Interspecific Mating Is Trivial and Asymmetrical Between Two Destructive Anoplophora Beetles
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources of Insects and Plants
2.2. Mounting and Movement Activity of Anoplophora Adults in a Cage
2.3. Aggregation-Sex Pheromone Release in Male Anoplophora Adults
2.4. Data Analyses
3. Results
3.1. Mounting and Movement Activity of Anoplophora Adults in a Cage
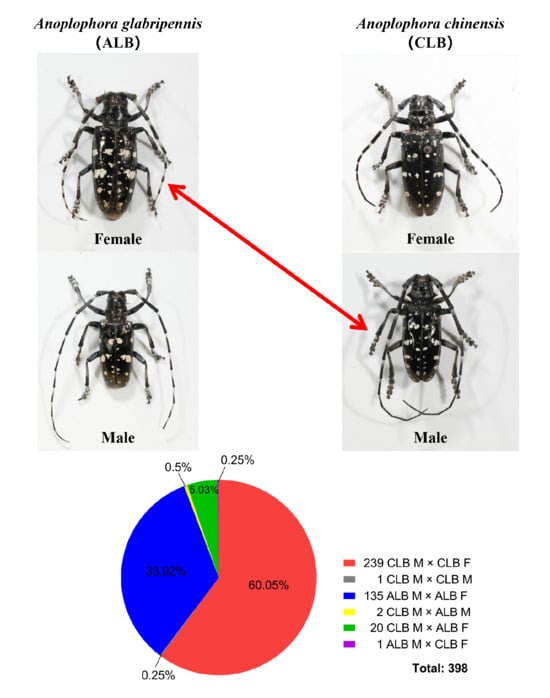
3.1.1. Mounting
3.1.2. Movement
3.2. Aggregation-Sex Pheromone Release in Male Anoplophora Adults
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALB | Asian longhorn beetle |
| CLB | Citrus longhorn beetle |
References
- Haack, R.A. Feeding biology of cerambycids. In Cerambycidae of the World: Biology and Pest Management; Wang, Q., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 105–131. [Google Scholar]
- Haack, R.A.; Keena, M.A.; Eyre, D. Life history and population dynamics of cerambycids. In Cerambycidae of the World: Biology and Pest Management; Wang, Q., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 71–103. [Google Scholar]
- Eyre, D.; Haack, R.A. Invasive cerambycid pests and biosecurity measures. In Cerambycidae of the World: Biology and Pest Management; Wang, Q., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 563–619. [Google Scholar]
- Haack, R.A. Cerambycid pests in forests and urban trees. In Cerambycidae of the World: Biology and Pest Management; Wang, Q., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 351–407. [Google Scholar]
- Branco, S.; Faccoli, M.; Brockerhoff, E.G.; Roux, G.; Jactel, H.; Desneux, N.; Gachet, E.; Mouttet, R.; Streito, J.-C.; Branco, M. Preventing invasions of Asian longhorn beetle and citrus longhorn beetle: Are we on the right track? J. Pest Sci. 2021, 95, 41–66. [Google Scholar]
- Wang, L.; Li, C.; Luo, Y.; Wang, G.; Dou, Z.; Haq, I.U.; Shang, S.; Cui, M. Current and future control of the wood-boring pest Anoplophora glabripennis. Insect Sci. 2023, 30, 1534–1551. [Google Scholar] [CrossRef] [PubMed]
- Hérard, F.; Maspero, M. History of discoveries and management of the citrus longhorned beetle, Anoplophora chinensis, in Europe. J. Pest Sci. 2018, 92, 117–130. [Google Scholar] [CrossRef]
- Haack, R.A.; Hérard, F.; Sun, J.; Turgeon, J.J. Managing invasive populations of Asian longhorned beetle and citrus longhorned beetle: A worldwide perspective. Annu. Rev. Entomol. 2010, 55, 521–546. [Google Scholar] [CrossRef]
- Lingafelter, S.W.; Hoebeke, E.R. Revision of Anoplophora (Coleoptera: Cerambycidae); Entomological Society of Washington: Washington, DC, USA, 2002. [Google Scholar]
- EPPO. Anoplophora glabripennis. EPPO Datasheets on Pests Recommended for Regulation. 2025. Available online: https://gd.eppo.int/taxon/ANOLGL (accessed on 23 March 2025).
- EPPO. Anoplophora chinensis. EPPO Datasheets on Pests Recommended for Regulation. 2025. Available online: https://gd.eppo.int/taxon/ANOLCN (accessed on 23 March 2025).
- Kim, S.; Farrell, B.D. Target enrichment museomics of the Asian long-horned beetle and its relatives (Cerambycidae: Anoplophora) reveals two independent origins of life in the cold. Syst. Entomol. 2024, 50, 139–153. [Google Scholar] [CrossRef]
- Wei, J. Prevetion and Control Techniques of Anoplophora glabripennis; Hebei University Press: Baoding, China, 2025. [Google Scholar]
- Wei, J.R.; Zhao, W.X.; Zhang, Y.A. Research progress on Anoplophora chinensis. Plant Quar. 2011, 25, 81–85. [Google Scholar]
- Zhang, Y. The Analyses of the Species Diversity and Faunal Distribution of Coleoptera in Beijing-Tianjin-Hebei of China. Master’s Thesis, Hebei University, Baoding, China, 2014. [Google Scholar]
- Lyu, F.; Hai, X.; Wang, Z. A review of the host plant location and recognition mechanisms of Asian longhorn beetle. Insects 2023, 14, 292. [Google Scholar] [CrossRef]
- Wu, J.H.; Huang, B.; Wang, D.H.; Gu, Y.T.; Du, Y.B.; Zheng, K.W.; Fan, J.T. Study on the interspecific competition behaviors between Anoplophora glabripennis and Anoplophora chinensis. J. Environ. Entomol. 2022, 44, 651–657. [Google Scholar]
- Liu, H. Distribution and Risk Assessment of Forestry Pests in Nanjing City. Master’s Thesis, Nanjing Forestry University, Nanjing, China, 2023. [Google Scholar]
- Chen, P.; Lu, L.; Wang, C. Large maple varieties introduced from North America and their main pests in Shanghai. For. Pest Dis. 2009, 28, 24–26,32. [Google Scholar]
- Qin, H.; Xu, H.; Capron, A.; Porth, I.; Cui, M.; Keena, M.A.; Deng, X.; Shi, J.; Hamelin, R.C. Is there hybridization between 2 species of the same genus in sympatry?—The genetic relationships between Anoplophora glabripennis, Anoplophora chinensis, and putative hybrids. Insect Sci. 2023, 31, 633–645. [Google Scholar] [CrossRef]
- Hansen, L.; Xu, T.; Wickham, J.; Chen, Y.; Hao, D.; Hanks, L.M.; Milllar, J.G.; Teale, S.A. Identification of a male-produced pheromone component of the citrus longhorned beetle, Anoplophora chinensis. PLoS ONE 2015, 10, e0134358. [Google Scholar]
- Zhang, A.; Oliver, J.E.; Aldrich, J.R.; Wang, B.; Mastro, V.C. Stimulatory beetle volatiles for the Asian longhorned beetle, Anoplophora glabripennis (Motschulsky). Z. Naturforschung C 2002, 57, 553–558. [Google Scholar]
- Wang, X.; Keena, M.A. Hybridization potential of two invasive Asian longhorn beetles. Insects 2021, 12, 1139. [Google Scholar] [CrossRef]
- Sunamura, E.; Tamura, S.; Mukai, H.; Tokoro, M.; Shoda-Kagaya, E. Mating behavior between alien Asian longhorned beetle Anoplophora glabripennis (Coleoptera: Cerambycidae) and a native related species Anoplophora chinensis in Japan. Appl. Entomol. Zool. 2022, 57, 275–281. [Google Scholar]
- Yasui, H.; Uechi, N.; Fujiwara-Tsujii, N. Differences in male mate recognition between the invasive Anoplophora glabripennis (Coleoptera: Cerambycidae) and Japanese native A. malasiaca. Insects 2023, 14, 171. [Google Scholar] [CrossRef]
- Hanks, L.M.; Wang, Q. Reproductive biology of cerambycids. In Cerambycidae of the World: Biology and Pest Management; Wang, Q., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 133–159. [Google Scholar]
- Iwata, R.; Maro, T.; Yonezawa, Y.; Yahagi, T.; Fujikawa, Y. Period of adult activity and response to wood moisture content as major segregating factors in the coexistence of two conifer longhorn beetles, Callidiellum rufipenne and Semanotus bifasciatus (Coleoptera: Cerambycidae). Eur. J. Entomol. 2007, 104, 341–345. [Google Scholar]
- Mazor, M.; Dunkelblum, E. Circadian rhythms of sexual behavior and pheromone titers of two closely related moth species Autographa gamma and Cornutiplusia circumflexa. J. Chem. Ecol. 2005, 31, 2153–2168. [Google Scholar]
- Mitchell, R.F.; Reagel, P.F.; Wong, J.C.; Meier, L.R.; Silva, W.D.; Mongold-Diers, J.; Millar, J.G.; Hanks, L.M. Cerambycid beetle species with similar pheromones are segregated by phenology and minor pheromone components. J. Chem. Ecol. 2015, 41, 431–440. [Google Scholar]
- Crook, D.; Maynard, E.; Furtado, M. Verification and evaluation of male-produced pheromone components from the citrus long-horned beetle, Anoplophora chinensis (Forster) (Insecta: Coleoptera: Cerambycidae). Insects 2024, 15, 692. [Google Scholar] [CrossRef]
- Crook, D.; Wickham, J.; Ren, L.; Xu, Z.; Jones, T.H.; Warden, M.; Cossé, A. Further evidence that female Anoplophora glabripennis (Coleoptera: Cerambycidae) utilizes photo-degradation to produce volatiles that are attractive to adult males. Insects 2024, 15, 923. [Google Scholar] [CrossRef]
- Crook, D.J.; Lance, D.R.; Mastro, V.C. Identifcation of a potential third component of the male-produced pheromone of Anoplophora glabripennis and its effect on behavior. J. Chem. Ecol. 2014, 40, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Wickham, J.D.; Xu, Z.; Teale, S.A. Evidence for a female-produced, long range pheromone of Anoplophora glabripennis (Coleoptera: Cerambycidae). Insect Sci. 2012, 19, 355–371. [Google Scholar]
- Xu, T.; Hansen, L.; Cha, D.H.; Hao, D.; Zhang, L.; Teale, S.A. Identification of a female-produced pheromone in a destructive invasive species: Asian longhorn beetle, Anoplophora glabripennis. J. Pest Sci. 2020, 93, 1321–1332. [Google Scholar] [CrossRef]
- Xu, T.; Teale, S.A. Chemical Ecology of the Asian Longhorn Beetle, Anoplophora glabripennis. J. Chem. Ecol. 2021, 47, 489–503. [Google Scholar] [CrossRef]
- Millar, J.G.; Hanks, L.M. Chemical ecology of cerambycids. In Cerambycidae of the World: Biology and Pest Management; Wang, Q., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 161–208. [Google Scholar]
- Keena, M.A.; Sánchez, V. Reproductive behaviors of Anoplophora glabripennis (Coleoptera: Cerambycidae) in the laboratory. J. Econ. Entomol. 2018, 111, 620–628. [Google Scholar] [CrossRef]
- Keena, M.A.; Sánchez, V. Inter- and intrasexual interactions in Anoplophora glabripennis (Coleoptera: Cerambycidae) and the impact of different sex ratios. J. Econ. Entomol. 2018, 111, 2163–2171. [Google Scholar] [CrossRef]
- Skabeikis, D.D.; Teale, S.A.; Fierke, M.K. Diel rhythms in Monochamus (Coleoptera: Cerambycidae): Production of and response to a male-produced aggregation pheromone. Environ. Entomol. 2016, 45, 1017–1021. [Google Scholar] [CrossRef]
- Kim, J.; Jung, Y.; Lee, S. Diel rhythmicity of field responses to synthetic pheromone lures in the pine sawyer Monochamus saltuarius. Insects 2021, 12, 441. [Google Scholar] [CrossRef]
- Meng, P.S.; Trotter, R.T.; Keena, M.A.; Baker, T.C.; Yan, S.; Schwartzberg, E.G.; Hoover, K. Effects of pheromone and plant volatile release rates and ratios on trapping Anoplophora glabripennis (Coleoptera: Cerambycidae) in China. Environ. Entomol. 2014, 43, 1379–1388. [Google Scholar] [CrossRef]
- Allison, J.D.; Borden, J.H.; Seybold, S.J. A review of the chemical ecology of the Cerambycidae (Coleoptera). Chemoecology 2004, 14, 123–150. [Google Scholar] [CrossRef]
- Hanks, L.M. Influence of the larval host plant on reproductive strategies of cerambycid beetles. Annu. Rev. Entomol. 1999, 44, 483–505. [Google Scholar] [PubMed]
- Fukaya, M.; Yasui, H.; Yasuda, T.; Akino, T.; Wakamura, S. Female orientation to the male in the white-spotted longicorn beetle, Anoplophora malasiaca (Thomson) (Coleoptera: Cerambycidae) by visual and olfactory cues. Appl. Entomol. Zool. 2005, 40, 63–68. [Google Scholar]
- Ji, B.; Wei, Y.; Huang, Z. Present situations and prospects of researches on adult’s behavior of longicorn beetles. J. Nanjing For. Univ. Nat. Sci. Ed. 2002, 26, 79–83. [Google Scholar]






| City | # | Site | Longitude | Latitude | CLB | ALB | Host Species * |
|---|---|---|---|---|---|---|---|
| Nanjing | 1 | Campus of Nanjing Forestry University | 118°48′28.44″E | 32°04′44.76″ N | × | Platanus sp. Lagerstroemia indica Melia azedarach | |
| 2 | Xuanwuhu Park | 118°47′34.44″ E | 32°04′41.16″ N | × | × | Salix babylonica | |
| 3 | Zhenghe Park | 118°47′20.04″ E | 32°02′36.6″ N | × | × | Salix babylonica Acer sp. | |
| 4 | Bailuzhou Park | 118°47′21.48″ E | 32°0′38.52″ N | × | Salix babylonica | ||
| 5 | Yangshan Park | 118°56′0.6″ E | 32°06′32.76″ N | × | × | Salix babylonica | |
| 6 | Tangshan outlets | 119°02′57.48″ E | 32°01′52.68″ N | × | Salix babylonica Acer sp. | ||
| 7 | Nanjing Botanical Garden | 118°49′53.76″ E | 32°03′22.32″ N | × | Acer negundo Lagerstroemia indica | ||
| 8 | Lijiang Road | 118°43′52.32″ E | 32°03′45″ N | × | Platanus sp. | ||
| Jurong | 9 | Campus of Jiangsu Vocational College of Agriculture and Forestry | 119°09′55.44″ E | 31°57′46.44″ N | × | Lagerstroemia indica Salix babylonica | |
| 10 | Xiashu Forestry Station | 119°12′38.16″ E | 32°07′39.36″ N | × | Koelreuteria bipinnata Populus sp. | ||
| 11 | Jiangsu Agricultural Expo Park | 119°14′26.52″ E | 32°01′15.6″ N | × | Acer negundo | ||
| 12 | Shishi Road | 119°08′1.32″ E | 31°57′13.68″ N | × | Lagerstroemia indica Melia azedarach Koelreuteria bipinnata Acer sp. | ||
| 13 | Fudi Road | 119°08′0.24″ E | 31°58′14.52″ N | × | Lagerstroemia indica |
| Score | Position Shift * |
|---|---|
| 1 | ① from a branch to the other within a region ② from a branch to the cage within a region ③ from the cage to a branch within a region |
| 2 | ④ between the cages of different regions |
| 3 | ⑤ from a branch to the cage of other regions ⑥ from the cage to a branch in other regions |
| 4 | ⑦ from a branch to a branch across regions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Wang, W.; Chen, X.; Ma, J.; Chen, R.; Sun, X.; Yang, Y.; Li, G.; Deng, Y.; Hao, D. Interspecific Mating Is Trivial and Asymmetrical Between Two Destructive Anoplophora Beetles. Insects 2025, 16, 352. https://doi.org/10.3390/insects16040352
Xu T, Wang W, Chen X, Ma J, Chen R, Sun X, Yang Y, Li G, Deng Y, Hao D. Interspecific Mating Is Trivial and Asymmetrical Between Two Destructive Anoplophora Beetles. Insects. 2025; 16(4):352. https://doi.org/10.3390/insects16040352
Chicago/Turabian StyleXu, Tian, Wenbo Wang, Xiaoyuan Chen, Jing Ma, Ruixu Chen, Xue Sun, Yang Yang, Guohao Li, Yadi Deng, and Dejun Hao. 2025. "Interspecific Mating Is Trivial and Asymmetrical Between Two Destructive Anoplophora Beetles" Insects 16, no. 4: 352. https://doi.org/10.3390/insects16040352
APA StyleXu, T., Wang, W., Chen, X., Ma, J., Chen, R., Sun, X., Yang, Y., Li, G., Deng, Y., & Hao, D. (2025). Interspecific Mating Is Trivial and Asymmetrical Between Two Destructive Anoplophora Beetles. Insects, 16(4), 352. https://doi.org/10.3390/insects16040352