Temperature Effects on the Survival and Oviposition of an Invasive Blow Fly Chrysomya rufifacies Macquart (Diptera: Calliphoridae)
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Colony Care
2.2. Survival and Oviposition Performance
2.3. Heat Shock Exposure
2.4. Statistical Analyses
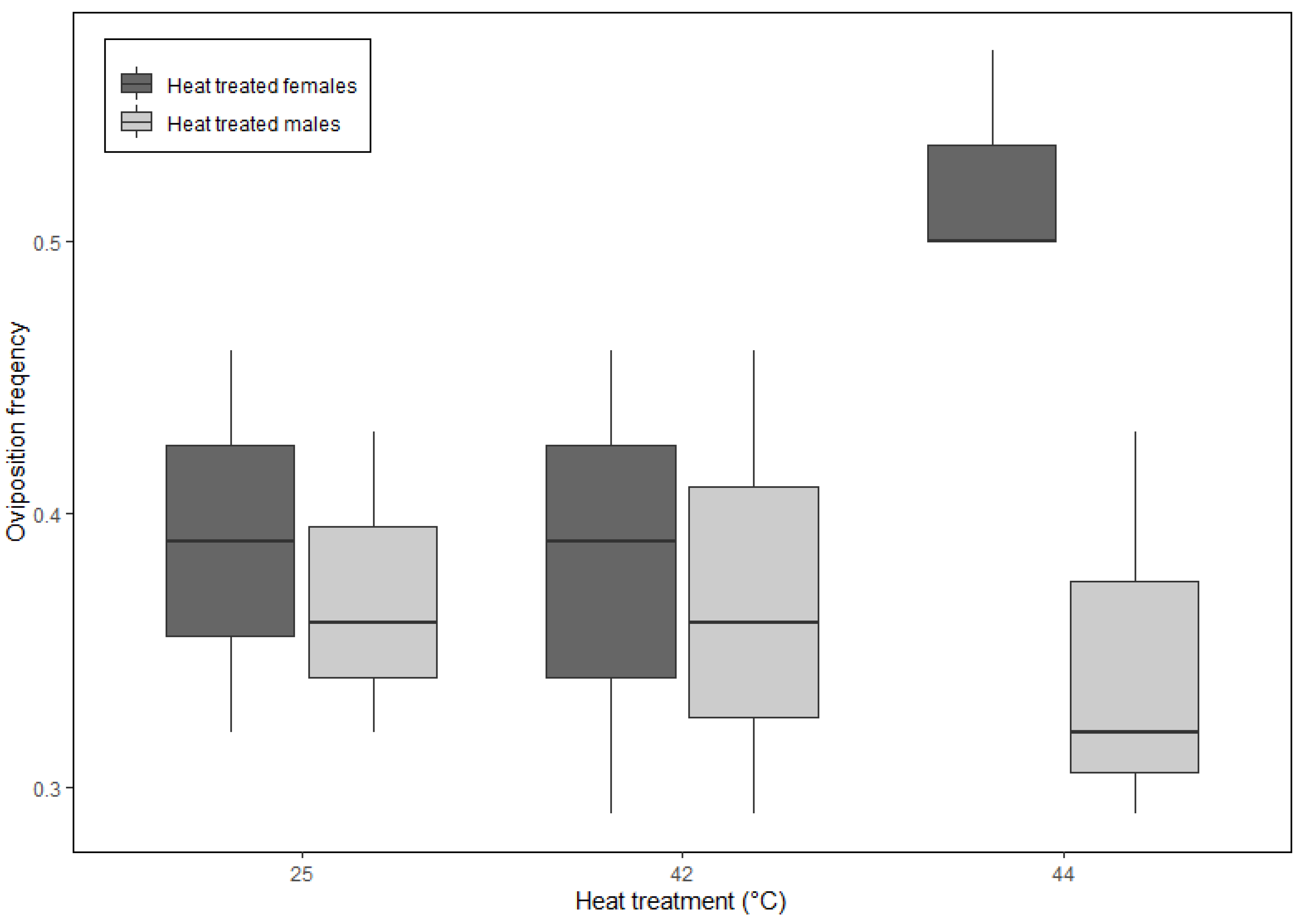
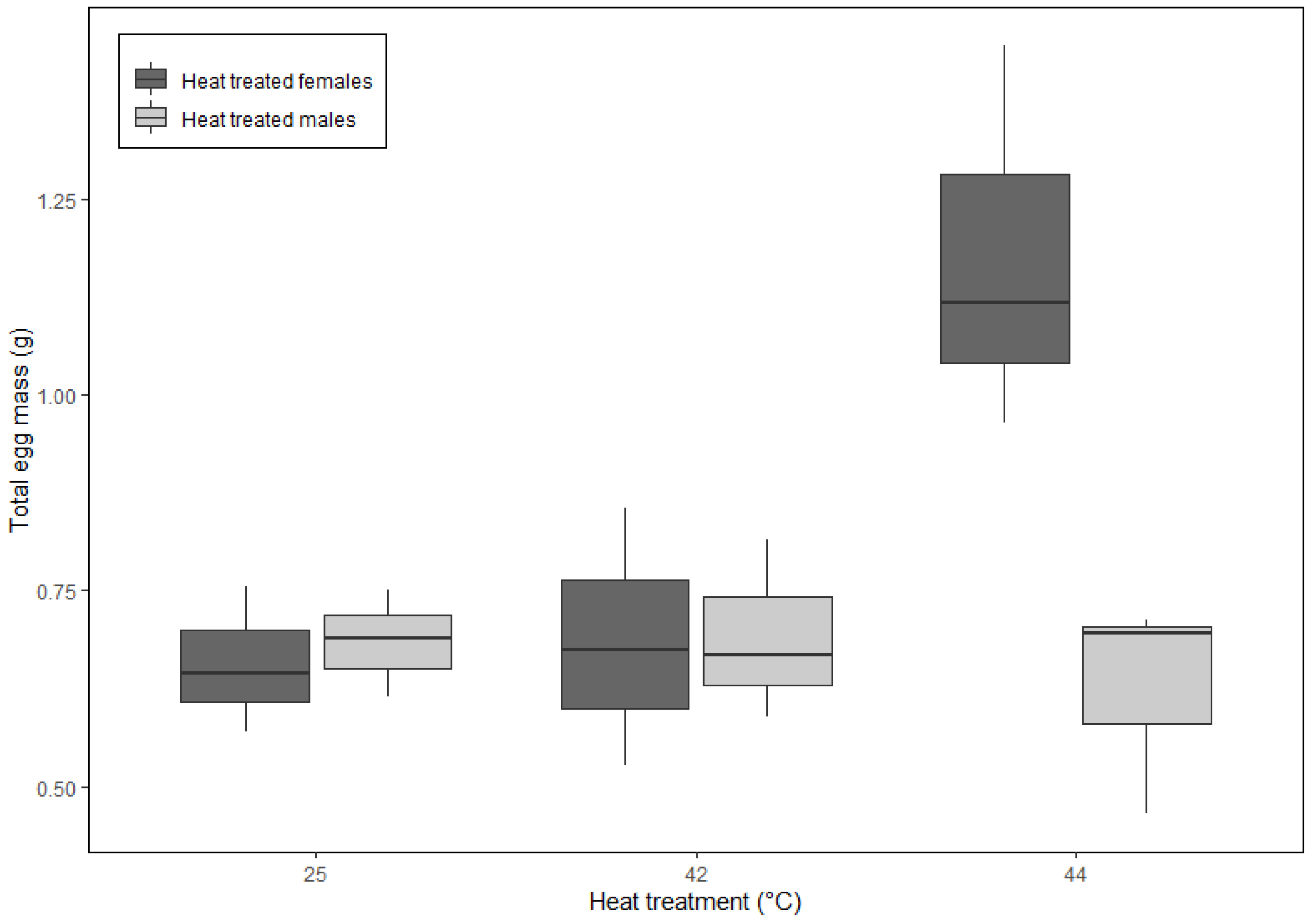
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marshall, K.E.; Sinclair, B.J. The sub-lethal effects of repeated freezing in the woolly bear caterpillar Pyrrharctia isabella. J. Exp. Biol. 2011, 214, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.F.; Woods, H.A.; Roberts, S.P. Ecological and Environmental Physiology of Insects; Oxford University Press: Oxford, UK, 2012; Volume 3. [Google Scholar]
- González-Tokman, D.; Córdoba-Aguilar, A.; Dáttilo, W.; Lira-Noriega, A.; Sánchez-Guillén, R.A.; Villalobos, F. Insect responses to heat: Physiological mechanisms, evolution and ecological implications in a warming world. Biol. Rev. 2020, 95, 802–821. [Google Scholar] [CrossRef] [PubMed]
- Roitberg, B.D.; Mangel, M. Cold snaps, heatwaves, and arthropod growth. Ecol. Entomol. 2016, 41, 653–659. [Google Scholar] [CrossRef]
- Stillman, J.H. Heat waves, the new normal: Summertime temperature extremes will impact animals, ecosystems, and human communities. Physiology 2019, 34, 86–100. [Google Scholar] [CrossRef]
- IPOCC. Climate change 2014: Synthesis report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Buckley, L.B.; Hurlbert, A.H.; Jetz, W. Broad-scale ecological implications of ectothermy and endothermy in changing environments. Glob. Ecol. Biogeogr. 2012, 21, 873–885. [Google Scholar] [CrossRef]
- Sales, K.; Vasudeva, R.; Dickinson, M.E.; Godwin, J.L.; Lumley, A.J.; Michalczyk, Ł.; Hebberecht, L.; Thomas, P.; Franco, A.; Gage, M.J. Experimental heatwaves compromise sperm function and cause transgenerational damage in a model insect. Nat. Commun. 2018, 9, 4771. [Google Scholar] [CrossRef]
- Huey, R.B.; Kingsolver, J.G. Climate warming, resource availability, and the metabolic meltdown of ectotherms. Am. Nat. 2019, 194, E140–E150. [Google Scholar] [CrossRef]
- Grant, B.W.; Dunham, A.E. Thermally imposed time constraints on the activity of the desert lizard Sceloporus merriami. Ecology 1988, 69, 167–176. [Google Scholar] [CrossRef]
- Angilletta, M.J. Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Bozinovic, F.; Bastías, D.A.; Boher, F.E.; Clavijo-Baquet, S.; Estay, S.A.; Angilletta, M.J. The mean and variance of environmental temperature interact to determine physiological tolerance and fitness Physiol. Biochem. Zool. 2011, 84, 543–552. [Google Scholar] [CrossRef]
- Estay, S.A.; Lima, M.; Bozinovic, F. The role of temperature variability on insect performance and population dynamics in a warming world. Oikos 2014, 123, 131–140. [Google Scholar] [CrossRef]
- Arbogast, R.T. Mortality and reproduction of Ephestia cautella and Plodia interpunctella exposed as pupae to high temperatures. Environ. Entomol. 1981, 10, 708–711. [Google Scholar] [CrossRef]
- Saxena, B.; Sharma, P.; Thappa, R.; Tikku, K. Temperature induced sterilization for control of three stored grain beetles. J. Stored Prod. Res. 1992, 28, 67–70. [Google Scholar] [CrossRef]
- Horgan, F.G.; Arida, A.; Ardestani, G.; Almazan, M.L.P. Temperature-dependent oviposition and nymph performance reveal distinct thermal niches of coexisting planthoppers with similar thresholds for development. PLoS ONE 2020, 15, e0235506. [Google Scholar] [CrossRef] [PubMed]
- Lister, B.C.; Garcia, A. Climate-driven declines in arthropod abundance restructure a rainforest food web. Proc. Natl. Acad. Sci. USA 2018, 115, E10397–E10406. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Wagner, D.L. Insect declines in the Anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef]
- Goulson, D. The insect apocalypse, and why it matters. Curr. Biol. 2019, 29, R967–R971. [Google Scholar] [CrossRef]
- Saunders, M.E.; Janes, J.K.; O’Hanlon, J.C. Moving on from the insect apocalypse narrative: Engaging with evidence-based insect conservation. BioScience 2020, 70, 80–89. [Google Scholar] [CrossRef]
- Montgomery, G.A.; Dunn, R.R.; Fox, R.; Jongejans, E.; Leather, S.R.; Saunders, M.E.; Shortall, C.R.; Tingley, M.W.; Wagner, D.L. Is the insect apocalypse upon us? How to find out. Biol. Conserv. 2020, 241, 108327. [Google Scholar] [CrossRef]
- Denlinger, D.L.; Yocum, G.D. Physiology of heat sensitivity. In Temperature Sensitivity in Insects and Application in Integrated Pest Management; Hallman, G.J., Denlinger, D.L., Eds.; Westview Press: Boulder, CO, USA, 1998. [Google Scholar]
- Stürup, M.; Baer-Imhoof, B.; Nash, D.R.; Boomsma, J.J.; Baer, B. When every sperm counts: Factors affecting male fertility in the honeybee Apis mellifera. Behav. Ecol. 2013, 24, 1192–1198. [Google Scholar] [CrossRef]
- Chevrier, C.; Nguyen, T.M.; Bressac, C. Heat shock sensitivity of adult male fertility in the parasitoid wasp Anisopteromalus calandrae (Hymenoptera, Pteromalidae). J. Therm. Biol. 2019, 85, 102419. [Google Scholar] [CrossRef]
- Proverbs, M. Induced sterilization and control of insects. Annu. Rev. Entomol. 1969, 14, 81–102. [Google Scholar] [CrossRef]
- Vollmer, J.; Sarup, P.; Kaersgaard, C.; Dahlgaard, J.; Loeschcke, V. Heat and cold-induced male sterility in Drosophila buzzatii: Genetic variation among populations for the duration of sterility. Heredity 2004, 92, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Bonebrake, T.C.; Boggs, C.L.; McNally, J.M.; Ranganathan, J.; Ehrlich, P.R. Oviposition behavior and offspring performance in herbivorous insects: Consequences of climatic and habitat heterogeneity. Oikos 2010, 119, 927–934. [Google Scholar] [CrossRef]
- Ody, H.; Bulling, M.T.; Barnes, K.M. Effects of environmental temperature on oviposition behavior in three blow fly species of forensic importance. Forensic Sci. Int. 2017, 275, 138–143. [Google Scholar] [CrossRef]
- Slatkin, M. Hedging one's evolutionary bets. Nature 1974, 250, 704–705. [Google Scholar] [CrossRef]
- Byrd, J.H.; Tomberlin, J.K. Forensic Entomology: The Utility of Arthropods in Legal Investigations; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Baumgartner, D.L. Review of Chrysomya rufifacies (Diptera: Calliphoridae). J. Med. Entomol. 1993, 30, 338–352. [Google Scholar] [CrossRef]
- Greenberg, B. Flies as forensic indicators. J. Med. Entomol. 1991, 28, 565–577. [Google Scholar] [CrossRef]
- Sukontason, K.L.; Sukontason, K.; Narongchai, P.; Lertthamnongtham, S.; Piangjai, S.; Olson, J.K. Chrysomya rufifacies (Macquart) as a forensically-important fly species in Thailand: A case report. J. Vector Ecol. J. Soc. Vector Ecol. 2001, 26, 162–164. [Google Scholar]
- Engelmann, F. Reproduction in insects. Ecol. Entomol. 1984, 113–147. [Google Scholar]
- Wells, J.D.; Greenberg, B. Interaction between Chrysomya rufifacies and Cochliomyia macellaria (Diptera: Calliphoridae): The possible consequences of an invasion. Bull. Entomol. Res. 1992, 82, 133–137. [Google Scholar] [CrossRef]
- Hans, K.R.; LeBouthillier, R.; VanLaerhoven, S. Effect of temperature on oviposition behavior and egg load of blow flies (Diptera: Calliphoridae). J. Med. Entomol. 2019, 56, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Monzon, M.A.; Weidner, L.M.; Rusch, T.W.; Nehrozoglu, S.; Hamilton, G. High temperature limits of survival and oviposition of Phormia regina (Meigen) and Lucilia sericata (Meigen). Insects 2022, 13, 991. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wan, F.; Xie, M.; Liu, T. Effects of heat shock on survival and reproduction of two whitefly species, Trialeurodes vaporariorum and Bemisia tabaci biotype B. J. Insect Sci. 2008, 8, 24. [Google Scholar] [CrossRef]
- Wolda, H. The effect of temperature on reproduction in some morphs of the landsnail Cepaea nemoralis (L.). Evolution 1967, 21, 117–129. [Google Scholar] [CrossRef]
- Whitworth, T. Keys to the genera and species of blow flies (Diptera: Calliphoridae) of the West Indies and description of a new species of Lucilia Robineau-Desvoidy. Zootaxa 2010, 2663, 1–35. [Google Scholar] [CrossRef]
- Wood, S.N.; Pya, N.; Säfken, B. Smoothing parameter and model selection for general smooth models. J. Am. Stat. Assoc. 2016, 111, 1548–1563. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman and Hall/CRC: Boca Raton, FL, USA, 2017. [Google Scholar]
- Zuur, A.F.; Leno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B. Lme4: Linear Mixed-Effects Models Using S4 Classes, R Package Version 0.999999-0; R Foundation for Statistical Computing: Vienna, Austria, 2012; Available online: http://CRAN.R-project.org/package=lme4 (accessed on 11 November 2024).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Rusch, T.W.; Faris, A.M.; Beebe, L.E.; Tomberlin, J.K.; Tarone, A.M. The upper thermal tolerance for a Texas population of the hairy maggot blow fly Chrysomya rufifacies Macquart (Diptera: Calliphoridae). Ecol. Entomol. 2020, 45, 1146–1157. [Google Scholar] [CrossRef]
- Richards, C.S.; Price, B.W.; Villet, M.H. Thermal ecophysiology of seven carrion-feeding blowflies in Southern Africa. Entomol. Exp. Appl. 2009, 131, 11–19. [Google Scholar] [CrossRef]
- Canavoso, L.E.; Jouni, Z.E.; Karnas, K.J.; Pennington, J.E.; Wells, M.A. Fat metabolism in insects. Annu. Rev. Nutr. 2001, 21, 23–46. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Lease, H.M.; Wolf, B.O. Lipid content of terrestrial arthropods in relation to body size, phylogeny, ontogeny and sex. Physiol. Entomol. 2011, 36, 29–38. [Google Scholar] [CrossRef]
- Davies, L.; Ratcliffe, G. Development rates of some pre-adult stages in blowflies with reference to low temperatures. Med. Vet. Entomol. 1994, 8, 245–254. [Google Scholar] [CrossRef]
- McLaughlin, L.G.; Wasserberg, G. Spatial bet hedging in sand fly oviposition: Factors affecting skip oviposition in Phlebotomus papatasi sand flies. Vector-Borne Zoonotic Dis. 2021, 21, 280–288. [Google Scholar] [CrossRef]
- Arribas, P.; Abellán, P.; Velasco, J.; Bilton, D.T.; Millán, A.; Sánchez-Fernández, D. Evaluating drivers of vulnerability to climate change: A guide for insect conservation strategies. Glob. Change Biol. 2012, 18, 2135–2146. [Google Scholar] [CrossRef]
- Foden, W.B.; Butchart, S.H.; Stuart, S.N.; Vié, J.-C.; Akçakaya, H.R.; Angulo, A.; DeVantier, L.M.; Gutsche, A.; Turak, E.; Cao, L. Identifying the world's most climate change vulnerable species: A systematic trait-based assessment of all birds, amphibians and corals. PLoS ONE 2013, 8, e65427. [Google Scholar] [CrossRef]
- Kellermann, V.; van Heerwaarden, B. Terrestrial insects and climate change: Adaptive responses in key traits. Physiol. Entomol. 2019, 44, 99–115. [Google Scholar] [CrossRef]
- Blomefield, T.; Giliomee, J. Effect of temperature on the oviposition, longevity and mating of codling moth, Cydia pomonella (L.)(Lepidoptera: Tortricidae). Afr. Entomol. 2011, 19, 42–60. [Google Scholar] [CrossRef]
- Fouche, Q.; Hedouin, V.; Charabidze, D. Communication in necrophagous Diptera larvae: Interspecific effect of cues left behind by maggots and implications in their aggregation. Sci. Rep. 2018, 8, 2844. [Google Scholar] [CrossRef]
- Simons, A.M.; Johnston, M.O. Developmental instability as a bet-hedging strategy. Oikos 1997, 80, 401–406. [Google Scholar] [CrossRef]
- Einum, S.; Fleming, I.A. Environmental unpredictability and offspring size: Conservative versus diversified bet-hedging. Evol. Ecol. Res. 2004, 6, 443–455. [Google Scholar]
- Clark, B.R.; Faeth, S.H. The evolution of egg clustering in butterflies: A test of the egg desiccation hypothesis. Evol. Ecol. 1998, 12, 543–552. [Google Scholar] [CrossRef]
- Jaenike, J. On optimal oviposition behavior in phytophagous insects. Theor. Popul. Biol. 1978, 14, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.N. Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomol. Exp. Appl. 1988, 47, 3–14. [Google Scholar] [CrossRef]
- Riemann, J.G. A cytological study of radiation effects in testes of the screw-worm fly, Cochliomyia hominivorax (Diptera: Calliphoridae). Ann. Entomol. Soc. Am. 1967, 60, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, J.J.; Byers, J.E.; Bierwagen, B.G.; Dukes, J.S. Five potential consequences of climate change for invasive species. Conserv. Biol. 2008, 22, 534–543. [Google Scholar] [CrossRef]
- Sutherst, R.W. Climate change and invasive species: A conceptual framework. Invasive Species Chang. World 2000, 211–240. [Google Scholar]
- Mooney, H.; Larigauderie, A.; Cesario, M.; Elmquist, T.; Hoegh-Guldberg, O.; Lavorel, S.; Mace, G.M.; Palmer, M.; Scholes, R.; Yahara, T. Biodiversity, climate change, and ecosystem services. Curr. Opin. Environ. Sustain. 2009, 1, 46–54. [Google Scholar] [CrossRef]
- Montoya, J.M.; Raffaelli, D. Climate change, biotic interactions and ecosystem services. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2013–2018. [Google Scholar] [CrossRef]
- Somero, G.N. The physiology of climate change: How potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 2010, 213, 912–920. [Google Scholar] [CrossRef]
- Domisch, S.; Jaehnig, S.C.; Haase, P. Climate-change winners and losers: Stream macroinvertebrates of a submontane region in Central Europe. Freshw. Biol. 2011, 56, 2009–2020. [Google Scholar] [CrossRef]
- Tewksbury, J.J.; Huey, R.B.; Deutsch, C.A. Putting the heat on tropical animals. Science 2008, 320, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef]
- Bellard, C.; Thuiller, W.; Leroy, B.; Genovesi, P.; Bakkenes, M.; Courchamp, F. Will climate change promote future invasions? Glob. Change Biol. 2013, 19, 3740–3748. [Google Scholar] [CrossRef] [PubMed]
- Brundage, A.; Benbow, M.E.; Tomberlin, J.K. Priority effects on the life-history traits of two carrion blow fly (Diptera, Calliphoridae) species. Ecol. Entomol. 2014, 39, 539–547. [Google Scholar] [CrossRef]
- Lihou, K.; Wall, R. Sheep blowfly strike: The cost of control in relation to risk. Animal 2019, 13, 2373–2378. [Google Scholar] [CrossRef]
- Hall, M. Traumatic myiasis of sheep in Europe: A review. Parassitologia 1997, 39, 409–413. [Google Scholar]
- Hallman, G.J.; Denlinger, D.L. Temperature Sensitivity in Insects and Application in Integrated Pest Management; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusch, T.W.; Sawyer, S.J.; Orr, A.E.; Richter, N.; Sohn, D.; Gagner, L.; Smith, A.; Tomberlin, J.K.; Tarone, A.M. Temperature Effects on the Survival and Oviposition of an Invasive Blow Fly Chrysomya rufifacies Macquart (Diptera: Calliphoridae). Insects 2025, 16, 310. https://doi.org/10.3390/insects16030310
Rusch TW, Sawyer SJ, Orr AE, Richter N, Sohn D, Gagner L, Smith A, Tomberlin JK, Tarone AM. Temperature Effects on the Survival and Oviposition of an Invasive Blow Fly Chrysomya rufifacies Macquart (Diptera: Calliphoridae). Insects. 2025; 16(3):310. https://doi.org/10.3390/insects16030310
Chicago/Turabian StyleRusch, Travis W., Samantha J. Sawyer, Abigail E. Orr, Nicholas Richter, David Sohn, Lauren Gagner, Alexandria Smith, Jeffery K. Tomberlin, and Aaron M. Tarone. 2025. "Temperature Effects on the Survival and Oviposition of an Invasive Blow Fly Chrysomya rufifacies Macquart (Diptera: Calliphoridae)" Insects 16, no. 3: 310. https://doi.org/10.3390/insects16030310
APA StyleRusch, T. W., Sawyer, S. J., Orr, A. E., Richter, N., Sohn, D., Gagner, L., Smith, A., Tomberlin, J. K., & Tarone, A. M. (2025). Temperature Effects on the Survival and Oviposition of an Invasive Blow Fly Chrysomya rufifacies Macquart (Diptera: Calliphoridae). Insects, 16(3), 310. https://doi.org/10.3390/insects16030310




