Exploring the Genetic Diversity of the Jewel Beetles Sternocera aequisignata Saunders, 1866, and S. ruficornis Saunders, 1866 (Coleoptera: Buprestidae) in Thailand and Lao PDR
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Molecular Analyses
2.2. DNA Sequence Analyses
2.3. Phylogenetic Tree Analyses
2.4. Delineation of Genetic Groups
3. Results
3.1. Molecular Diversity Indices
3.1.1. Green-Legged S. aequisignata
3.1.2. Red-Legged S. ruficornis
3.2. Neutrality Test
3.2.1. Green-Legged S. aequisignata
3.2.2. Red-Legged S. ruficornis
3.3. Haplotype Network
3.4. Genetic Differences and Genetic Structure Analyses
3.5. Phylogenetic Trees
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, A.; Mckenna, D.D.; Bellamy, C.L.; Farrell, B.D. Large-scale molecular phylogeny of metallic wood-boring beetles (Coleoptera: Buprestoidea) provides new insights into relationships and reveals multiple evolutionary origins of the larval leaf-mining habit. Syst. Entomol. 2014, 40, 385–400. [Google Scholar] [CrossRef]
- Virayaidya, P.; Annes, P. Some Splendid Crafts; Bangkok Printing Co., Ltd.: Bangkok, Thailand, 1994. [Google Scholar]
- Bellamy, C.L. The World of Jewel Beetles (version Aug 2014). In Catalogue of Life; (Version 2024-12-19); Bánki, O., Roskov, Y., Döring, M., Ower, G., Hernández Robles, D.R., Plata Corredor, C.A., Stjernegaard Jeppesen, T., Örn, A., Pape, T., Hobern, D., et al., Eds.; Catalogue of Life: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Pinkaew, N. Some biological aspects of Sternocera ruficornis Saunder, 1866 in dry dipterocarp forest at Sakaerat Environmental Research Station. Kasetsart J. 2001, 35, 132–138. [Google Scholar]
- Hawkeswood, T.J.; Sommung, B. Review of the biology of Sternocera aequisignata Saunders, 1866 (Coleoptera: Buprestidae) in Thailand. Calodema 2016, 414, 1–6. [Google Scholar]
- Jaroenkietkajorn, U.; Gheewala, S.H.; Scherer, L. Species loss from land use of oil palm plantations in Thailand. Ecol. Indic. 2021, 133, 108444. [Google Scholar] [CrossRef]
- Pellegrino, I.; Curletti, G.; Liberatore, F.; Cucco, M. Cryptic diversity of the jewel beetles Agrilus viridis (Coleoptera: Buprestidae) hosted on hazelnut. Eur. Zool. J. 2017, 84, 465–472. [Google Scholar] [CrossRef]
- Muanglen, N.; Sribenja, K.; Chaiyasan, P.; Tanomtong, A.; Nuchjangreed, C. A new report on karyological analysis of the jewel-beetle, Sternocera ruficornis (Coleoptera, Buprestidae) from Thailand. Sci. Technol. Eng. J. 2021, 7, 49–57. [Google Scholar]
- Pradit, N.; Saijuntha, W.; Pilap, W.; Suksavate, W.; Agatsuma, T.; Jongsomchai, K.; Kongbuntad, W.; Tantrawatpan, C. Genetic variation of Tarbinskiellus portentosus (Lichtenstein 1796) (Orthoptera: Gryllidae) in mainland Southeast Asia examined by mitochondrial DNA sequences. Int. J. Trop. Insect Sci. 2022, 42, 955–964. [Google Scholar] [CrossRef]
- Pradit, N.; Tantrawatpan, C.; Thanee, I.; Jayareon, P.; Pilap, W.; Saijuntha, W. Genetic variation of the giant water bug Lethocerus indicus (Lepeletier and Serville, 1825) (Hemiptera: Belostomatidae) collected from natural habitats in northeastern Thailand. Aquat. Insects. 2022, 43, 307–318. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/ NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 18, 3022–3027. [Google Scholar] [CrossRef]
- Bandelt, H.J.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Molec. Ecol. Res. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Res. 2021, 21, 609–620. [Google Scholar] [CrossRef]
- Zhang, J.; Kapli, R.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placments. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef]
- Vangno, W.; Pradit, N.; Pilap, W.; Jaroenchaiwattanachote, C.; Duangkhamchan, W.; Saijuntha, J.; Thanee, I.; Plant, A.R.; Tawong, W.; Saijuntha, W. Revealing a cryptic species of mole cricket genus Gryllotalpa Latreille, 1802 (Orthoptera: Gryllotalpidae) in Roi Et Province, Thailand, detected by mitochondrial DNA sequences. Biologia 2025, 80, 323–333. [Google Scholar] [CrossRef]
- Krovi, R.S.; Amer, N.; Oczkowicz, M.; Kajtoch, Ł. Meta-analysis of spatial genetic patterns among European saproxylic beetles. Biodivers. Conserv. 2025, 34, 1–27. [Google Scholar] [CrossRef]
- Moctezuma, V. Spatial autocorrelation in a Mexican dung beetle ensemble: Implications for biodiversity assessment and monitoring. Ecol. Indic. 2021, 125, 107548. [Google Scholar] [CrossRef]
- Rousset, F. Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics 1997, 145, 1219–1228. [Google Scholar] [CrossRef]
- Crispo, E.; Bentzen, P.; Reznick, D.N.; Kinnison, M.T.; Hendry, A.P. The relative influence of natural selection and geography on gene flow in guppies. Mol. Ecol. 2006, 15, 49–62. [Google Scholar] [CrossRef]
- Spear, S.F.; Balkenhol, N.; Fortin, M.J.; McRae, B.H.; Scribner, K. Use of resistance surfaces for landscape genetic studies: Considerations for parameterization and analysis. Mol. Ecol. 2010, 19, 3576–3591. [Google Scholar] [CrossRef]
- Revell, L.J. Phylogenetic signal and linear regression on species data. Methods Ecol. Evol. 2010, 1, 319–329. [Google Scholar] [CrossRef]
- Tantrawatpan, C.; Saijuntha, W.; Pilab, W.; Sakdakham, K.; Pasorn, P.; Thanonkeo, S.; Thiha; Satrawaha, R.; Petney, T. Genetic differentiation among populations of Brachytrypes portentosus (Lichtenstein 1796) (Orthoptera: Gryllidae) in Thailand and the Lao PDR: The Mekong River as a biogeographic barrier. Bull. Entomol. Res. 2011, 101, 687–696. [Google Scholar] [CrossRef]
- Sareein, N.; Kang, J.H.; Jung, S.W.; Phalaraksh, C.; Bae, Y.J. Taxonomic review and distribution of giant water bugs (Hemiptera: Belostomatidae: Lethocerinae) in the Palearctic, Oriental, and Australian regions. Entomol. Res. 2019, 49, 462–473. [Google Scholar] [CrossRef]
- Ivy, J.A.; Lacy, R.C. A Comparison of Strategies for Selecting Breeding Pairs to Maximize Genetic Diversity Retention in Managed Populations. J. Hered. 2012, 103, 186–196. [Google Scholar] [CrossRef]
- Willoughby, J.R.; Ivy, J.A.; Lacy, R.C.; Doyle, J.M.; DeWoody, J.A. Inbreeding and selection shape genomic diversity in captive populations: Implications for the conservation of endangered species. PLoS ONE 2017, 12, e0175996. [Google Scholar] [CrossRef]
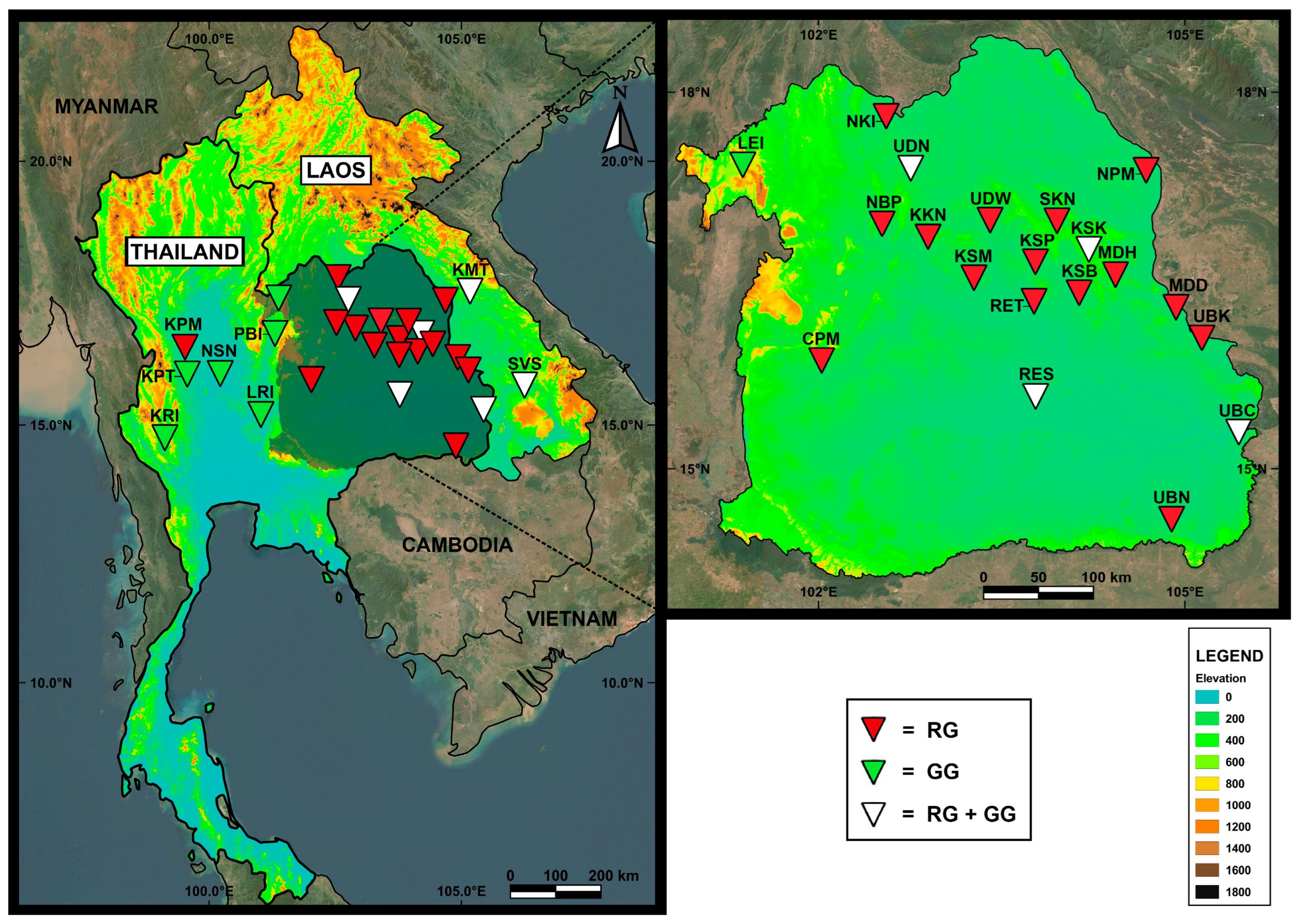
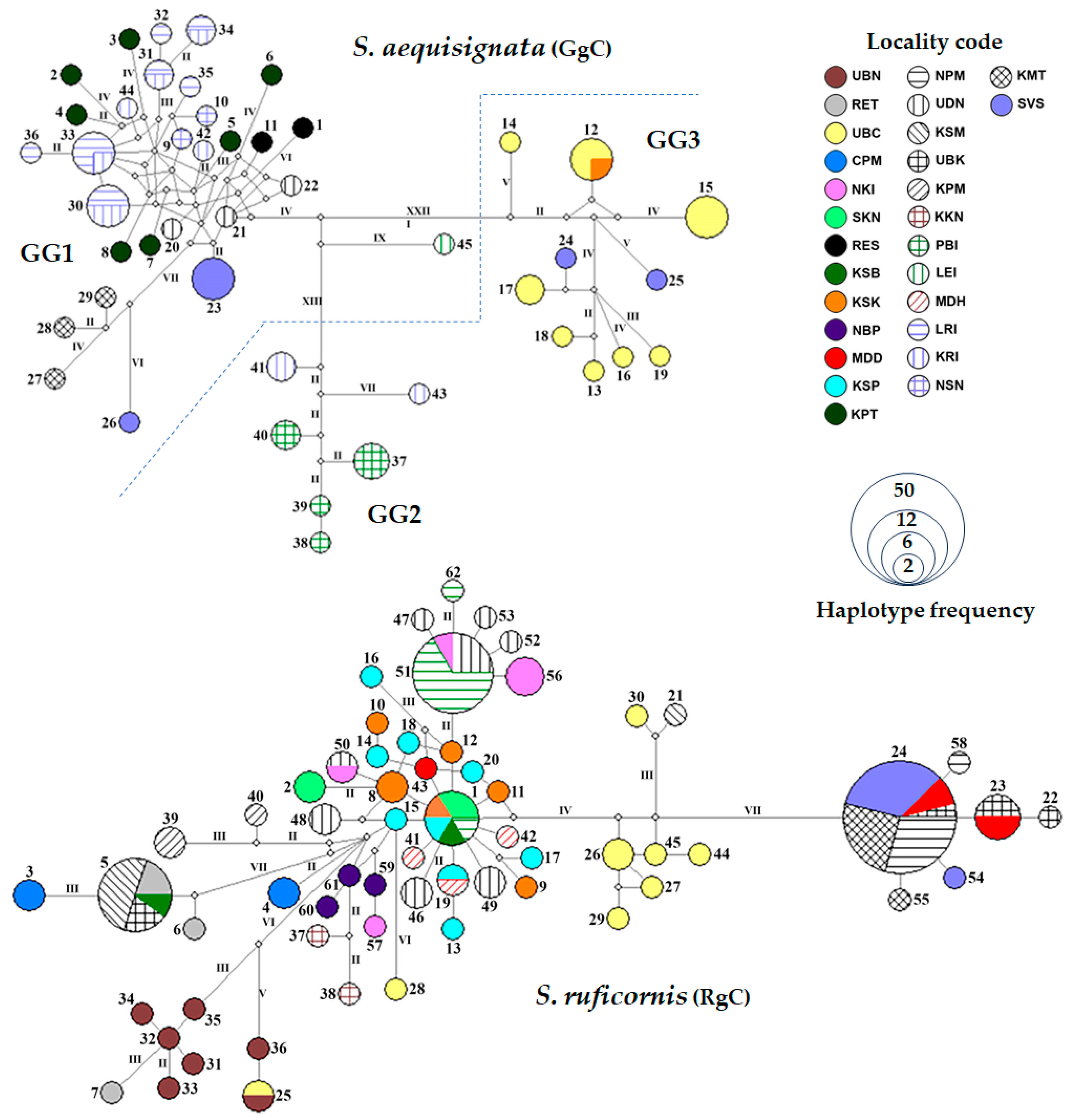

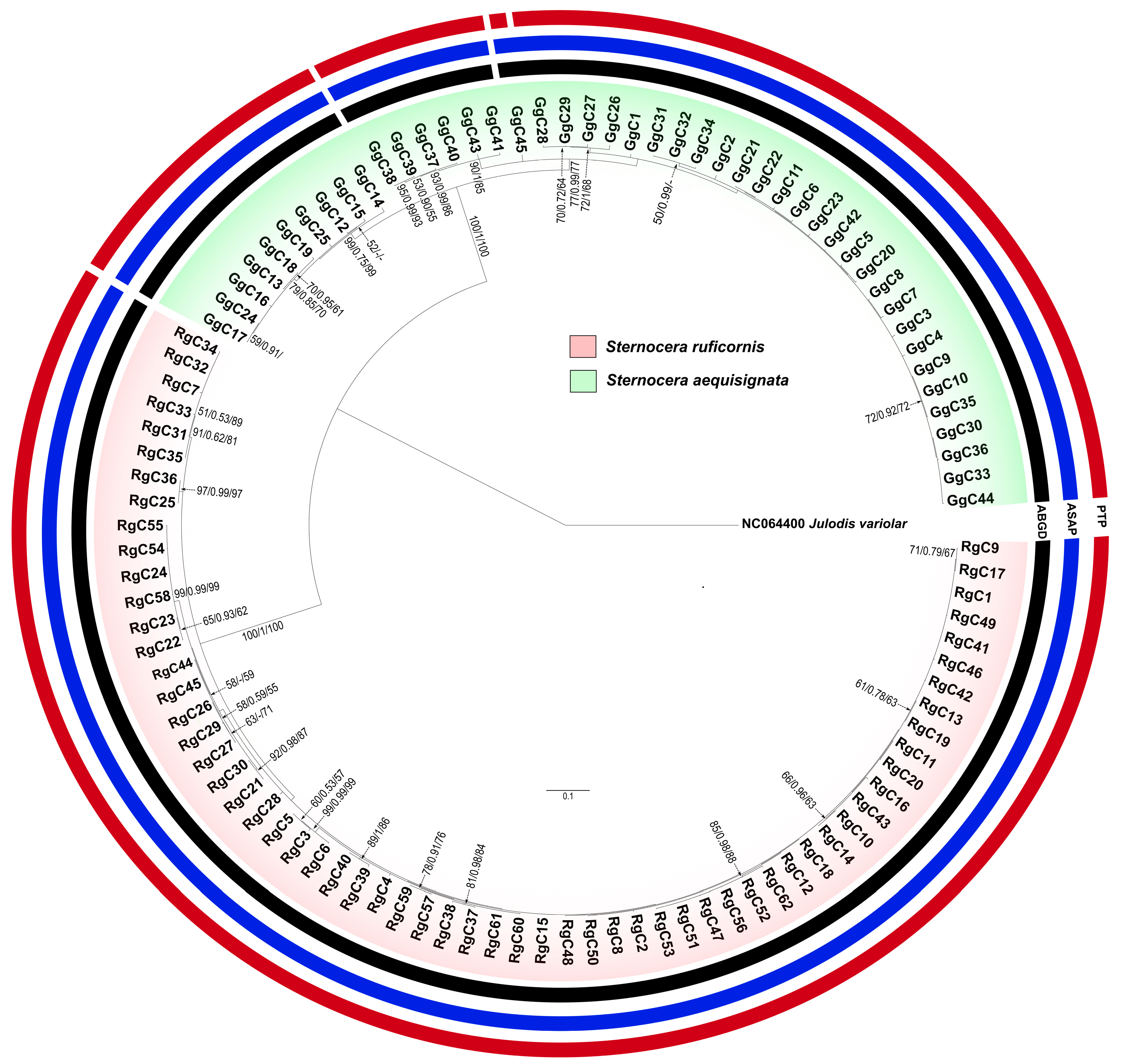
| Country | Province | District | Code | Region | 16S rDNA | CO1 | ||
|---|---|---|---|---|---|---|---|---|
| RG a | GG b | RG | GG | |||||
| THAILAND | Nakhon Sawan | Banphot Phisai | NSN | Central | - | 2 | - | 2 |
| Lopburi | Chaibadan | LRI | Central | - | 12 | - | 10 | |
| Kampang Phet | Khanu Woralaksaburi | KPT | North | - | 10 | - | 7 | |
| Mueang | KPM | North | 3 | - | 3 | - | ||
| Phetchabun | Lom Sak | PBI | North | - | 14 | - | 7 | |
| Kanchanaburi | Si Sawat | KRI | West | - | 12 | - | 10 | |
| Chaiyaphum | Mueang | CPM | Northeast | 7 | - | 4 | - | |
| Kalasin | Khao Wong | KSK | Northeast | 7 | - | 7 | 1 | |
| Huai Mek | KSM | Northeast | 8 | - | 6 | - | ||
| Huai Phueng | KSP | Northeast | 9 | - | 9 | - | ||
| Baukhao | KSB | Northeast | 2 | - | - | - | ||
| Khon Kaen | Khao Suan Kwang | KKN | Northeast | 5 | - | 2 | - | |
| Loei | Phu Ruea | LEI | Northeast | - | 1 | - | 1 | |
| Mukdahan | Kamcha-i | MDH | Northeast | 3 | - | 3 | - | |
| Dontan | MDD | Northeast | 5 | - | 5 | - | ||
| Nakhon Phanom | Mueang | NPM | Northeast | 11 | - | 8 | - | |
| Nong Bua Lam Phu | Non Sang | NBP | Northeast | 9 | - | 3 | - | |
| Nong Khai | Tha Bo | NKI | Northeast | 11 | - | 6 | - | |
| Roi Et | Pho Chai | RET | Northeast | 9 | - | 4 | - | |
| Suwannaphumm | RES | Northeast | 1 | 2 | - | 2 | ||
| Sakon Nakhon | Phu Phan | SKN | Northeast | 7 | - | 4 | - | |
| Ubon Ratchathani | Khemarat | UBK | Northeast | 8 | - | 6 | - | |
| Khong Chiam | UBC | Northeast | 8 | 13 | 9 | 14 | ||
| Nam Khun | UBN | Northeast | 9 | - | 7 | - | ||
| Udonthani | Mueang | UDN | Northeast | 19 | 3 | 13 | 3 | |
| Wang Sam Mo | UDW | Northeast | - | - | 10 | - | ||
| LAO PDR | Khammouan | Tha Khek | KMT | South | 9 | 4 | 7 | 3 |
| Svannakhet | Songkon | SVS | South | 11 | 7 | 9 | 7 | |
| Total | 161 | 80 | 125 | 67 | ||||
| Populations | CO1 | 16S rDNA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | S | H | Uh | Hd ± SD | Nd ± SD | n | S | H | Uh | Hd ± SD | Nd ± SD | |
| NSN | 2 | 2 | 2 | 2 | 1.000 ± 0.500 | 0.0032 ± 0.0016 | 2 | 1 | 2 | 0 | 1.000 ± 0.500 | 0.0021 ± 0.0011 |
| LRI | 10 | 13 | 7 | 3 | 0.911 ± 0.077 | 0.0066 ± 0.0013 | 12 | 13 | 8 | 3 | 0.924 ± 0.057 | 0.0063 ± 0.0019 |
| KPT | 7 | 26 | 7 | 7 | 1.000 ± 0.076 | 0.0143 ± 0.0014 | 10 | 11 | 7 | 5 | 0.911 ± 0.077 | 0.0070 ± 0.0010 |
| PBI | 7 | 7 | 4 | 4 | 0.810 ± 0.130 | 0.0053 ± 0.0009 | 14 | 5 | 3 | 3 | 0.484 ± 0.142 | 0.0028 ± 0.0010 |
| KRI | 10 | 39 | 8 | 4 | 0.956 ± 0.059 | 0.0236 ± 0.0047 | 12 | 13 | 7 | 2 | 0.894 ± 0.063 | 0.0087 ± 0.0015 |
| RES | 2 | 9 | 2 | 2 | 1.000 ± 0.500 | 0.0144 ± 0.0072 | 2 | 1 | 2 | 1 | 1.000 ± 0.500 | 0.0021 ± 0.0011 |
| UBC | 14 | 29 | 8 | 7 | 0.890 ± 0.060 | 0.0134 ± 0.0013 | 13 | 5 | 7 | 5 | 0.885 ± 0.064 | 0.0032 ± 0.0005 |
| UDN | 3 | 7 | 3 | 3 | 1.000 ± 0.272 | 0.0074 ± 0.0027 | 3 | 3 | 3 | 2 | 1.000 ± 0.272 | 0.0042 ± 0.0015 |
| KMT | 3 | 7 | 3 | 3 | 1.000 ± 0.272 | 0.0074 ± 0.0023 | 4 | 2 | 3 | 3 | 0.833 ± 0.222 | 0.0025 ± 0.0008 |
| SVS | 7 | 51 | 4 | 4 | 0.714 ± 0.181 | 0.0324 ± 0.0092 | 7 | 9 | 4 | 1 | 0.714 ± 0.181 | 0.0088 ± 0.0020 |
| Total | 67 | 105 | 45 | 39 | 0.983 ± 0.006 | 0.0361 ± 0.0020 | 80 | 29 | 35 | 25 | 0.958 ± 0.010 | 0.0116 ± 0.0004 |
| Populations | CO1 | 16S rDNA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | S | H | Uh | Hd ± SD | Nd ± SD | n | S | H | Uh | Hd ± SD | Nd ± SD | |
| KPM | 3 | 4 | 2 | 2 | 0.667 ± 0.314 | 0.0043 ± 0.0020 | 3 | 0 | 1 | 0 | 0 | 0 |
| CPM | 4 | 13 | 2 | 2 | 0.667 ± 0.204 | 0.0138 ± 0.0042 | 7 | 0 | 1 | 0 | 0 | 0 |
| KSK | 7 | 8 | 6 | 5 | 0.952 ± 0.096 | 0.0040 ± 0.0009 | 7 | 2 | 2 | 1 | 0.286 ± 0.196 | 0.0012 ± 0.0008 |
| KSM | 6 | 16 | 2 | 1 | 0.333 ± 0.215 | 0.0085 ± 0.0055 | 8 | 2 | 3 | 2 | 0.607 ± 0.164 | 0.0014 ± 0.0005 |
| KSP | 9 | 13 | 9 | 7 | 1.000 ± 0.052 | 0.0056 ± 0.0010 | 10 | 0 | 1 | 0 | 0 | 0 |
| KSB | 2 | 11 | 2 | 0 | 1.000 ± 0.500 | 0.0175 ± 0.0088 | 2 | 2 | 2 | 1 | 1.000 ± 0.500 | 0.0042 ± 0.0021 |
| KKN | 2 | 3 | 2 | 2 | 1.000 ± 0.500 | 0.0048 ± 0.0024 | 5 | 0 | 1 | 1 | 0 | 0 |
| MDH | 3 | 3 | 3 | 2 | 1.000 ± 0.272 | 0.0032 ± 0.0009 | 3 | 0 | 1 | 0 | 0 | 0 |
| MDD | 5 | 15 | 3 | 1 | 0.800 ± 0.164 | 0.0099 ± 0.0053 | 6 | 4 | 3 | 1 | 0.600 ± 0.215 | 0.0028 ± 0.0014 |
| NPM | 8 | 1 | 2 | 1 | 0.250 ± 0.180 | 0.0004 ± 0.0003 | 11 | 0 | 1 | 0 | 0 | 0 |
| NBP | 3 | 3 | 3 | 3 | 1.000 ± 0.272 | 0.0032 ± 0.0011 | 9 | 2 | 3 | 3 | 0.556 ± 0.165 | 0.0013 ± 0.0005 |
| NKI | 6 | 10 | 4 | 2 | 0.800 ± 0.172 | 0.0067 ± 0.0022 | 11 | 1 | 2 | 0 | 0.436 ± 0.133 | 0.0009 ± 0.0003 |
| RET | 4 | 23 | 3 | 2 | 0.833 ± 0.222 | 0.0186 ± 0.0089 | 9 | 5 | 3 | 1 | 0.417 ± 0.191 | 0.0027 ± 0.0014 |
| SKN | 4 | 3 | 2 | 1 | 0.667 ± 0.204 | 0.0032 ± 0.0010 | 7 | 2 | 3 | 1 | 0.524 ± 0.209 | 0.0012 ± 0.0005 |
| UBK | 6 | 20 | 4 | 1 | 0.867 ± 0.129 | 0.0168 ± 0.0050 | 8 | 1 | 2 | 1 | 0.250 ± 0.180 | 0.0005 ± 0.0004 |
| UBC | 9 | 26 | 8 | 7 | 0.972 ± 0.064 | 0.0120 ± 0.0038 | 11 | 8 | 6 | 5 | 0.727 ± 0.144 | 0.0034 ± 0.0010 |
| UBN | 7 | 13 | 7 | 6 | 1.000 ± 0.076 | 0.0087 ± 0.0021 | 9 | 4 | 5 | 4 | 0.861 ± 0.087 | 0.0028 ± 0.0006 |
| UDN | 13 | 13 | 8 | 6 | 0.923 ± 0.050 | 0.0064 ± 0.0006 | 19 | 4 | 5 | 3 | 0.591 ± 0.118 | 0.0015 ± 0.0004 |
| UDW | 10 | 5 | 3 | 1 | 0.378 ± 0.181 | 0.0016 ± 0.0008 | n/a | n/a | n/a | n/a | n/a | n/a |
| KMT | 7 | 1 | 2 | 1 | 0.286 ± 0.196 | 0.0005 ± 0.0003 | 9 | 0 | 1 | 0 | 0 | 0 |
| SVS | 9 | 1 | 2 | 1 | 0.222 ± 0.166 | 0.0004 ± 0.0003 | 11 | 1 | 2 | 1 | 0.182 ± 0.144 | 0.0004 ± 0.0003 |
| Total | 127 | 81 | 62 | 54 | 0.947 ± 0.012 | 0.0174 ± 0.0007 | 166 | 29 | 28 | 25 | 0.706 ± 0.029 | 0.0030 ± 0.0003 |
| Source of Variation | d.f. | Sum of Squares | Variance Components | Percentage of Variation | Fixation Index |
|---|---|---|---|---|---|
| Among groups | 2 | 479.653 | 12.1708 | 73.06 | FCT = 0.73059 * |
| Among populations within groups | 10 | 88.739 | 1.3709 | 8.23 | FSC = 0.30545 * |
| Within populations | 53 | 165.214 | 3.11725 | 18.71 | FST = 0.81288 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thaenasa, A.; Pradit, N.; Pilap, W.; Jaroenchaiwattanachote, C.; Wongpakam, K.; Inkhavilay, K.; Saijuntha, J.; Tawong, W.; Suksavate, W.; Tantrawatpan, C.; et al. Exploring the Genetic Diversity of the Jewel Beetles Sternocera aequisignata Saunders, 1866, and S. ruficornis Saunders, 1866 (Coleoptera: Buprestidae) in Thailand and Lao PDR. Insects 2025, 16, 322. https://doi.org/10.3390/insects16030322
Thaenasa A, Pradit N, Pilap W, Jaroenchaiwattanachote C, Wongpakam K, Inkhavilay K, Saijuntha J, Tawong W, Suksavate W, Tantrawatpan C, et al. Exploring the Genetic Diversity of the Jewel Beetles Sternocera aequisignata Saunders, 1866, and S. ruficornis Saunders, 1866 (Coleoptera: Buprestidae) in Thailand and Lao PDR. Insects. 2025; 16(3):322. https://doi.org/10.3390/insects16030322
Chicago/Turabian StyleThaenasa, Anisanee, Nakorn Pradit, Warayutt Pilap, Chavanut Jaroenchaiwattanachote, Komgrit Wongpakam, Khamla Inkhavilay, Jatupon Saijuntha, Wittaya Tawong, Warong Suksavate, Chairat Tantrawatpan, and et al. 2025. "Exploring the Genetic Diversity of the Jewel Beetles Sternocera aequisignata Saunders, 1866, and S. ruficornis Saunders, 1866 (Coleoptera: Buprestidae) in Thailand and Lao PDR" Insects 16, no. 3: 322. https://doi.org/10.3390/insects16030322
APA StyleThaenasa, A., Pradit, N., Pilap, W., Jaroenchaiwattanachote, C., Wongpakam, K., Inkhavilay, K., Saijuntha, J., Tawong, W., Suksavate, W., Tantrawatpan, C., & Saijuntha, W. (2025). Exploring the Genetic Diversity of the Jewel Beetles Sternocera aequisignata Saunders, 1866, and S. ruficornis Saunders, 1866 (Coleoptera: Buprestidae) in Thailand and Lao PDR. Insects, 16(3), 322. https://doi.org/10.3390/insects16030322







