Seasonal Dynamics and Factors Shaping Aquatic Insect Assemblages in Mountain Streams of the Pannonian Lowland Ecoregion
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Aquatic Insect Assemblage Analysis
2.3. Environmental Conditions Analysis
2.4. Data Analysis
3. Results
3.1. Aquatic Insect Assemblages
3.2. Papuk Mountain Streams
3.3. Psunj Mountain Streams
3.4. Medvednica Mountain Streams
3.5. Spatial and Seasonal Diversity
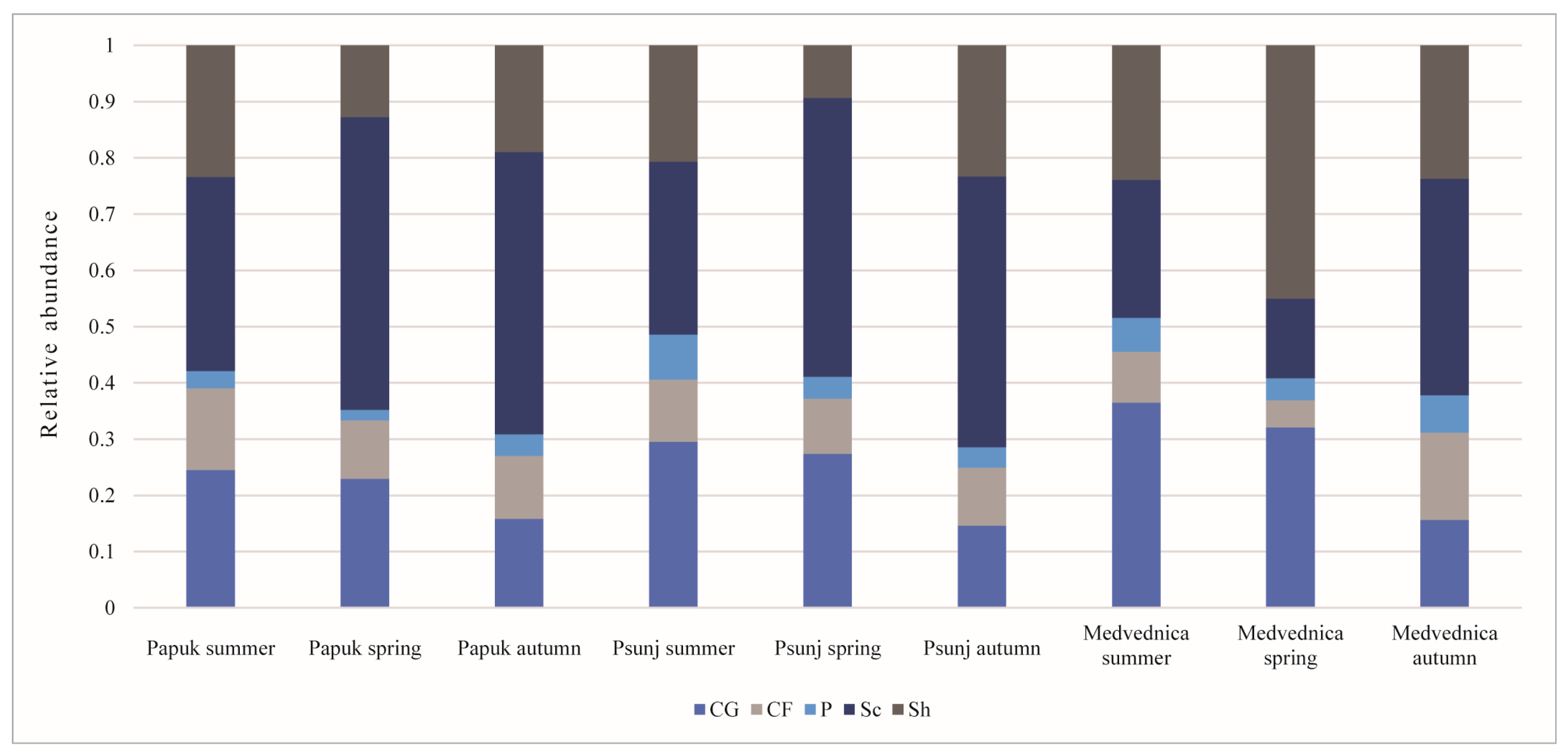
3.6. Functional Feeding Groups
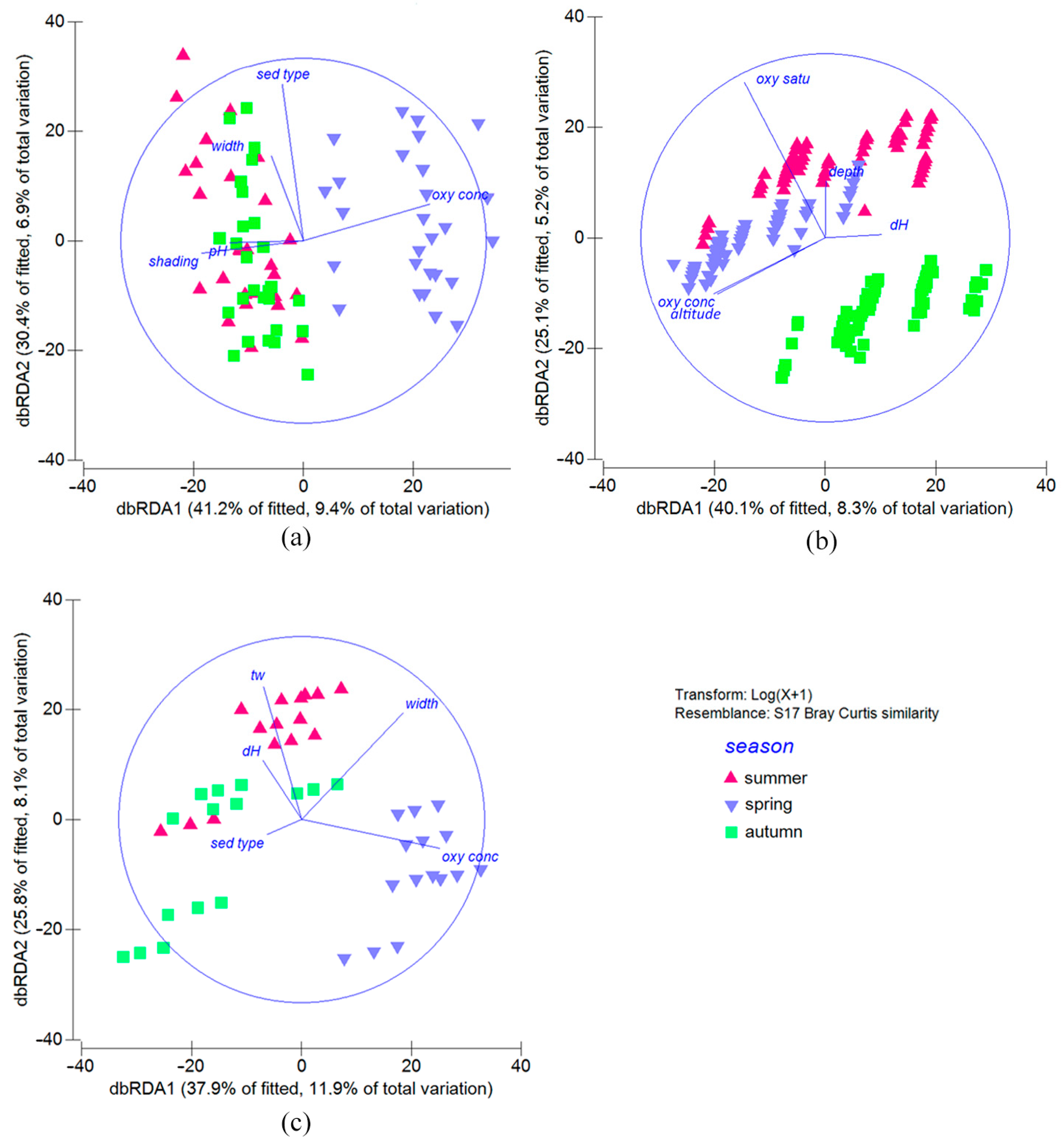
3.7. Environmental Conditions Affecting Aquatic Insect Assemblages Related to Seasonality
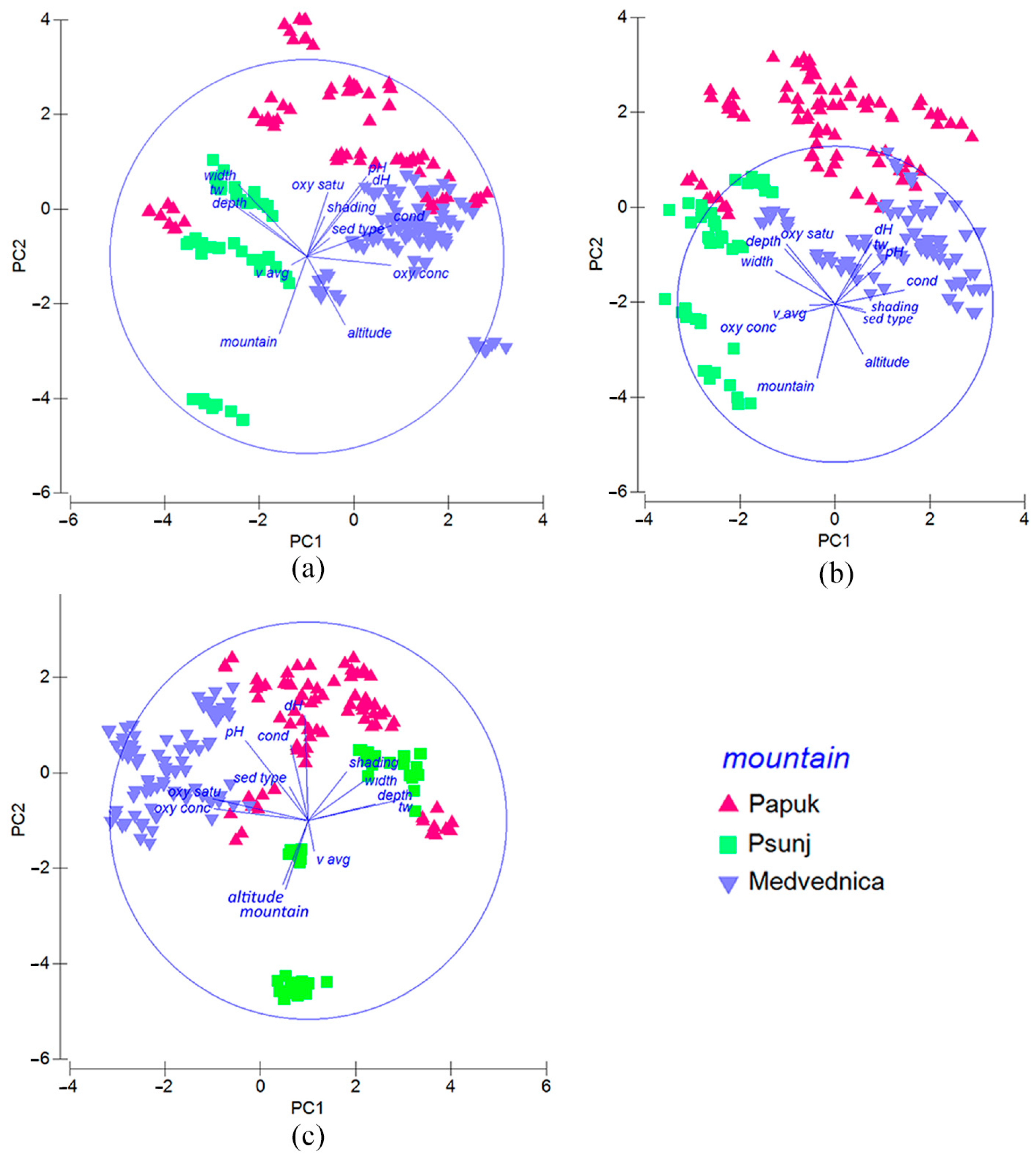
3.8. Environmental Parameters Influencing Aquatic Insect Assemblages as a Function of Spatial Factor
4. Discussion
4.1. Contribution to Species Diversity
4.2. Spatial and Seasonal Variability in Aquatic Insect Assemblages
4.3. Influence of Environmental Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moore, R.D.; Richardson, J.S. Progress towards Understanding the Structure, Function, and Ecological Significance of Small Stream Channels and Their Riparian Zones. Can. J. For. Res. 2003, 1, 1349–1351. [Google Scholar] [CrossRef]
- Richardson, J.S. Biological Diversity in Headwater Streams. Water 2019, 11, 366. [Google Scholar] [CrossRef]
- Pero, E.J.I.; Georgieff, S.M.; de Lourdes Gultemirian, M.; Romero, F.; Hankel, G.E.; Domínguez, E. Ecoregions, climate, topography, physicochemical, or a combination of all: Which criteria are the best to define river types based on abiotic variables and macroinvertebrates in neotropical rivers? Sci. Total Environ. 2020, 738, 140303. [Google Scholar] [CrossRef] [PubMed]
- Bylak, A.; Kukuła, K. Impact of Fine-Grained Sediment on Mountain Stream Macroinvertebrate Communities: Forestry Activities and Beaver-Induced Sediment Management. Sci. Total Environ. 2022, 832, 155079. [Google Scholar] [CrossRef] [PubMed]
- Owens, P.N.; Batalla, R.J.; Collins, A.J.; Gomez, B.; Hicks, D.M.; Horowitz, A.J.; Kondolf, G.M.; Marden, M.; Page, M.J.; Peacock, D.H.; et al. Fine-Grained Sediment in River Systems: Environmental Significance and Management Issues. River Res. Appl. 2005, 21, 693–717. [Google Scholar] [CrossRef]
- Walling, D.E.; Fang, D. Recent Trends in the Suspended Sediment Loads of the World’s Rivers. Glob. Planet Chang. 2003, 39, 111–126. [Google Scholar] [CrossRef]
- Power, M.E.; Dietrich, W.E. Food webs in river networks. Ecol. Res. 2002, 17, 451–471. [Google Scholar]
- Lamouroux, N.; Dolédec, S.; Gayraud, S. Biological Traits of Stream Macroinvertebrate Communities: Effects of Microhabitat, Reach, and Basin Filters. J. N. Am. Benthol. Soc. 2004, 23, 449–466. [Google Scholar] [CrossRef]
- Petrović, A.; Milošević, D.; Paunović, M.; Simić, S.; Đorđević, N.; Stojković, M.; Simić, V. New Data on the Distribution and Ecology of the Mayfly Larvae (Insecta: Ephemeroptera) of Serbia (Central Part of the Balkan Peninsula). Turk. J. Zool. 2015, 39, 195–209. [Google Scholar] [CrossRef]
- Beisel, J.-N.; Usseglio-Polatera, P.; Thomas, S.; Moreteau, J.-C. Stream Community Structure in Relation to Spatial Variation: The Influence of Mesohabitat Characteristics. Hydrobiologia 1998, 389, 73–88. [Google Scholar]
- Adler, P.H.; Courtney, G.W. Ecological and Societal Services of Aquatic Diptera. Insects 2019, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Rimcheska, B.; Vidinova, Y. Diversity and Structure of Macroinvertebrate Communities in Permanent Small Streams and Rivers in Eastern Balkans. Hydrobiologia 2023, 850, 3341–3357. [Google Scholar] [CrossRef]
- Tubić, B.; Andjus, S.; Zorić, K.; Vasiljević, B.; Jovičić, K.; Čanak Atlagić, J.; Paunović, M. Aquatic Insects (Ephemeroptera, Plecoptera and Trichoptera) Metric as an Important Tool in Water Quality Assessment in Hilly and Mountain Streams. Water 2024, 16, 849. [Google Scholar] [CrossRef]
- Milner, A.M.; Petts, G.E. Glacial Rivers: Physical Habitat and Ecology. Freshw. Biol. 1994, 32, 295–307. [Google Scholar]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The River Continuum Concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar]
- Lancaster, J.; Downes, B.J. Aquatic Entomology, 1st ed.; Oxford University Press: Oxford, UK, 2013; p. 296. [Google Scholar]
- Graça, M.A.S. The role of invertebrates on leaf litter decomposition in streams—A review. Int. Rev. Hydrobiol. 2001, 86, 383–393. [Google Scholar]
- Bauernfeind, E.; Soldán, T. The Mayflies of Europe; Apollo Books: Ollerup, Denmark, 2012; p. 779. [Google Scholar]
- Rimcheska, B.; Vidinova, Y.; Varadinova, E. Trophic Structure of Macrozoobenthos in Permanent Streams in the Eastern Balkans. Diversity 2022, 14, 1121. [Google Scholar] [CrossRef]
- Ward, J.V. Ecology of alpine streams. Freshw. Biol. 1994, 32, 277–294. [Google Scholar]
- Dettinger, M.D.; Diaz, H.F. Global Characteristics of Stream Flow Seasonality and Variability. J. Hygrometeor. 2000, 1, 289–310. [Google Scholar]
- Bereczki, C.; Szivák, I.; Móra, A.; Csabai, Z. Variation of aquatic insect assemblages among seasons and microhabitats in Hungarian second-order streams. Aquat. Insects 2012, 34, 103–112. [Google Scholar] [CrossRef]
- Duran, M. Monitoring Water Quality Using Benthic and Physicochemical of Behzat Stream in Turkey. Pol. J. Environ. Stud. 2006, 15, 709–717. [Google Scholar]
- Chagnon, M.; Kreutzweiser, D.; Mitchell, E.A.D.; Morrissey, C.A.; Noome, D.A.; Van Der Sluijs, J.P. Risks of Large-Scale Use of Systemic Insecticides to Ecosystem Functioning and Services. Environ. Sci. Pollut. Res. 2015, 22, 119–134. [Google Scholar] [CrossRef]
- Akamagwuna, F.C.; Odume, O.N. Ephemeroptera, Plecoptera and Trichoptera (EPT) Functional Feeding Group Responses to Fine Grain Sediment Stress in a River in the Eastern Cape, South Africa. Environ. Monit. Assess 2020, 192, 214. [Google Scholar] [CrossRef] [PubMed]
- El Yaagoubi, S.; El Alami, M.; Harrak, R.; Azmizem, A.; Ikssi, M.; Mansour, M.R.A. Assessment of Functional Feeding Groups (FFG) Structure of Aquatic Insects in North-Western Rif—Morocco. Biodivers. Data J. 2023, 11, e104218. [Google Scholar] [CrossRef]
- Illies, J. Limnofauna Europaea. Eine Zusammenstellung Aller Die Europäische Binnengewässer Bewohnenden Mehrzelligen Tierarten Mit Angaben Über Ihre Verbreitung Und Ökologie, 2nd ed.; Gustav Fischer Verlag: Stuttgart, Germany, 1978; p. 532. [Google Scholar]
- Pamić, J.; Radonić, G.; Pavić, G. Geološki Vodič Kroz Park Prirode Papuk; JU PP Papuk: Velika, Croatia, 2003; pp. 1–67. [Google Scholar]
- Balen, D. Evolution of Columnar Joint Polygonal Patterns at Rupnica Geosite (Papuk Geopark, Croatia). In Proceedings of the 10th Alpine Workshop “CorseAlp 2011”, Saint-Florent (Corsica), France, 10–16 April 2011; p. 5. [Google Scholar]
- Pandža, M. The Flora of the Papuk Nature Park (Slavonia, Croatia). Šumarski List 2010, 134, 25–44. [Google Scholar]
- Slovenec, D.; Halamić, J.; Šegvić, B. Middle Triassic Basaltic Pyroclastic Rocks from the Mt. Medvednica Ophiolitic Mélange (NW Croatia): Petrology, Geochemistry and Tectono-Magmatic Setting. Geol. Croat. 2024, 77, 57–68. [Google Scholar] [CrossRef]
- Glavačević, A.M.; Turković Čakalić, I.; Ergović, V.; Koh, M.; Vuić, N.; Vlaičević, B. Turbellarian Fauna (Platyhelminthes, Turbellaria) in the Water Habitats of Slavonian Mountains. In Book of abstracts, Proceedings of 12th Symposium with international participation, Kopači Rit, Past Present Future, Osijek, Croatia, 28–29 September; Public Institution Nature Park Kopački Rit: Osijek, Croatia, 2023; p. 64. [Google Scholar]
- AQEM Consortium. Manual for the Application of the AQEM System: A Comprehensive Method to Assess European Streams Using Benthic Macroinvertebrates, Developed for the Purpose of the Water Framework Directive. Published 2003. Available online: https://www.eugris.info/displayproject.asp?Projectid=4422 (accessed on 15 April 2020).
- Hering, D.; Moog, O.; Sandin, L.; Verdonschot, P.F.M. Overview and Application of the AQEM Assessment System. Hydrobiologia 2004, 516, 1–20. [Google Scholar]
- Askew, R.R. The Dragonflies of Europe, 2nd ed.; Brill: Essex, UK, 2004; p. 308. [Google Scholar]
- Eiseler, B. Bildbestimmungsschlüssel Für Die Eintagsfliegenlarven Der Deutschen Mittelgebirge Und Des Tieflandes, Identification Key to the Mayfly Larvae of the German Highlands Und Lowlands; Lauterbornia: Dinkelscherben, Germany, 2005; p. 112. [Google Scholar]
- Nilsson, A.N. Aquatic Insects of North Europe: A Taxonomic Handbook. Volume 1: Ephemeroptera, Plecoptera, Heteroptera, Neuroptera, Megaloptera, Coleoptera, Trichoptera, Lepidoptera; Apollo Books: Stenstrup, Denmark, 1996; p. 274. [Google Scholar]
- Waringer, J.; Graf, W. Atlas Der Mitteleuropliischen Kocherfliegenlarven—Atlas of Central European Trichoptera Larvae; Erik Mauch Verlag: Oinkelscherben, Germany, 2011. [Google Scholar]
- Zwick, P. Key to the West Palaearctic Genera of Stoneflies (Plecoptera) in the Larval Stage. Limnologica 2004, 34, 315–348. [Google Scholar] [CrossRef]
- Moog, O. Fauna Aquatica Austriaca. Katalog Zur Autökologischen Einstufung Aquatischer Organismen Österreichs, 2nd ed.; Bundesministerium für Land- und Forstwirtschaft, Umwelt und Wasserwirtschaft Wasserwirtschaftskataster: Wien, Austria, 2002; p. 670. [Google Scholar]
- Merritt, R.W.; Cummins, K.W.; Berg, M.B. Chapter 20—Trophic Relationships of Macroinvertebrates. In Methods in Stream Ecology, 3rd ed.; Hauer, F.R., Lamberti, G.A., Eds.; Academic Press: Boston, MA, USA, 2017; Volume 1, pp. 413–433. [Google Scholar] [CrossRef]
- Boyd, C.E.; Tucker, C.S.; Somridhivej, B. Alkalinity and Hardness: Critical but Elusive Concepts in Aquaculture. J. World Aquac. Soc. 2016, 47, 6–41. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. Primer V6: User Manual—Tutorial; PRIMER-E Limited: Plymouth, UK, 2006; p. 190. [Google Scholar]
- ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and User’s Guide to Canoco for Windows: Software for Canonical Community Ordination (Version 4); Microcomputer Power: New York, NY, USA, 1998; p. 352. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E Limited: Plymouth, UK, 2001; p. 168. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; The University of Illinois Press: Urbana, IL, USA, 1949; p. 117. [Google Scholar]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Anderson, M.J. Distance-Based Tests for Homogeneity of Multivariate Dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.R. Non-Parametric Multivariate Analyses of Changes in Community Structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Schröder, M.; Kiesel, J.; Schattmann, A.; Jähnig, S.C.; Lorenz, A.W.; Kramm, S.; Keizer-Vlek, H.; Rolauffs, P.; Graf, W.; Leitner, P.; et al. Substratum associations of benthic invertebrates in lowland and mountain streams. Ecol. Indic. 2013, 30, 178–189. [Google Scholar] [CrossRef]
- Mullner, S.A.; Hubert, W.A. Low Summer Water Temperatures Influence Occurrence of Naturalized Salmonids across a Mountain Watershed. N. Am. J. Fish Manag. 2005, 25, 1034–1040. [Google Scholar] [CrossRef]
- Clarke, A.; Mac Nally, R.; Bond, N.; Lake, P.S. Macroinvertebrate Diversity in Headwater Streams: A Review. Freshw. Biol. 2008, 53, 1707–1721. [Google Scholar] [CrossRef]
- Viviroli, D.; Archer, D.R.; Buytaert, W.; Fowler, H.J.; Greenwood, G.B.; Hamlet, A.F.; Huang, Y.; Koboltschnig, G.; Litaor, M.I.; López-Moreno, J.I.; et al. Climate Change and Mountain Water Resources: Overview and Recommendations for Research, Management and Policy. Hydrol. Earth Syst. Sci. 2011, 15, 471–504. [Google Scholar] [CrossRef]
- Vilenica, M.; Ergović, V.; Mihaljević, Z. Mayfly (Ephemeroptera) Assemblages of a Pannonian Lowland Mountain, with First Records of the Parasite Symbiocladius rhithrogenae (Zavrel, 1924) (Diptera: Chironomidae). Ann. Limnol. 2018, 545, 31. [Google Scholar] [CrossRef]
- Principe, R.E.; Márquez, J.A.; Cibils-Martina, L. Distribution and Habitat Preference of Ephemeroptera and Trichoptera in Subtropical Mountain Streams: Implications for Monitoring and Conservation. Acad. Bras. Cienc. 2019, 91, e20180692. [Google Scholar] [CrossRef]
- Vilenica, M.; Gattolliat, J.L.; Mihaljević, Z.; Sartori, M. Croatian mayflies (Insecta, Ephemeroptera): Species diversity and distribution patterns. Zookeys 2015, 523, 99–127. [Google Scholar] [CrossRef]
- Previšić, A.; Ivković, M.; Miliša, M.; Kerovec, M. Caddisfly (Insecta: Trichoptera) fauna of Papuk Nature Park in Croatia. Nat. Croat. 2013, 22, 104428. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2024-2. Available online: https://www.iucnredlist.org (accessed on 15 October 2024).
- Simović, P.; Simić, V.; Milošević, D.; Petrović, A. New Records of Species Taeniopteryx hubaulti Aubert, 1946 and Taeniopteryx schoenemundi (Mertense, 1923) (Plecoptera: Taeniopterygidae) in Serbia. J. Entomol. Res. Soc. 2023, 25, 155–166. [Google Scholar] [CrossRef]
- Balázs, A.; Fric, Z.F.; Holuša, O. Flying Activity and Population Dynamics of Cordulegaster Heros Theischinger, 1979 (Insecta: Odonata: Cordulegastridae) in Slovakia. Int. J. Odonatol. 2020, 23, 155–163. [Google Scholar] [CrossRef]
- Holuša, O.; Holušová, K.; Balázs, A. Is the Current Forest Management to the Northernmost Population of Cordulegaster Heros (Anisoptera: Cordulegastridae) in Central Europe (Czech Republic) Threatening? Forests 2023, 14, 228. [Google Scholar] [CrossRef]
- Holuša, O.; Holušová, K. Population Density and Abundance of the Northernmost Population of Cordulegaster Heros (Anisoptera: Cordulegastridae) in Europe (Czech Republic) with Notes on Its Biogeographical Range. Diversity 2022, 14, 854. [Google Scholar] [CrossRef]
- Bried, J.T.; Ervin, G.N. Distribution of Adult Odonata among Localized Wetlands in East-Central Mississippi. Southeast. Nat. 2005, 4, 731–744. [Google Scholar] [CrossRef]
- Louton, J.; Garrison, R.; Flint, O. The Odonata of Parque Nacional Manu, Madre de Dios, Peru; Natural History, Species Richness and Comparisons with Other Peruvian Sites. In The Biodiversity of Southeastern Peru; Wilsonn, D., Sandoval, A., Eds.; Smithsonian Institution: Washington, DC, USA, 1996; pp. 431–439. [Google Scholar]
- Dijkstra, K.D.; Lewington, R. Field Guide to the Dragonflies of Britain and Europe, 1st ed.; British Wildlife Publishing: Totnes, UK, 2006; p. 320. [Google Scholar]
- Vilenica, M.; Brigić, A.; Ergović, V.; Koh, M.; Alegro, A.; Šegota, V.; Rimac, A.; Rummišek, M.; Mihaljević, Z. Taxonomic and functional Odonata assemblage metrics: Macrophyte–driven changes in anthropogenically disturbed floodplain habitats. Hydrobiologia 2024, 851, 3787–3807. [Google Scholar] [CrossRef]
- Vilenica, M.; Rebrina, F.; Kepčija, R.M.; Šegota, V.; Rumišek, M.; Ružanović, L.; Brigić, A. Aquatic Macrophyte Vegetation Promotes Taxonomic and Functional Diversity of Odonata Assemblages in Intermittent Karst Rivers in the Mediterranean. Diversity 2022, 14, 31. [Google Scholar] [CrossRef]
- Vilenica, M.; Katar, M.; Koren, T.; Koren, A.Š. Dragonfly Fauna (Insecta: Odonata) of Papuk Nature Park, Croatia. Nat. Croat. 2022, 31, 351–364. [Google Scholar] [CrossRef]
- Reding, J.; Vinçon, G.; Graf, W. Notes on Leuctra Signifera Kempny, 1899 and Leuctra Austriaca Aubert, 1954 (Plecoptera: Leuctridae), with the Description of a New Species. Zootaxa 2023, 5296, 1–15. [Google Scholar] [CrossRef]
- Marion, J.L.; Leung, Y.F.; Eagleston, H.; Burroughs, K.A. Review and Synthesis of Recreation Ecology Research Findings on Visitor Impacts to Wilderness and Protected Natural Areas. J. For. 2016, 114, 352–362. [Google Scholar] [CrossRef]
- Buffagni, A. The Lentic and Lotic Characteristics of Habitats Determine the Distribution of Benthic Macroinvertebrates in Mediterranean Rivers. Freshw. Biol. 2021, 66, 13–34. [Google Scholar] [CrossRef]
- Tierno de Figueroa, J.M.; López-Rodríguez, M.J.; Villar-Argaiz, M. Spatial and Seasonal Variability in the Trophic Role of Aquatic Insects: An Assessment of Functional Feeding Group Applicability. Freshw. Biol. 2019, 64, 954–966. [Google Scholar] [CrossRef]
- Scharf, I.; Ruxton, G.D. Shadow Competition: Its Definition, Prevalence, Causes and Measurement. Oikos 2023, 2023, e09774. [Google Scholar] [CrossRef]
- Larned, S.T.A. Prospectus for Periphyton: Recent and Future Ecological Research. J. N. Am. Benthol. Soc. 2010, 29, 182–206. [Google Scholar] [CrossRef]
- Vesterinen, J.; Strandberg, U.; Taipale, S.J.; Kainz, M.J.; Kankaala, P. Periphyton as a Key Diet Source of Essential Fatty Acids for Macroinvertebrates across a Nutrient and Dissolved Organic Carbon Gradient in Boreal Lakes. Limnol. Ocean. 2022, 67, 1604–1616. [Google Scholar] [CrossRef]
- Heino, J.; Melo, A.S.; Bini, L.M. Reconceptualising the Beta Diversity-Environmental Heterogeneity Relationship in Running Water Systems. Freshw. Biol. 2015, 60, 223–235. [Google Scholar] [CrossRef]
- Winegardner, A.K.; Jones, B.K.; Ng, I.S.; Siqueira, T.; Cottenie, K. The Terminology of Metacommunity Ecology. Trends Ecol. Evol. 2012, 27, 253–254. [Google Scholar] [CrossRef]
- Dodds, R.; Butler, R. The Phenomena of Overtourism: A Review. Int. J. Tour. Cities 2019, 5, 519–528. [Google Scholar] [CrossRef]
- Consoli, G.; Haller, R.M.; Doering, M.; Hashemi, S.; Robinson, C.T. Tributary Effects on the Ecological Responses of a Regulated River to Experimental Floods. J. Environ. Manag. 2022, 303, 114–122. [Google Scholar] [CrossRef]
- Milošević, D.; Medeiros, A.S.; Cvijanović, D.; Jenačković Gocić, D.; Đurđević, A.; Čerba, D.; Stojković Piperac, M. Implications of Local Niche- and Dispersal-Based Factors That May Influence Chironomid Assemblages in Bioassessment. Environ. Sci. Pollut. Res. 2022, 29, 51951–51963. [Google Scholar] [CrossRef]
- Cheney, K.N.; Roy, A.H.; Smith, R.F.; Dewalt, R.E.; Murphy, S. Effects of Stream Temperature and Substrate Type on Emergence Patterns of Plecoptera and Trichoptera from Northeastern United States Headwater Streams. Environ. Entomol. 2019, 48, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Douville, H.; Raghavan, K.; Renwick, J.; Allan, R.P.; Arias, P.A.; Barlow, M.; Cerezo-Mota, R.; Cherchi, A.; Gan, T.Y.; Gergis, J.; et al. Water cycle changes. In Climate Change 2021: The Physical Science Basis; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 1055–1210. [Google Scholar] [CrossRef]
- Li, Z.; Fang, H. Impacts of climate change on water erosion: A review. Earth Sci. Rev. 2016, 163, 94–117. [Google Scholar] [CrossRef]
- Dorić, V.; Ivković, M.; Baranov, V.; Pozojević, I.; Mihaljević, Z. Extreme freshwater discharge events exacerbated by climate change influence the structure and functional response of the chironomid community in a biodiversity hotspot. Sci. Total Environ. 2023, 879, 163110. [Google Scholar] [CrossRef] [PubMed]
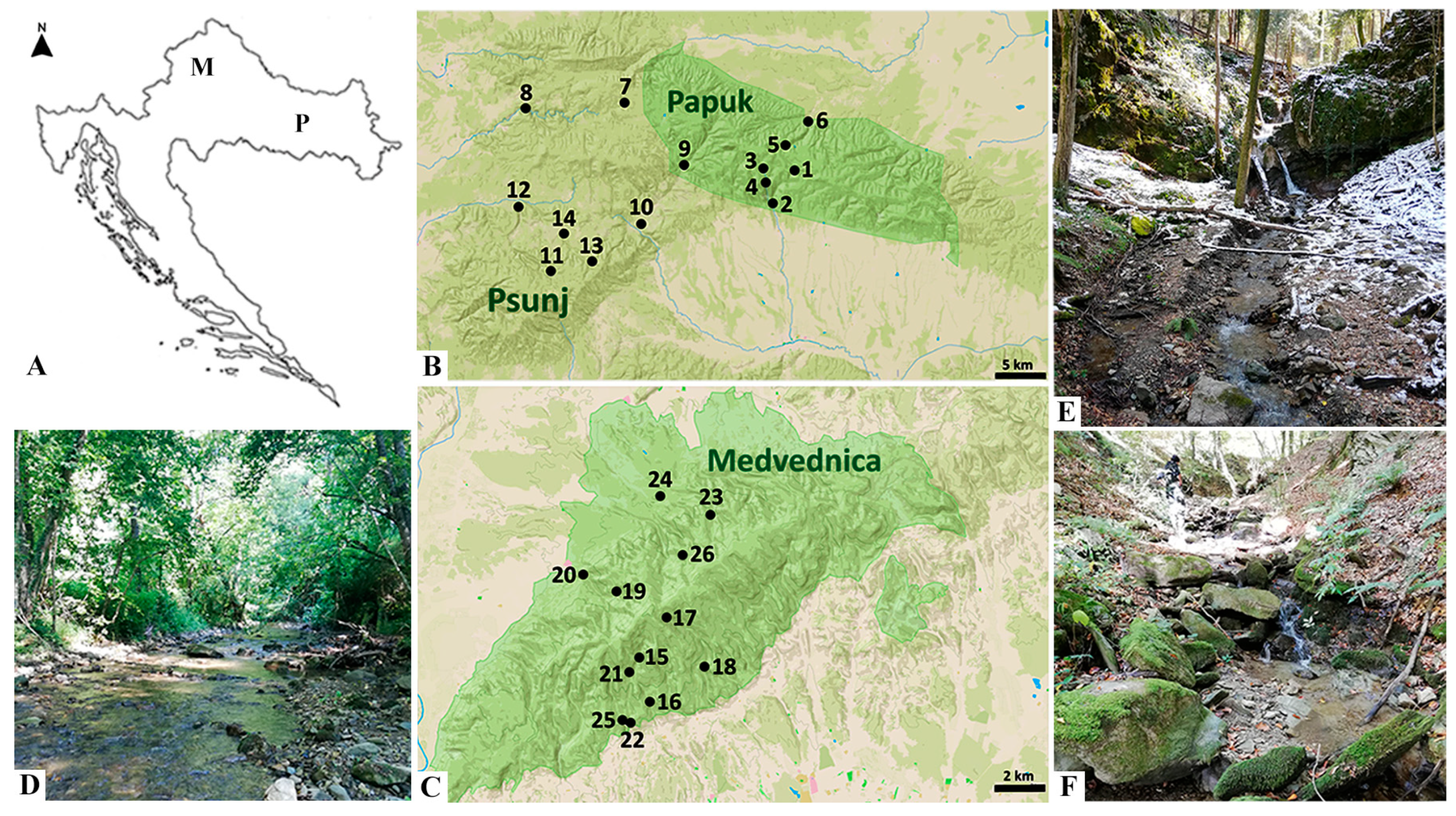

| Mountain | Site Number | Sampling Sites | Latitude | Longitude | Elevation (m) | Substrate Type Sampled | ||
|---|---|---|---|---|---|---|---|---|
| Papuk | 1 | Dubočanka HA | 45°29′53.0402″ N | 17°41′06.3962″ E | 510 | macrolithal | mesolithal | akal |
| 2 | Dubočanka LA | 45°28′05.0584″ N | 17°39′25.7028″ E | 313 | macrolithal | mesolithal | akal/ psammal | |
| 3 | Veličanka HA | 45°29′58.9226″ N | 17°38′39.9127″ E | 540 | mesolithal | microlithal | phytal (moss) | |
| 4 | Veličanka LA | 45°29′12.0196″ N | 17°38′51.6752″ E | 350 | macrolithal | mesolithal | akal | |
| 5 | Kovačica HA | 45°31′16.2020″ N | 17°40′25.8704″ E | 587 | mesolithal | microlithal | akal/ psammal | |
| 6 | Kovačica LA | 45°32′34.6322″ N | 17°42′12.7032″ E | 216 | macrolithal | mesolithal | akal/ psammal | |
| 7 | Bijela HA | 45°33′37.5947″ N | 17°27′40.2850″ E | 567 | macrolithal | mesolithal/ microlithal | psammal | |
| 8 | Bijela LA | 45°33′19.6970″ N | 17°19′52.9951″ E | 273 | mesolithal | microlithal | akal/ psammal | |
| 9 | Brzaja HA | 45°30′08.4380″ N | 17°32′23.6873″ E | 326 | macrolithal | mesolithal | psammal | |
| Psunj | 10 | Brzaja LA | 45°26′55.7606″ N | 17°28′58.9086″ E | 241 | macrolithal | mesolithal | psammal |
| 11 | Sivornica HA | 45°24′17.3901″ N | 17°21′50.3550″ E | 765 | macrolithal | mesolithal | microlithal | |
| 12 | Sivornica LA | 45°27′52.4345″ N | 17°19′18.4885″ E | 334 | macrolithal | mesolithal | microlithal | |
| 13 | Cikotska HA | 45°24′50.71″ N | 17°25′08.68″ E | 725 | macrolithal | mesolithal | akal | |
| 14 | Cikotska LA | 45°26′24.2445″ N | 17°22′53.2382″ E | 366 | macrolithal | mesolithal | microlithal | |
| Medvednica | 15 | Kraljevec HA | 45°52′54.9058″ N | 15°56′33.7911″ E | 589 | mesolithal | microlithal | xylal/CPOM |
| 16 | Kraljevec LA | 45°51′56.4801″ N | 15°56′53.9527″ E | 384 | mesolithal | microlithal | xylal/CPOM | |
| 17 | Bliznec HA | 45°53′48.9760″ N | 15°57′25.5210″ E | 819 | macrolithal | mesolithal | xylal/CPOM | |
| 18 | Bliznec LA | 45°52′43.7938″ N | 15°58′37.0039″ E | 402 | macrolithal | mesolithal | microlithal | |
| 19 | Bistra HA | 45°54′22.3981″ N | 15°55′49.4613″ E | 519 | macrolithal | mesolithal | akal/ psammal | |
| 20 | Bistra LA | 45°54′45.7918″ N | 15°54′45.2363″ E | 320 | macrolithal | mesolithal | akal/ psammal | |
| 21 | Mali Potok HA | 45°52′35.6642″ N | 15°56′13.4719″ E | 593 | mesolithal | xylal/CPOM | ||
| 22 | Mali Potok LA | 45°51′28.5240″ N | 15°56′09.8860″ E | 310 | mesolithal | microlithal | xylal/CPOM | |
| 23 | Rakova Noga HA | 45°56′05.5498″ N | 15°58′49.6007″ E | 534 | mesolithal | microlithal | xylal/CPOM | |
| 24 | Vidak LA | 45°56′30.6217″ N | 15°57′14.7400″ E | 315 | mesolithal | microlithal | xylal/CPOM | |
| 25 | Veliki Potok LA | 45°51′29.7565″ N | 15°56′05.7794″ E | 301 | mesolithal | microlithal | xylal | |
| 26 | Bistra 2 HA | 45°55′12.1207″ N | 15°57′56.1521″ E | 694 | macrolithal | mesolithal | microlithal | |
| Sampling Site | Shannon–Wiener (H’) | Simpson’s (D) | ||||
|---|---|---|---|---|---|---|
| Summer | Spring | Autumn | Summer | Spring | Autumn | |
| Dubočanka HA | 2.526 | 2.632 | 3.103 | 0.851 | 0.892 | 0.944 |
| Dubočanka LA | 2.988 | 2.655 | 2.807 | 0.933 | 0.891 | 0.918 |
| Veličanka HA | 2.255 | 2.714 | 2.535 | 0.780 | 0.878 | 0.820 |
| Veličanka LA | 2.893 | 2.454 | 2.695 | 0.909 | 0.834 | 0.899 |
| Kovačica HA | 2.388 | 2.673 | 2.529 | 0.843 | 0.898 | 0.878 |
| Kovačica LA | 2.486 | 2.870 | 2.85 | 0.863 | 0.916 | 0.904 |
| Bijela HA | 2.848 | 2.788 | 2.536 | 0.919 | 0.914 | 0.869 |
| Bijela LA | 2.280 | 2.268 | 2.865 | 0.813 | 0.833 | 0.920 |
| Brzaja HA | 2.718 | 2.674 | 2.843 | 0.857 | 0.893 | 0.918 |
| Brzaja LA | 2.954 | 2.967 | 2.942 | 0.919 | 0.931 | 0.924 |
| Sivornica HA | 2.956 | 2.346 | 2.782 | 0.914 | 0.846 | 0.902 |
| Sivornica LA | 2.733 | 2.764 | 2.249 | 0.902 | 0.908 | 0.821 |
| Cikotska HA | 2.855 | 2.325 | 2.455 | 0.926 | 0.784 | 0.857 |
| Cikotska LA | 2.891 | 2.866 | 2.839 | 0.918 | 0.902 | 0.897 |
| Kraljevec HA | 2.663 | 1.931 | 2.761 | 0.898 | 0.728 | 0.902 |
| Kraljevec LA | 1.740 | 1.621 | 2.712 | 0.741 | 0.711 | 0.901 |
| Bliznec HA | 2.47 | 1.435 | 2.473 | 0.862 | 0.602 | 0.846 |
| Bliznec LA | 2.446 | 2.042 | 2.329 | 0.884 | 0.789 | 0.853 |
| Bistra HA | 2.617 | 2.023 | 2.801 | 0.901 | 0.793 | 0.911 |
| Bistra LA | 1.962 | 1.996 | 2.754 | 0.743 | 0.784 | 0.912 |
| Mali Potok HA | 2.106 | 1.978 | 2.532 | 0.824 | 0.790 | 0.897 |
| Mali Potok LA | 2.023 | 2.417 | 2.856 | 0.774 | 0.879 | 0.916 |
| Rakova Noga HA | 2.245 | 2.556 | 2.986 | 0.766 | 0.867 | 0.934 |
| Vidak LA | 1.954 | 2.570 | 2.587 | 0.719 | 0.874 | 0.869 |
| Veliki Potok LA | 2.687 | 2.636 | 2.286 | 0.905 | 0.894 | 0.819 |
| Bistra 2 HA | 2.030 | * | * | 0.822 | * | * |
| Sampling Campaign Groups | t | p-Value |
|---|---|---|
| Papuk, Medvednica | 2.5926 | 0.014 |
| Papuk, Psunj | 0.7627 | 0.475 |
| Medvednica, Psunj | 1.3882 | 0.181 |
| summer, spring | 4.6651 | 0.001 |
| summer, autumn | 0.6674 | 0.553 |
| spring, autumn | 5.4371 | 0.001 |
| Sampling Campaign | Average | SE |
|---|---|---|
| summer | 47.920 | 0.62207 |
| spring | 51.962 | 0.60177 |
| autumn | 47.343 | 0.59951 |
| Papuk | 49.245 | 0.62672 |
| Medvednica | 51.269 | 0.48503 |
| Psunj | 50.028 | 0.78804 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ergović, V.; Čerba, D.; Tubić, B.; Novaković, B.; Koh, M.; Mihaljević, Z. Seasonal Dynamics and Factors Shaping Aquatic Insect Assemblages in Mountain Streams of the Pannonian Lowland Ecoregion. Insects 2025, 16, 344. https://doi.org/10.3390/insects16040344
Ergović V, Čerba D, Tubić B, Novaković B, Koh M, Mihaljević Z. Seasonal Dynamics and Factors Shaping Aquatic Insect Assemblages in Mountain Streams of the Pannonian Lowland Ecoregion. Insects. 2025; 16(4):344. https://doi.org/10.3390/insects16040344
Chicago/Turabian StyleErgović, Viktorija, Dubravka Čerba, Bojana Tubić, Boris Novaković, Miran Koh, and Zlatko Mihaljević. 2025. "Seasonal Dynamics and Factors Shaping Aquatic Insect Assemblages in Mountain Streams of the Pannonian Lowland Ecoregion" Insects 16, no. 4: 344. https://doi.org/10.3390/insects16040344
APA StyleErgović, V., Čerba, D., Tubić, B., Novaković, B., Koh, M., & Mihaljević, Z. (2025). Seasonal Dynamics and Factors Shaping Aquatic Insect Assemblages in Mountain Streams of the Pannonian Lowland Ecoregion. Insects, 16(4), 344. https://doi.org/10.3390/insects16040344







