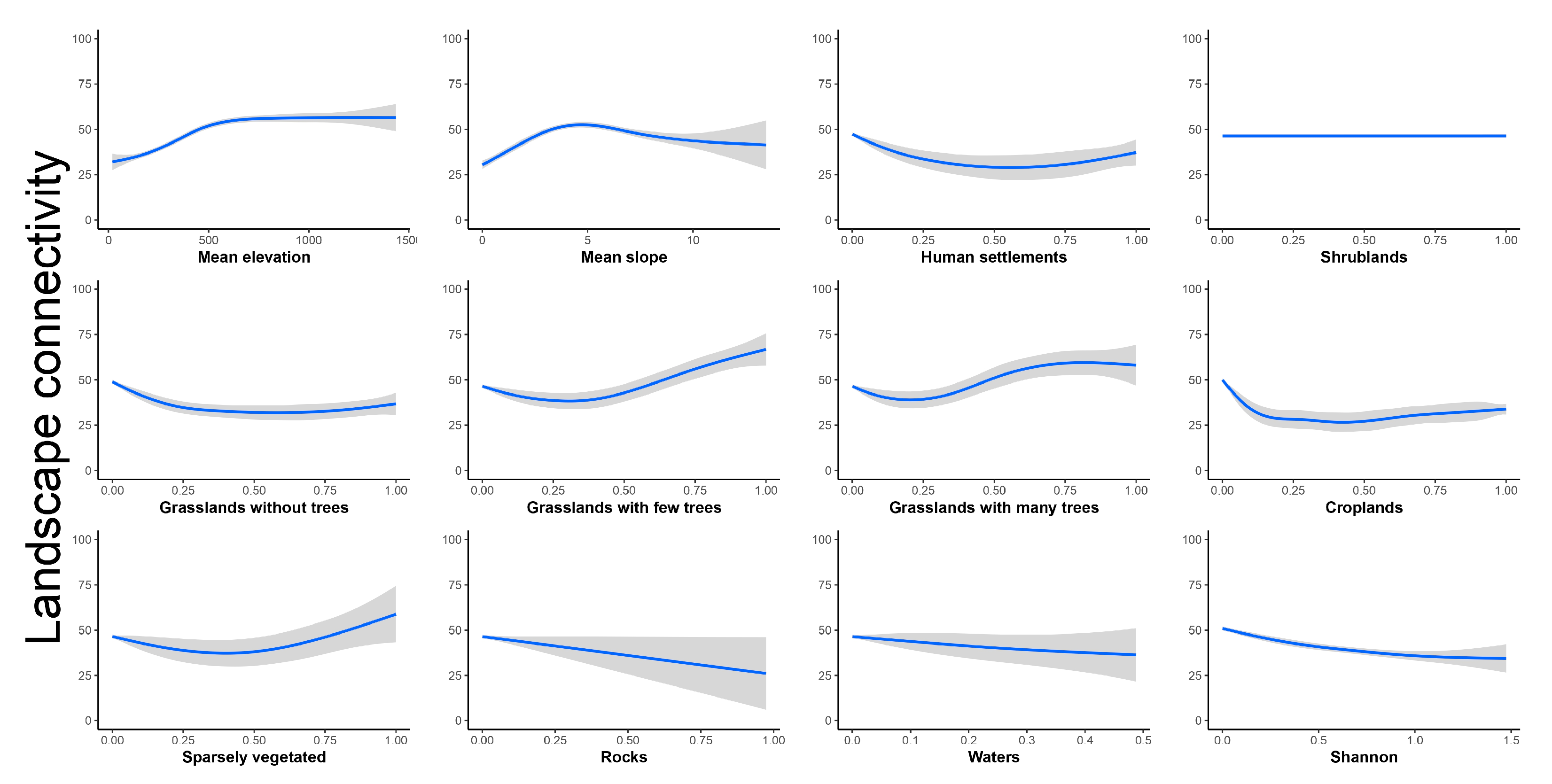
3. Results
4. Discussion
4.1. Probability of Occurrence and Landscape Connectivity of S. pedo
4.2. INLA-SPDE and Omniscape.jl for the Conservation of S. pedo
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Della Rocca, F.; Tagliani, A.; Milanesi, P.; Barcella, M.; Assini, S.P. Contrasting Response of Mountain Plant-Pollinator Network to Fragmented Semi-Natural Grasslands. Land 2023, 12, 356. [Google Scholar] [CrossRef]
- Socher, S.A.; Prati, D.; Boch, S.; Müller, J.; Baumbach, H.; Gockel, S.; Hemp, A.; Schöning, I.; Wells, K.; Buscot, F.; et al. Interacting Effects of Fertilization, Mowing and Grazing on Plant Species Diversity of 1500 Grasslands in Germany Differ between Regions. Basic Appl. Ecol. 2013, 14, 126–136. [Google Scholar] [CrossRef]
- Bullock, J.M.; Jefferson, R.G.; Blackstock, T.H.; Pakeman, R.J.; Emmett, B.A.; Pywell, R.J.; Grime, J.P.; Silvertown, J. Semi-Natural Grasslands. In UNEP-WCMC; UK Centre for Ecology and Hydrology: Cambridge, UK, 2011; pp. 161–196. ISBN 978-92-807-3164-4. [Google Scholar]
- Wilson, J.B.; Peet, R.K.; Dengler, J.; Pärtel, M. Plant Species Richness: The World Records. J. Veg. Sci. 2012, 23, 796–802. [Google Scholar] [CrossRef]
- Galvagni, A.; Prosser, F. Saga pedo (Pallas, 1771) Rinvenuta in Trentino, Italia Settentrionale (Insecta Orthoptera Tettigoniidae Saginae). Atti Della Accad. Roveretana Degli Agiati Ser 8 B Classe di Scienze Matematiche Fisiche e Naturali 2004, 4B, 97–106. [Google Scholar]
- Magistretti, M.; Ruffo, S. Primo Contributo Alla Conoscenza Della Fauna Delle Oasi Xerotermiche Prealpine (Coleotteri Carabidi, Scarabeidi, Crisomelidi). Mem. Mus. Civ. Stor. Nat. Verona 2022, 7, 99–125. [Google Scholar]
- Vergari, S.; Vergari, S.; Dondini, G.; Carotti, G. First Record of Saga pedo (Pallas, 1771) for Tuscany (Orthoptera: Tettigoniidae). Onychium 2017, 13, 35–37. [Google Scholar]
- Massa, B.; Bologna, M.; Rovelli, V.; Zapparoli, M. Saga pedo Pallas, 1771 (Stregona Dentellata). In Manuali Per il Monitoraggio di Specie e Habitat di Interesse Comunitario (Direttiva 92/43/CEE) in Italia: Specie Animali. Serie Manuali e Linee Guida; Stoch, F., Genovesi, P., Eds.; ISPRA: Rome, Italy, 2016; p. 141. [Google Scholar]
- Evju, M.; Blumentrath, S.; Skarpaas, O.; Stabbetorp, O.E.; Sverdrup-Thygeson, A. Plant Species Occurrence in a Fragmented Grassland Landscape: The Importance of Species Traits. Biodivers. Conserv. 2015, 24, 547–561. [Google Scholar] [CrossRef]
- Butaye, J.; Adriaens, D.; Honnay, O. Conservation and Restoration of Calcareous Grasslands: A Concise Review of the Effects of Fragmentation and Management on Plant Species. Biotechnol. Agron. Soc. Environ. 2005, 9, 111–118. [Google Scholar]
- Holuša, J.; Kočárek, P.; Vlk, R. Monitoring and Conservation of Saga pedo (Orthoptera: Tettigoniidae) in an Isolated Nothwestern Population. J. Insect. Conserv. 2013, 17, 663–669. [Google Scholar] [CrossRef]
- Anselmo, L. A Field Study on Saga pedo (Ensifera, Tettigoniidae, Saginae): Spatial Behavior of Adult Specimens. J. Orthoptera Res. 2022, 31, 41–46. [Google Scholar] [CrossRef]
- Kaltenbach, A. Unterlagen für eine Monographie der Saginae. II. Beiträge zur Autökologie der Gattung Saga Charpentier (Saltatoria: Tettigoniidae). Zool. Beiträge 1970, 16, 155–245. [Google Scholar]
- Krištín, A.; Kaňuch, P. Population, Ecology and Morphology of Saga pedo (Orthoptera: Tettigoniidae) at the Northern Limit of Its Distribution. Eur. J. Entomol. 2007, 104, 73–79. [Google Scholar] [CrossRef]
- Orthopteroid Specialist Group Saga pedo. The IUCN Red List of Threatened Species 1996: e.T19811A9018679. Available online: https://www.iucnredlist.org/species/19811/9018679 (accessed on 11 December 2024).
- Hochkirch, A.; Massa, B.; Skejo, J.; Presa, J.J.; Zuna-Kratky, T.; Kristin, A.; Ivkovic, S.; Korsunovskaya, O.; Monnerat, C.; Puskas, G.; et al. IUCN Red List of Threatened Species: Saga pedo 2016: e.T19811A74624296. Available online: https://www.iucnredlist.org/species/19811/74624296 (accessed on 11 December 2024).
- Adžić, K.; Deranja, M.; Mihaljević, M.; Rebrina, F.; Skejo, J.; Jelić, D.; Pavlović, M.; Kirin, K.; Tvrtković, N. Distribution and Ecology of the Predatory Katydid Saga pedo (Pallas, 1771) in Croatia with the First Record in the Continental Region. Nat. Croat. Period. Musei Hist. Nat. Croat. 2023, 32, 35–47. [Google Scholar] [CrossRef]
- New, T.R.; Habel, J.C.; Meyer, M.; Schmitt, T. Jewels in the Mist: A Synopsis on the Highly Endangered Butterfly Species the Violet Copper, Lycaena helle. J. Insect. Conserv. 2016, 20, 163–164. [Google Scholar] [CrossRef]
- Saura, S.; Pascual-Hortal, L. A New Habitat Availability Index to Integrate Connectivity in Landscape Conservation Planning: Comparison with Existing Indices and Application to a Case Study. Landsc. Urban Plan. 2007, 83, 91–103. [Google Scholar] [CrossRef]
- Vai, L. SIC IT1180030 Calanchi di Rigoroso, Sottovalle e Carrosio—Aree Protette 2020. Available online: https://www.areeprotetteappenninopiemontese.it/2020/10/30/sic-it1180030-calanchi-di-rigoroso-sottovalle-e-carrosio/ (accessed on 11 December 2024).
- Rossi, R.; Bonifacino, M.; Sindaco, R. Le Farfalle (Lepidoptera Papilionoidea) Del Sito Di Interesse Comunitario IT1180030 “Calanchi Di Rigoroso, Sottovalle e Carrosio” (Provincia Di Alessandria, Piemonte). Riv. Piemont. Stor. Nat. 2021, 42, 85–91. [Google Scholar] [CrossRef]
- Anselmo, L. Habitat Selection and Morphology of Saga pedo (Pallas, 1771) in Alps (Susa Valley, Piedmont, NW Italy) (Insecta: Orthoptera, Tettigoniidae, Saginae). Fragm. Entomol. 2019, 51, 63–74. [Google Scholar] [CrossRef]
- Repetto, E.; Milanesi, P.; De Caria, L.; Della Rocca, F. Ecology and Abundance of a Relict Population of the Bush Cricket Saga pedo in the Northern Apennines, Italy. Ecol. Evol. 2024, 14, e11381. [Google Scholar] [CrossRef]
- Della Rocca, F.; Musiani, M.; Galaverni, M.; Milanesi, P. Improving Online Citizen Science Platforms for Biodiversity Monitoring. J. Biogeogr. 2024, 51, 2412–2423. [Google Scholar] [CrossRef]
- Milanesi, P.; Mori, E.; Menchetti, M. Observer-Oriented Approach Improves Species Distribution Models from Citizen Science Data. Ecol. Evol. 2020, 10, 12104–12114. [Google Scholar] [CrossRef]
- Della Rocca, F.; Milanesi, P. The New Dominator of the World: Modeling the Global Distribution of the Japanese Beetle under Land Use and Climate Change Scenarios. Land 2022, 11, 567. [Google Scholar] [CrossRef]
- Della Rocca, F.; Milanesi, P. The Spread of the Japanese Beetle in a European Human-Dominated Landscape: High Anthropization Favors Colonization of Popillia japonica. Diversity 2022, 14, 658. [Google Scholar] [CrossRef]
- Barve, V.; Hart, E.; Guillou, S. Rinat: Access “iNaturalist” Data Through APIs 0.1.9. 2022. Available online: https://cran.r-project.org/web/packages/rinat/rinat.pdf (accessed on 11 December 2024).
- European Environment Agency. CLC+ Backbone Product Specification and User Manual, Issue 1.2.; Framework Service Contract No. EEA/DIS/R0/19/012. 2024. Available online: https://land.copernicus.eu/en/technical-library/product-user-manual-clc-backbone-2021/@@download/file (accessed on 11 December 2024).
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A Protocol for Data Exploration to Avoid Common Statistical Problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Naimi, B. Usdm: Uncertainty Analysis for Species Distribution Models 2.1-7. 2012. Available online: https://cran.r-project.org/web/packages/usdm/usdm.pdf (accessed on 11 December 2024).
- Rue, H.; Martino, S.; Chopin, N. Approximate Bayesian Inference for Latent Gaussian Models by Using Integrated Nested Laplace Approximations. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2009, 71, 319–392. [Google Scholar] [CrossRef]
- Beguin, J.; Martino, S.; Rue, H.; Cumming, S.G. Hierarchical Analysis of Spatially Autocorrelated Ecological Data Using Integrated Nested Laplace Approximation. Methods Ecol. Evol. 2012, 3, 921–929. [Google Scholar] [CrossRef]
- Lindgren, F.; Rue, H.; Lindström, J. An Explicit Link between Gaussian Fields and Gaussian Markov Random Fields: The Stochastic Partial Differential Equation Approach. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2011, 73, 423–498. [Google Scholar] [CrossRef]
- Blangiardo, M.; Cameletti, M.; Baio, G.; Rue, H. Spatial and Spatio-Temporal Models with R-INLA. Spat. Spatio-Temporal Epidemiol. 2013, 7, 39–55. [Google Scholar] [CrossRef]
- Erickson, K.D.; Smith, A.B. Modeling the rarest of the rare: A comparison between multi-species distribution models, ensembles of small models, and single-species models at extremely low sample sizes. Ecography 2023, 46, e06500. [Google Scholar] [CrossRef]
- Breiner, F.T.; Guisan, A.; Bergamini, A.; Nobis, M.P. Overcoming limitations of modelling rare species by using ensembles of small models. Methods Ecol. Evol. 2015, 6, 1210–1218. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the Accuracy of Species Distribution Models: Prevalence, Kappa and the True Skill Statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F. Package ‘Biomod2’. Species Distribution Modeling within an Ensemble Forecasting Framework. 2016. Available online: https://cran.r-project.org/web/packages/biomod2/biomod2.pdf (accessed on 11 December 2024).
- Landau, V.; Shah, V.; Anantharaman, R.; Hall, K. Omniscape.Jl: Software to Compute Omnidirectional Landscape Connectivity. JOSS 2021, 6, 2829. [Google Scholar] [CrossRef]
- McRae, B.H.; Dickson, B.G.; Keitt, T.H.; Shah, V.B. Using Circuit Theory to Model Connectivity in Ecology, Evolution, and Conservation. Ecology 2008, 89, 2712–2724. [Google Scholar] [CrossRef]
- Richard, M.-A. Expérimentation et Suivi de Population Chez Saga pedo Sur La Plaine Du Regard (07). Master’s Thesis, Université Toulouse III—Paul Sabatier, Toulouse, France, 2010. [Google Scholar]
- McRae, B.; Popper, K.; Jones, A.; Schindel, M.; Buttrick, S.; Hall, K.; Unnasch, R.; Platt, J. Conserving Nature’s Stage: Mapping Omnidirectional Connectivity for Resilient Terrestrial Landscapes in the Pacific Northwest. 2016. Available online: https://www.conservationgateway.org/ConservationByGeography/NorthAmerica/UnitedStates/oregon/science/Documents/McRae_et_al_2016_PNW_CNS_Connectivity.pdf (accessed on 11 December 2024).
- Başnou, C.; Álvarez, E.; Bagaria, G.; Guardiola, M.; Isern, R.; Vicente, P.; Pino, J. Spatial Patterns of Land Use Changes Across a Mediterranean Metropolitan Landscape: Implications for Biodiversity Management. Environ. Manag. 2013, 52, 971–980. [Google Scholar] [CrossRef]
- Pörtner, H.-O.; Roberts, D.C.; Tignor, M.; Poloczanska, E.S.; Mintenbeck, K.; Alegría, A.; Craig, M.; Langsdorf, S.; Löschke, S.; Möller, V. (Eds.) Annex IV: Contributors to the Working Group II Contribution to the IPCC Sixth Assessment Report. In Climate Change 2022: Impacts, Adaptation and Vulnerability; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; pp. 2939–2963. [Google Scholar] [CrossRef]
- Genovese, D.; Ostellino, I.; Battaglini, L. Chapter 2. The Conflict of Itinerant Pastoralism in the Piedmont Po Plain (Collina Po Biosphere Reserve, Italy). In Environmental Anthropology and Ethnobiology; Berghahn: New York, NY, USA, 2022; Volume 29, pp. 44–60. ISBN 978-1-80073-667-2. [Google Scholar]
- Orlandi, S.; Probo, M.; Sitzia, T.; Trentanovi, G.; Garbarino, M.; Lombardi, G.; Lonati, M. Environmental and Land Use Determinants of Grassland Patch Diversity in the Western and Eastern Alps under Agro-Pastoral Abandonment. Biodivers. Conserv. 2016, 25, 275–293. [Google Scholar] [CrossRef]
- Mairota, P.; Leronni, V.; Xi, W.; Mladenoff, D.J.; Nagendra, H. Using Spatial Simulations of Habitat Modification for Adaptive Management of Protected Areas: Mediterranean Grassland Modification by Woody Plant Encroachment. Environ. Conserv. 2014, 41, 144–156. [Google Scholar] [CrossRef]
- Prangel, E.; Kasari-Toussaint, L.; Neuenkamp, L.; Noreika, N.; Karise, R.; Marja, R.; Ingerpuu, N.; Kupper, T.; Keerberg, L.; Oja, E.; et al. Afforestation and Abandonment of Semi-Natural Grasslands Lead to Biodiversity Loss and a Decline in Ecosystem Services and Functions. J. Appl. Ecol. 2023, 60, 825–836. [Google Scholar] [CrossRef]
- Ancillotto, L.; Labadessa, R. Can Protected Areas and Habitats Preserve the Vulnerable Predatory Bush Cricket Saga pedo? J. Insect Conserv. 2023, 27, 615–624. [Google Scholar] [CrossRef]
- Habel, J.C.; Dengler, J.; Janišová, M.; Török, P.; Wellstein, C.; Wiezik, M. European Grassland Ecosystems: Threatened Hotspots of Biodiversity. Biodivers. Conserv. 2013, 22, 2131–2138. [Google Scholar] [CrossRef]
- Maioglio, O.; Repetto, E. Nuova Segnalazione Di Saga pedo (Pallas, 1771) in Provincia Di Alessandria, Piemonte e Relative Osservazioni Ecologiche (Orthoptera: Tettigoniidae). Riv. Piemont. Stor. Nat. 2022, 43, 49–58. [Google Scholar]
- Lemonnier-Darcemont, M.; Darcemont, C.; Heller, K.-G.; Dutrillaux, A.-M.; Dutrillaux, B. Saginae of Europe—Les Saginae d’Europe; Pemberley: Iver, UK, 2016; ISBN 978-2-9537533-9-4. [Google Scholar]
- Zema, D.A.; Bombino, G.; Zimbone, S.M. Agricultural Land Degradation in Italy. In Impact of Agriculture on Soil Degradation II: A European Perspective; Pereira, P., Muñoz-Rojas, M., Bogunovic, I., Zhao, W., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 179–222. ISBN 978-3-031-32052-1. [Google Scholar]
- Romano, B.; Zullo, F.; Fiorini, L.; Marucci, A.; Ciabò, S. Land Transformation of Italy Due to Half a Century of Urbanization. Land Use Policy 2017, 67, 387–400. [Google Scholar] [CrossRef]
- Baggio, J.A.; Salau, K.; Janssen, M.A.; Schoon, M.L.; Bodin, Ö. Landscape Connectivity and Predator–Prey Population Dynamics. Landsc. Ecol. 2011, 26, 33–45. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat Fragmentation and Its Lasting Impact on Earth’s Ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef]
- Gebhardt, S.; van Dijk, J.; Wassen, M.J.; Bakker, M. Agricultural Intensity Interacts with Landscape Arrangement in Driving Ecosystem Services. Agric. Ecosyst. Environ. 2023, 357, 108692. [Google Scholar] [CrossRef]
- Della Rocca, F.; Bogliani, G.; Breiner, F.T.; Milanesi, P. Identifying Hotspots for Rare Species under Climate Change Scenarios: Improving Saproxylic Beetle Conservation in Italy. Biodivers. Conserv. 2019, 28, 433–449. [Google Scholar] [CrossRef]
- Domisch, S.; Friedrichs, M.; Hein, T.; Borgwardt, F.; Wetzig, A.; Jähnig, S.C.; Langhans, S.D. Spatially Explicit Species Distribution Models: A Missed Opportunity in Conservation Planning? Divers. Distrib. 2019, 25, 758–769. [Google Scholar] [CrossRef]
- Milanesi, P.; Holderegger, R.; Caniglia, R.; Fabbri, E.; Galaverni, M.; Randi, E. Expert-Based versus Habitat-Suitability Models to Develop Resistance Surfaces in Landscape Genetics. Oecologia 2017, 183, 67–79. [Google Scholar] [CrossRef]
- Balestrieri, A.; Mori, E.; Menchetti, M.; Ruiz-González, A.; Milanesi, P. Far from the Madding Crowd: Tolerance toward Human Disturbance Shapes Distribution and Connectivity Patterns of Closely Related Martes Spp. Popul. Ecol. 2019, 61, 289–299. [Google Scholar] [CrossRef]
- Phillips, P.; Clark, M.M.; Baral, S.; Koen, E.L.; Bowman, J. Comparison of Methods for Estimating Omnidirectional Landscape Connectivity. Landsc. Ecol 2021, 36, 1647–1661. [Google Scholar] [CrossRef]
- Unnithan Kumar, S.; Cushman, S.A. Connectivity Modelling in Conservation Science: A Comparative Evaluation. Sci. Rep. 2022, 12, 16680. [Google Scholar] [CrossRef]
- Della Rocca, F.; Bogliani, G.; Milanesi, P. Patterns of Distribution and Landscape Connectivity of the Stag Beetle in a Human-Dominated Landscape. Nat. Conserv. 2017, 19, 19–37. [Google Scholar] [CrossRef]

| Predictor | Unit | VIF |
|---|---|---|
| Mean elevation | m a.s.l. | 1.507 |
| Stand. dev. elevation | m a.s.l. | >3 |
| Mean slope | ° | 1.907 |
| Stand. dev. slope | ° | >3 |
| Mean roughness | average length of isoipses in the cell/cell side | >3 |
| Stand. dev. roughness | average length of isoipses in the cell/cell side | >3 |
| Mean tree cover density | n/m2 | >3 |
| Stand. dev. tree cover density | n/m2 | >3 |
| Shrublands | % | 1.117 |
| Grasslands without woody trees | % | 1.338 |
| Grasslands with few woody trees | % | 1.238 |
| Grasslands with many woody trees | % | 1.197 |
| Croplands | % | 2.388 |
| Sparsely vegetated areas | % | 1.048 |
| Waters | % | 1.055 |
| Woodlands | % | >3 |
| Rocky areas | % | 1.041 |
| Shannon habitat diversity index | H′ = −Σ (pi × lnpi) | 1.966 |
| Human settlements | % | 1.231 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Rocca, F.; Repetto, E.; De Caria, L.; Milanesi, P. Identifying Ecological Corridors of the Bush Cricket Saga pedo in Fragmented Landscapes. Insects 2025, 16, 279. https://doi.org/10.3390/insects16030279
Della Rocca F, Repetto E, De Caria L, Milanesi P. Identifying Ecological Corridors of the Bush Cricket Saga pedo in Fragmented Landscapes. Insects. 2025; 16(3):279. https://doi.org/10.3390/insects16030279
Chicago/Turabian StyleDella Rocca, Francesca, Emanuele Repetto, Livia De Caria, and Pietro Milanesi. 2025. "Identifying Ecological Corridors of the Bush Cricket Saga pedo in Fragmented Landscapes" Insects 16, no. 3: 279. https://doi.org/10.3390/insects16030279
APA StyleDella Rocca, F., Repetto, E., De Caria, L., & Milanesi, P. (2025). Identifying Ecological Corridors of the Bush Cricket Saga pedo in Fragmented Landscapes. Insects, 16(3), 279. https://doi.org/10.3390/insects16030279









