The Dangers of Growing Old: Adult Moths Face Higher Predation Pressures than Caterpillars in Hyles lineata
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasticine Replicas
2.1.1. Adult Moth Replica Development
2.1.2. Caterpillar Model Development
2.2. Model Deployment and Predation Quantification
2.3. Statistical Analyses
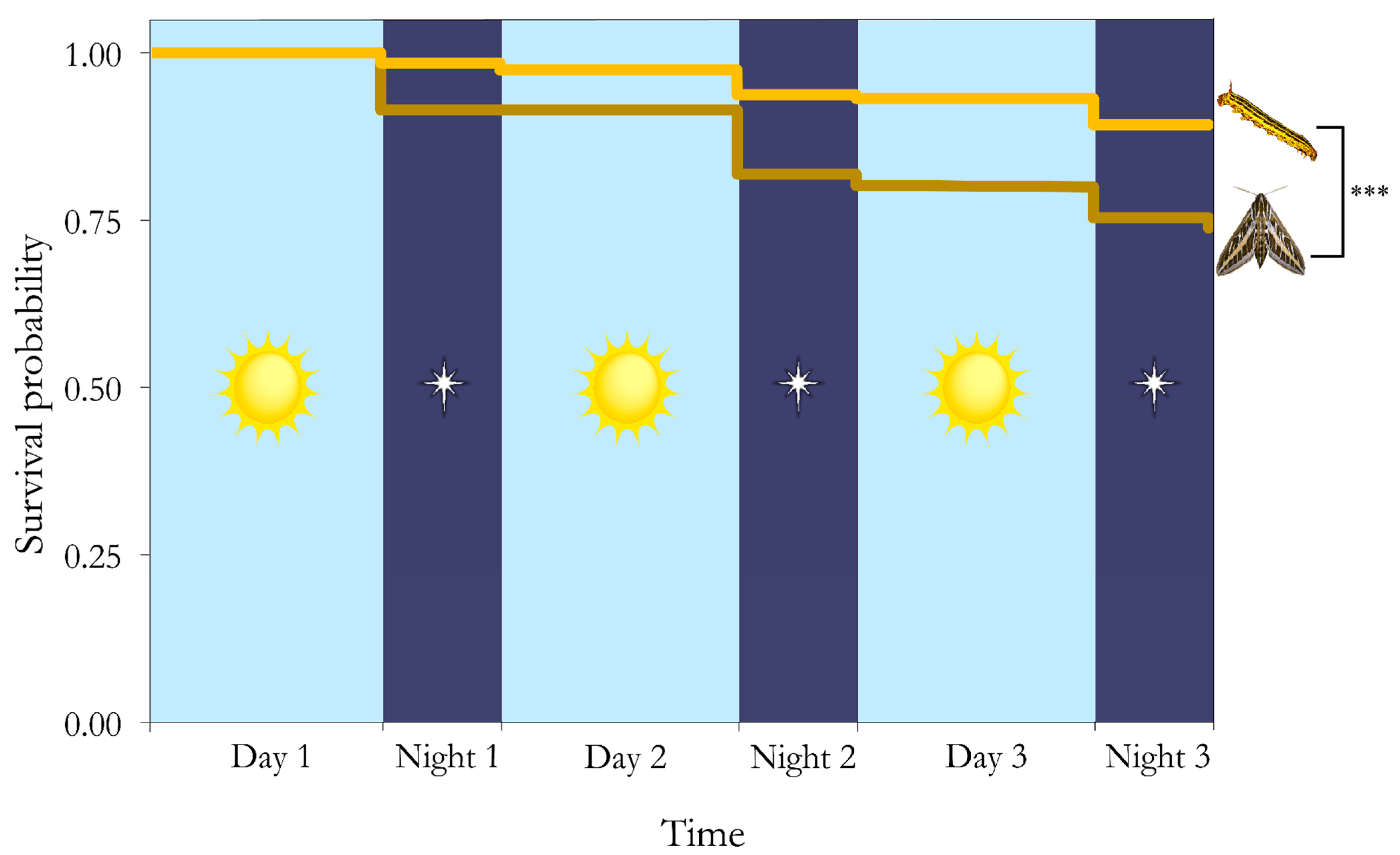
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jindra, M. Where Did the Pupa Come from? The Timing of Juvenile Hormone Signalling Supports Homology between Stages of Hemimetabolous and Holometabolous Insects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20190064. [Google Scholar]
- Hammer, T.J.; Moran, N.A. Links between Metamorphosis and Symbiosis in Holometabolous Insects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20190068. [Google Scholar] [CrossRef] [PubMed]
- Truman, J.W. The Evolution of Insect Metamorphosis. Curr. Biol. 2019, 29, R1252–R1268. [Google Scholar]
- Berenbaum, M.R.; Isman, M.B. Herbivory in Holometabolous and Hemimetabolous Insects: Contrasts between Orthoptera and Lepidoptera. Experientia 1989, 45, 229–236. [Google Scholar]
- Howe, A.; Lövei, G.L.; Nachman, G. Dummy Caterpillars as a Simple Method to Assess Predation Rates on Invertebrates in a Tropical Agroecosystem. Entomol. Exp. Appl. 2009, 131, 325–329. [Google Scholar]
- Seymoure, B.M.; Raymundo, A.; McGraw, K.J.; Owen McMillan, W.; Rutowski, R.L. Environment-Dependent Attack Rates of Cryptic and Aposematic Butterflies. Curr. Zool. 2018, 64, 663–669. [Google Scholar] [CrossRef]
- Weissflog, A.; Markesteijn, L.; Aiello, A.; Healey, J.; Geipel, I. Do Prey Shape, Time of Day, and Plant Trichomes Affect the Predation Rate on Plasticine Prey in Tropical Rainforests? Biotropica 2022, 54, 1259–1269. [Google Scholar] [CrossRef]
- Cozzer, G.D.; Rezende, R.d.S.; Lara, T.S.; Machado, G.H.; Dal Magro, J.; Albeny-Simões, D. Predation Risk Effects on Larval Development and Adult Life of Aedes Aegypti Mosquito. Bull. Entomol. Res. 2023, 113, 29–36. [Google Scholar]
- Hironori, Y.A.D.; Katsuhiro, S.A.D. Cannibalism and Interspecific Predation in Two Predatory Ladybirds in Relation to Prey Abundance in the Field. Biocontrol 1997, 42, 153–163. [Google Scholar] [CrossRef]
- Hagler, J.R.; Jackson, C.G.; Isaacs, R.; Machtley, S.A. Foraging Behavior and Prey Interactions by a Guild of Predators on Various Lifestages of Bemisia Tabaci. J. Insect Sci. 2004, 4, 1. [Google Scholar]
- Cronin, T.W.; Johnsen, S.; Marshall, N.J.; Warrant, E.J. Visual Ecology; Princeton University Press: Princeton, NJ, USA, 2014; ISBN 9780691151847. [Google Scholar]
- Stevens, M. Cheats and Deceits: How Animals and Plants Exploit and Mislead; Oxford University Press: London, UK, 2016; ISBN 9780191017605. [Google Scholar]
- Parker, A.R. On the Origin of Optics. Opt. Laser Technol. 2011, 43, 323–329. [Google Scholar]
- Martin, G.R. Through Birds’ Eyes: Insights into Avian Sensory Ecology. J. Ornithol. 2012, 153, 23–48. [Google Scholar]
- Seymoure, B.; Sanchez, B.A.; Pollard, K.J.; Horne, L.M.; Field, E.; Portz, A.D.; Savage, J.; Smith, C.; Duffendack, S.; Cotty, E.; et al. Predation of the White-lined Sphinx Moth (Hyles lineata) Is Dependent upon Time of Day but Not Human Disturbance. Ecol. Entomol. 2025. [Google Scholar] [CrossRef]
- Maor, R.; Dayan, T.; Ferguson-Gow, H.; Jones, K.E. Temporal Niche Expansion in Mammals from a Nocturnal Ancestor after Dinosaur Extinction. Nat. Ecol. Evol. 2017, 1, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M. Sensory Ecology, Behaviour, and Evolution; Oxford University Press: Oxford, UK, 2013; ISBN 9780191651465. [Google Scholar]
- Nokelainen, O.; de Moraes Rezende, F.; Valkonen, J.K.; Mappes, J. Context-Dependent Coloration of Prey and Predator Decision Making in Contrasting Light Environments. Behav. Ecol. 2022, 33, 77–86. [Google Scholar]
- Husak, J.F.; Macedonia, J.M.; Fox, S.F.; Sauceda, R.C. Predation Cost of Conspicuous Male Coloration in Collared Lizards (Crotaphytus collaris): An Experimental Test Using Clay-covered Model Lizards. Ethology 2006, 112, 572–580. [Google Scholar]
- Mason, L.D.; Wardell-Johnson, G.; Luxton, S.J.; Bateman, P.W. Predators Show Seasonal Predilections for Model Clay Spiders in an Urban Environment. Sci. Rep. 2018, 8, 12444. [Google Scholar] [CrossRef]
- Calderon-Chalco, K.A.; Putman, B.J. The Effect of Paint Marking on Predation Risk in Western Fence Lizards: A Test Using Clay Models. Herpetol. Conserv. Biol. 2019, 14, 80–90. [Google Scholar]
- Nimalrathna, T.S.; Solina, I.D.; Mon, A.M.; Pomoim, N.; Bhadra, S.; Zvereva, E.L.; Sam, K.; Nakamura, A. Estimating Predation Pressure in Ecological Studies: Controlling Bias Imposed by Using Sentinel Plasticine Prey. Entomol. Exp. Appl. 2023, 171, 56–67. [Google Scholar]
- Finkbeiner, S.D.; Briscoe, A.D.; Reed, R.D. The Benefit of Being a Social Butterfly: Communal Roosting Deters Predation. Proc. Biol. Sci. 2012, 279, 2769–2776. [Google Scholar]
- Vorobyev, M.; Osorio, D. Receptor Noise as a Determinant of Colour Thresholds. Proc. Biol. Sci. 1998, 265, 351–358. [Google Scholar] [CrossRef]
- Seymoure, B.; Parrish, T.; Egan, K.; Furr, M.; Irwin, D.; Brown, C.; Crump, M.; White, J.; Crooks, K.; Angeloni, L. Better Red than Dead: Plasticine Moths Are Attacked Less under HPS Streetlights than LEDs. Basic Appl. Ecol. 2024, 74, 66–73. [Google Scholar] [CrossRef]
- Powell, J.A.; Opler, P.A. Moths of Western North America; Univ of California Press: Berkeley, CA, USA, 2009; ISBN 9780520943773. [Google Scholar]
- Troscianko, J.; Stevens, M. Image Calibration and Analysis Toolbox—A Free Software Suite for Objectively Measuring Reflectance, Colour and Pattern. Methods Ecol. Evol. 2015, 6, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Cunha, O.D.; Fournier, C.; Horne, L.M.; Seymoure, B.M.; Johnson, J.D. You Can’t See Me: Background Matching in the Western Diamond-Backed Rattlesnake (Crotalus atrox). Res. Sq. 2023. [Google Scholar] [CrossRef]
- Francois, C.L.; Davidowitz, G. Genetic Color Polymorphism of the Whitelined Sphinx Moth Larva (Lepidoptera: Sphingidae). J. Insect Sci. 2020, 20, 19. [Google Scholar] [CrossRef]
- Low, P.A.; Sam, K.; McArthur, C.; Posa, M.R.C.; Hochuli, D.F. Determining Predator Identity from Attack Marks Left in Model Caterpillars: Guidelines for Best Practice. Entomol. Exp. Appl. 2014, 152, 120–126. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Therneau, T. A Package for Survival Analysis in R. R Package Version 2015, 2, 2014. [Google Scholar]
- Seymoure, B.M.; Aiello, A. Keeping the Band Together: Evidence for False Boundary Disruptive Coloration in a Butterfly. J. Evol. Biol. 2015, 28, 1618–1624. [Google Scholar] [CrossRef]
- Merrill, R.M.; Wallbank, R.W.R.; Bull, V.; Salazar, P.C.A.; Mallet, J.; Stevens, M.; Jiggins, C.D. Disruptive Ecological Selection on a Mating Cue. Proc. Biol. Sci. 2012, 279, 4907–4913. [Google Scholar] [CrossRef]
- Finkbeiner, S.D.; Briscoe, A.D.; Reed, R.D. Warning Signals Are Seductive: Relative Contributions of Color and Pattern to Predator Avoidance and Mate Attraction in Heliconius Butterflies. Evolution 2014, 68, 3410–3420. [Google Scholar] [CrossRef]
- Deitsch, J.F.; Kaiser, S.A. Artificial Light at Night Increases Top-down Pressure on Caterpillars: Experimental Evidence from a Light-Naive Forest. Proc. Biol. Sci. 2023, 290, 20230153. [Google Scholar] [CrossRef]
- Roeder, K.A.; Dorland, M.S.; Daniels, J.D. Importance of Color for Artificial Clay Caterpillars as Sentinel Prey in Maize, Soybean, and Prairie. Entomol. Exp. Appl. 2023, 171, 68–72. [Google Scholar] [CrossRef]
- Roslin, T.; Hardwick, B.; Novotny, V.; Petry, W.K.; Andrew, N.R.; Asmus, A.; Barrio, I.C.; Basset, Y.; Boesing, A.L.; Bonebrake, T.C.; et al. Higher Predation Risk for Insect Prey at Low Latitudes and Elevations. Science 2017, 356, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Seifert, C.L.; Schulze, C.H.; Dreschke, T.C.T.; Frötscher, H.; Fiedler, K. Day vs. Night Predation on Artificial Caterpillars in Primary Rainforest Habitats—An Experimental Approach. Entomol. Exp. Appl. 2016, 158, 54–59. [Google Scholar] [CrossRef]
- Antoine, J.; Brou, C. Distribution and Phenologies of Louisiana Sphingidae. J. Lepid. Soc. 1997, 51, 156–175. [Google Scholar]
- Tanaka, K.D. A Colour to Birds and to Humans: Why Is It so Different? J. Ornithol. 2015, 156, 433–440. [Google Scholar] [CrossRef]
- Ishay, J.; Motro, A.; Gitter, S.; Brown, M.B. Rhythms in Acoustical Communication by the Oriental Hornet, Vespa Orientalis. Anim. Behav. 1974, 22, 741–744. [Google Scholar] [CrossRef]
- Rydell, J.; Eklöf, J. Vision Complements Echolocation in an Aerial-Hawking Bat. Naturwissenschaften 2003, 90, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Rydell, J.; Jones, G.; Waters, D. Echolocating Bats and Hearing Moths: Who Are the Winners? Oikos 1995, 73, 419–424. [Google Scholar] [CrossRef]
- Jones, P.L.; Page, R.A.; Ratcliffe, J.M. To Scream or to Listen? Prey Detection and Discrimination in Animal-Eating Bats. In Bat Bioacoustics; Fenton, M.B., Grinnell, A.D., Popper, A.N., Fay, R.R., Eds.; Springer: New York, NY, USA, 2016; pp. 93–116. ISBN 9781493935277. [Google Scholar]
- Lewis, W.J.; Tumlinson, J.H. Host Detection by Chemically Mediated Associative Learning in a Parasitic Wasp. Nature 1988, 331, 257–259. [Google Scholar] [CrossRef]
- Dicke, M.; Sabelis, M.W. How Plants Obtain Predatory Mites as Bodyguards. Neth. J. Zool. 1987, 38, 148–165. [Google Scholar]
- Turlings, T.C.; Tumlinson, J.H.; Lewis, W.J. Exploitation of Herbivore-Induced Plant Odors by Host-Seeking Parasitic Wasps. Science 1990, 250, 1251–1253. [Google Scholar]
- Sam, K.; Koane, B.; Novotny, V. Herbivore Damage Increases Avian and Ant Predation of Caterpillars on Trees along a Complete Elevational Forest Gradient in Papua New Guinea. Ecography 2015, 38, 293–300. [Google Scholar]
- Ishii, Y.; Shimada, M. The Effect of Learning and Search Images on Predator–prey Interactions. Popul. Ecol. 2010, 52, 27–35. [Google Scholar] [CrossRef]
- Nokelainen, O.; Silvasti, S.A.; Strauss, S.Y.; Wahlberg, N.; Mappes, J. Predator Selection on Phenotypic Variability of Cryptic and Aposematic Moths. Nat. Commun. 2024, 15, 1678. [Google Scholar]
- Weir, J.C. The Evolution of Colour Polymorphism in British Winter-active Lepidoptera in Response to Search Image Use by Avian Predators. J. Evol. Biol. 2018, 31, 1109–1126. [Google Scholar] [CrossRef]
- Tuttle, J.P. The Hawk Moths of North America. A Natural History Study of the Sphingidae of the United States and Canada; The Wedge Entomological Research Foundation: Washington, DC, USA; Entomological Reprint Specialists: Tucson, AZ, USA, 2007. [Google Scholar]



| Caterpillars Attacked | Moths Attacked | Total Attacks | |
|---|---|---|---|
| Night | 3 | 6 | 9 |
| Day | 17 | 43 | 60 |
| Bird | 14 | 38 | 52 |
| Mammal | 2 | 2 | 4 |
| Insect | 0 | 4 | 4 |
| Unknown | 4 | 6 | 10 |
| Total | 20 | 50 | 70 |
| Missing | Caterpillars Missing | Moths Missing | Total Missing |
|---|---|---|---|
| Night | 4 | 6 | 10 |
| Day | 13 | 7 | 20 |
| Total | 17 | 13 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, B.A.; Da Cunha, O.; Savage, J.W.; Horne, L.M.; Saenz-Arreola, S.; Pollard, K.; Neria, O.; Duffendack, S.; Terrazas, S.; Diaz, J.M.; et al. The Dangers of Growing Old: Adult Moths Face Higher Predation Pressures than Caterpillars in Hyles lineata. Insects 2025, 16, 347. https://doi.org/10.3390/insects16040347
Sanchez BA, Da Cunha O, Savage JW, Horne LM, Saenz-Arreola S, Pollard K, Neria O, Duffendack S, Terrazas S, Diaz JM, et al. The Dangers of Growing Old: Adult Moths Face Higher Predation Pressures than Caterpillars in Hyles lineata. Insects. 2025; 16(4):347. https://doi.org/10.3390/insects16040347
Chicago/Turabian StyleSanchez, Braulio A., Oceane Da Cunha, Jackson W. Savage, L. Miles Horne, Sol Saenz-Arreola, Kajaya Pollard, Oliver Neria, Spencer Duffendack, Simon Terrazas, Javier M. Diaz, and et al. 2025. "The Dangers of Growing Old: Adult Moths Face Higher Predation Pressures than Caterpillars in Hyles lineata" Insects 16, no. 4: 347. https://doi.org/10.3390/insects16040347
APA StyleSanchez, B. A., Da Cunha, O., Savage, J. W., Horne, L. M., Saenz-Arreola, S., Pollard, K., Neria, O., Duffendack, S., Terrazas, S., Diaz, J. M., Deitsch, J., & Seymoure, B. M. (2025). The Dangers of Growing Old: Adult Moths Face Higher Predation Pressures than Caterpillars in Hyles lineata. Insects, 16(4), 347. https://doi.org/10.3390/insects16040347







