The Effects of Artificial Diets Containing Free Amino Acids Versus Intact Proteins on Biomarkers of Nutrition and Deformed Wing Virus Levels in the Honey Bee
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Caged Honey Bees
2.2. Honey Bee Diets and Age Collections
2.2.1. Food Consumption and Honey Bee Survival
2.2.2. Hemocytes Measurements
2.2.3. RNA Extraction and Quantitative PCR of vg, mrjp1 and DWV
2.2.4. Statistical Analysis
3. Results
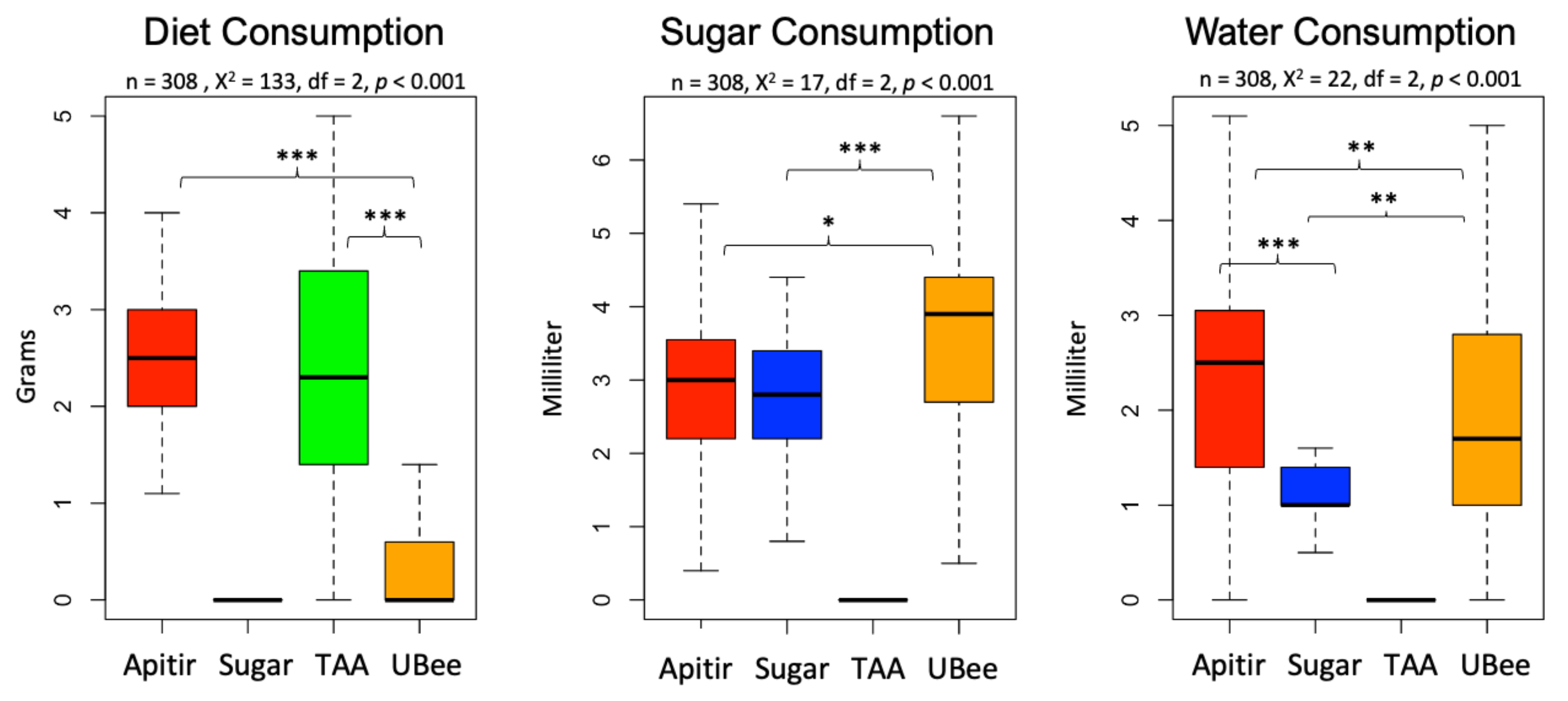
3.1. Bee Survival and Food Consumption
3.2. Hemocyte Number
3.3. Analyses of Gene Expression and Viral Levels
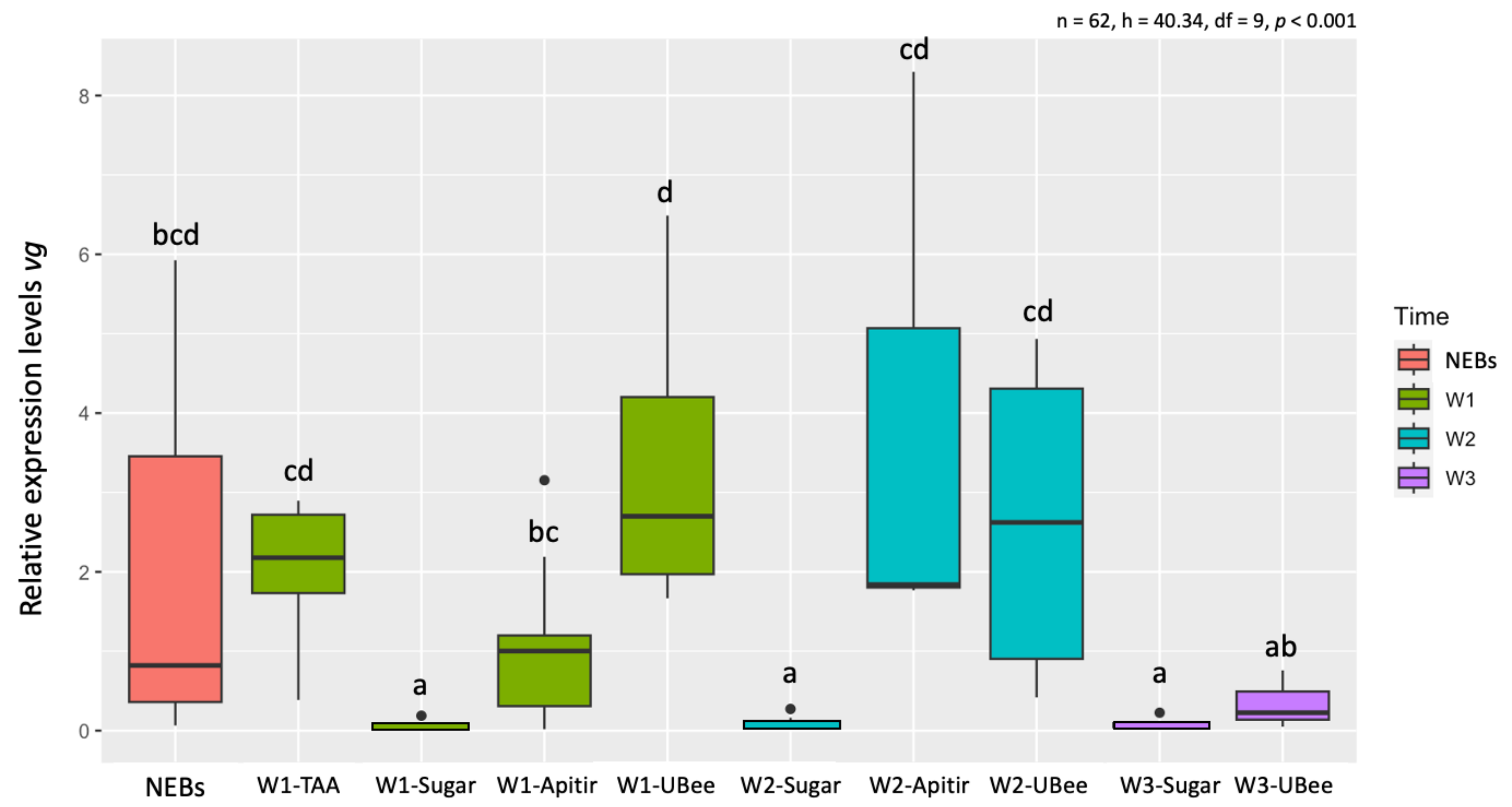
3.3.1. Vitellogenin
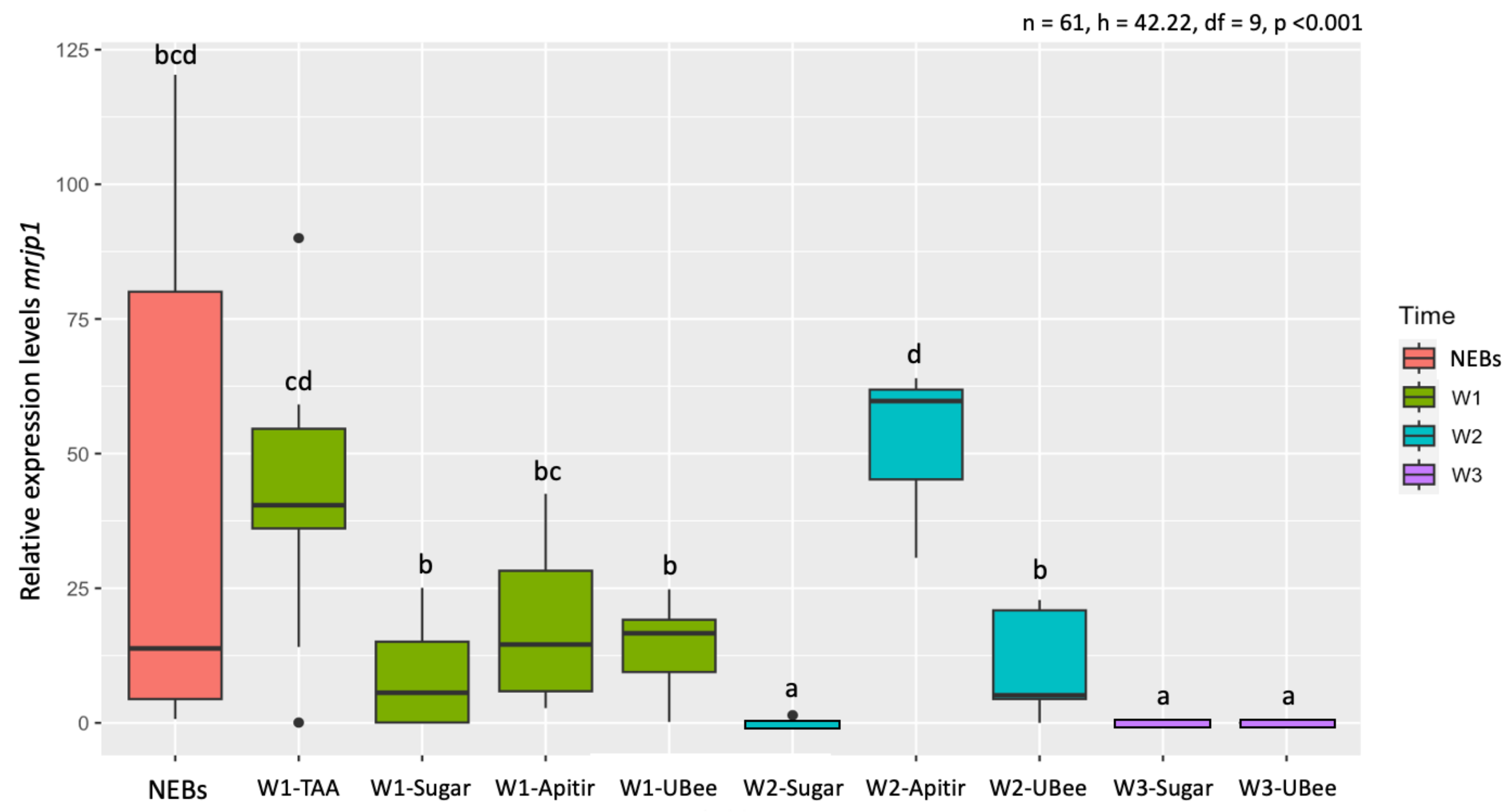
3.3.2. Major Royal Jelly Protein 1
3.3.3. Deformed Wing Virus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Klein, A.M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of crop pollinators in changing landscapes for world crops. Proc. Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef]
- Lorenzo-Felipe, I.; Blanco, C.; Corona, M. Impact of apoidea (Hymenoptera) on the world’s food production and diets. Ann. Entomol. Soc. Am. 2020, 113, 407–424. [Google Scholar] [CrossRef]
- Hristov, P.; Shumkova, R.; Palova, N.; Neov, B. Factors associated with honey bee colony losses: A mini-review. Vet. Sci. 2020, 7, 166. [Google Scholar] [CrossRef]
- Potts, S.G.; Roberts, S.P.M.; Dean, R.; Marris, G.; Brown, M.A.; Jones, R.; Neumann, P.; Settele, J. Declines of managed honey bees and beekeepers in Europe. J. Apic. Res. 2010, 49, 15–22. [Google Scholar] [CrossRef]
- Requier, F.; Leyton, M.S.; Morales, C.L.; Garibaldi, L.A.; Giacobino, A.; Porrini, M.P.; Rosso-Londoño, J.M.; Velarde, R.A.; Aignasse, A.; Aldea-Sánchez, P.; et al. First large-scale study reveals important losses of managed honey bee and stingless bee colonies in Latin America. Sci. Rep. 2024, 14, 10079. [Google Scholar] [CrossRef]
- Kulhanek, K.; Steinhauer, N.; Rennich, K.; Caron, D.; Sagili, R.R.; Pettis, J.S.; Ellis, J.D.; Wilson, M.E.; Wilkes, J.T.; Tarpy, D.R.; et al. A national survey of managed honey bee 2015–2016 annual colony losses in the USA. J. Apic. Res. 2017, 56, 328–340. [Google Scholar] [CrossRef]
- Guzman-Novoa, E.; Eccles, L.; Calvete, Y.; Mcgowan, J.; Kelly, P.G.; Correa-Benítez, A. Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie 2010, 41, 443–450. [Google Scholar] [CrossRef]
- Martin, S.J.; Highfield, A.C.; Brettell, L.; Villalobos, E.M.; Budge, G.E.; Powell, M.; Nikaido, S.; Schroeder, D.C. Global honey bee viral landscape altered by a parasitic mite. Science 2012, 336, 1304–1306. [Google Scholar] [CrossRef]
- Smart, M.; Pettis, J.; Rice, N.; Browning, Z.; Spivak, M. Linking measures of colony and individual honey bee health to survival among apiaries exposed to varying agricultural land use. PLoS ONE 2016, 11, e0152685. [Google Scholar] [CrossRef]
- Dolezal, A.G.; Carrillo-Tripp, J.; Miller, W.A.; Bonning, B.C.; Toth, A.L. Intensively cultivated landscape and Varroa mite infestation are associated with reduced honey bee nutritional state. PLoS ONE 2016, 11, e0153531. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Alaux, C.; Le Conte, Y.; Odoux, J.F.; Pioz, M.; Vaissière, B.E.; Belzunces, L.P.; Decourtye, A. Variations in the availability of pollen resources affect honey bee health. PLoS ONE 2016, 11, e0162818. [Google Scholar] [CrossRef]
- Ricigliano, V.A.; Mott, B.M.; Maes, P.W.; Floyd, A.S.; Fitz, W.; Copeland, D.C.; Meikle, W.G.; Anderson, K.E. Honey bee colony performance and health are enhanced by apiary proximity to US Conservation Reserve Program (CRP) lands. Sci. Rep. 2019, 9, 4894. [Google Scholar] [CrossRef]
- Haydak, M.H. Honey bee nutrition. Annu. Rev. Entomol. 1970, 15, 143–156. [Google Scholar] [CrossRef]
- Tsuruda, J.M.; Chakrabarti, P.; Sagili, R.R. Honey bee nutrition. Vet. Clin. N. Am. Food Anim. Pract. 2021, 37, 505–519. [Google Scholar] [CrossRef]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Keller, I.; Fluri, P.; Imdorf, I. Pollen nutrition and colony development in honey bees: Part 1. Bee World 2005, 86, 3–10. [Google Scholar]
- Huang, Z.Y. Pollen nutrition affects honey bee stress resistance. Terr. Arthropod. Rev. 2012, 5, 175–189. [Google Scholar] [CrossRef]
- Decourtye, A.; Alaux, C.; Odoux, J.-F.; Henry, M.; Vaissière, B.E.; Le Conte, Y. Why enhancement of floral resources in agro-ecosystems benefit honeybees and beekeepers? In Ecosystems Biodiversity; Oscar, G., Gianfranco, V., Eds.; IntechOpen: Rijeka, Zagreb, 2011; Chapter 16. [Google Scholar] [CrossRef]
- Weiner, C.N.; Hilpert, A.; Werner, M.; Linsenmair, K.E.; Blüthgen, N. Pollen amino acids and flower specialisation in solitary bees. Apidologie 2010, 41, 476–487. [Google Scholar] [CrossRef]
- Barraud, A.; Barascou, L.; Lefebvre, V.; Sene, D.; Le Conte, Y.; Alaux, C.; Grillenzoni, F.-V.; Corvucci, F.; Serra, G.; Costa, C.; et al. Variations in nutritional requirements across bee species. Front. Sustain. Food Syst. 2022, 6, 824750. [Google Scholar] [CrossRef]
- Yokota, S.C.; Broeckling, C.; Arathi, H.S. Pollen foraging preferences in honey bees and the nutrient profiles of the pollen. Sci. Rep. 2024, 14, 15028. [Google Scholar] [CrossRef]
- Höcherl, N.; Siede, R.; Illies, I.; Gätschenberger, H.; Tautz, J. Evaluation of the nutritive value of maize for honey bees. J. Insect. Physiol. 2012, 58, 278–285. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Human, H. Chemical composition of the ‘low quality’ pollen of sunflower (Helianthus annuus, Asteraceae). Apidologie 2013, 44, 144–152. [Google Scholar] [CrossRef]
- Herbert, E.W.; Bickley, W.E.; Shimanuki, H. The brood-rearing capability of caged honey bees fed dandelion and mixed pollen diets. J. Econ. Entomol. 1970, 63, 215–218. [Google Scholar] [CrossRef]
- Manning, R. Fatty acids in pollen: A review of their importance for honey bees. Bee World 2001, 82, 60–75. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef]
- Roulston, T.H.; Cane, J.H. Pollen nutritional content and digestibility for animals. Plant Syst. Evol. 2000, 222, 187–209. [Google Scholar] [CrossRef]
- Roulston, T.H.; Cane, J.H.; Buchmann, S.L. What governs protein content of pollen: Pollinator preferences, pollen–pistil interactions, or phylogeny? Ecol. Monogr. 2000, 70, 617–643. [Google Scholar] [CrossRef]
- de Groot, A.P. Amino acid requirements for growth of the honeybee (Apis mellifica L.). Experientia 1952, 8, 192–194. [Google Scholar] [CrossRef]
- De Groot, A.P. Protein and amino acid requirements of the honey bee (Apis mellifera). Physiol. Comp. Oecol. 1953, 3, 197–285. [Google Scholar]
- Wegener, J.; Jakop, U.; Schiller, J.; Müller, K. The membrane phospholipid composition of honeybee (Apis mellifera) workers reflects their nutrition, fertility, and vitellogenin stores. Insectes Soc. 2018, 65, 381–391. [Google Scholar] [CrossRef]
- Das, U.N. Essential fatty acids: Biochemistry, physiology and pathology. Biotechnol. J. 2006, 1, 420–439. [Google Scholar] [CrossRef]
- Canavoso, L.E.; Jouni, Z.E.; Karnas, K.J.; Pennington, J.E.; Wells, M.A. Fat metabolism in insects. Annu. Rev. Nutr. 2001, 21, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Arien, Y.; Dag, A.; Shafir, S. Omega-6:3 ratio more than absolute lipid level in diet affects associative learning in honey Bees. Front. Psychol. 2018, 9, 1001. [Google Scholar] [CrossRef]
- Kim, H.; Frunze, O.; Maigoro, A.Y.; Lee, M.L.; Lee, J.H.; Kwon, H.W. Comparative study of the effect of pollen substitute diets on honey bees during early spring. Insects 2024, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- DeGrandi-Hoffman, G.; Chen, Y.; Rivera, R.; Carroll, M.; Chambers, M.; Hidalgo, G.; de Jong, E.W. Honey bee colonies provided with natural forage have lower pathogen loads and higher overwinter survival than those fed protein supplements. Apidologie 2016, 47, 186–196. [Google Scholar] [CrossRef]
- Kumsa Gemeda, T. Testing the effect of dearth period supplementary feeding of honeybee (Apis mellifera) on brood development and honey production. Int. J. of Adv. Res. 2014, 2, 319–324. [Google Scholar]
- Noordyke, E.R.; Ellis, J.D. Reviewing the efficacy of pollen substitutes as a management tool for improving the health and productivity of western honey bee (Apis mellifera) colonies. Front. Sustain. Food Syst. 2021, 5, 772897. [Google Scholar] [CrossRef]
- Ricigliano, V.A.; Williams, S.T.; Oliver, R. Effects of different artificial diets on commercial honey bee colony performance, health biomarkers, and gut microbiota. BMC Vet. Res. 2022, 18, 52. [Google Scholar] [CrossRef]
- Lamas, Z.S.; Solmaz, S.; Stevens, C.; Bragg, J.; Ryabov, E.V.; Madella, S.; Corona, M.; Evans, J.D. Nano colonies: Rearing honey bee queens and their offspring in small laboratory arenas. Heliyon 2025, 11, e42042. [Google Scholar] [CrossRef]
- Hendriksma, H.P.; Pachow, C.D.; Nieh, J.C. Effects of essential amino acid supplementation to promote honey bee gland and muscle development in cages and colonies. J. Insect. Physiol. 2019, 117, 103906. [Google Scholar] [CrossRef]
- Kartik; Rana, A.; Singh, G. Artificial diet supplementation: A review for sustainable approach to boost honeybee health. J. Sci. Ind. Res. 2024, 83, 914–933. [Google Scholar] [CrossRef]
- Koopman, R.; Crombach, N.; Gijsen, A.P.; Walrand, S.; Fauquant, J.; Kies, A.K.; Lemosquet, S.; Saris, W.H.; Boirie, Y.; van Loon, L.J. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am. J. Clin. Nutr. 2009, 90, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Weijzen, M.E.G.; van Gassel, R.J.J.; Kouw, I.W.K.; Trommelen, J.; Gorissen, S.H.M.; van Kranenburg, J.; Goessens, J.P.B.; van de Poll, M.C.G.; Verdijk, L.B.; van Loon, L.J.C. Ingestion of free amino acids compared with an equivalent amount of intact protein results in more rapid amino acid absorption and greater postprandial plasma amino acid availability without affecting muscle protein synthesis rates in young adults in a double-blind randomized trial. J. Nutr. 2022, 152, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Eugenio, F.A.; van Milgen, J.; Duperray, J.; Sergheraert, R.; Le Floc’h, N. Feeding intact proteins, peptides, or free amino acids to monogastric farm animals. Amino Acids 2022, 54, 157–168. [Google Scholar] [CrossRef]
- Gwin, J.A.; Church, D.D.; Wolfe, R.R.; Ferrando, A.A.; Pasiakos, S.M. Muscle protein synthesis and whole-body protein turnover responses to ingesting essential amino acids, intact protein, and protein-containing mixed meals with considerations for energy deficit. Nutrients 2020, 12, 2457. [Google Scholar] [CrossRef]
- Smith, K.; Reynolds, N.; Downie, S.; Patel, A.; Rennie, M.J. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am. J. Physiol. 1998, 275, E73–E78. [Google Scholar] [CrossRef]
- Cleveland, B.M.; Radler, L.M. Essential amino acids exhibit variable effects on protein degradation in rainbow trout (Oncorhynchus mykiss) primary myocytes. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 229, 33–39. [Google Scholar] [CrossRef]
- Paoli, P.P.; Donley, D.; Stabler, D.; Saseendranath, A.; Nicolson, S.W.; Simpson, S.J.; Wright, G.A. Nutritional balance of essential amino acids and carbohydrates of the adult worker honeybee depends on age. Amino Acids 2014, 46, 1449–1458. [Google Scholar] [CrossRef]
- Pirk, C.W.W.; Boodhoo, C.; Human, H.; Nicolson, S.W. The importance of protein type and protein to carbohydrate ratio for survival and ovarian activation of caged honeybees (Apis mellifera scutellata). Apidologie 2010, 41, 62–72. [Google Scholar] [CrossRef]
- Corona, M.; Branchiccela, B.; Alburaki, M.; Palmer-Young, E.C.; Madella, S.; Chen, Y.; Evans, J.D. Decoupling the effects of nutrition, age, and behavioral caste on honey bee physiology, immunity, and colony health. Front. Physiol. 2023, 14, 1149840. [Google Scholar] [CrossRef]
- Piulachs, M.D.; Guidugli, K.R.; Barchuk, A.R.; Cruz, J.; Simões, Z.L.; Bellés, X. The vitellogenin of the honey bee, Apis mellifera: Structural analysis of the cDNA and expression studies. Insect Biochem. Mol. Biol. 2003, 33, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Schmitzová, J.; Klaudiny, J.; Albert, S.; Schröder, W.; Schreckengost, W.; Hanes, J.; Júdová, J.; Simúth, J. A family of major royal jelly proteins of the honeybee Apis mellifera L. Cell. Mol. Life Sci. 1998, 54, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Bitondi, M.M.G.; Simões, Z.L.P. The relationship between level of pollen in the diet, vitellogenin and juvenile hormone titres in Africanized Apis mellifera workers. J. Apic. Res. 1996, 35, 27–36. [Google Scholar] [CrossRef]
- Alaux, C.; Dantec, C.; Parrinello, H.; Le Conte, Y. Nutrigenomics in honey bees: Digital gene expression analysis of pollen’s nutritive effects on healthy and varroa-parasitized bees. BMC Genom. 2011, 12, 496. [Google Scholar] [CrossRef]
- Ament, S.A.; Corona, M.; Pollock, H.S.; Robinson, G.E. Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. Proc. Natl. Acad. Sci. USA 2008, 105, 4226–4231. [Google Scholar] [CrossRef]
- Li, J.; Heerman, M.C.; Evans, J.D.; Rose, R.; Li, W.; Rodríguez-García, C.; DeGrandi-Hoffman, G.; Zhao, Y.; Huang, S.; Li, Z.; et al. Pollen reverses decreased lifespan, altered nutritional metabolism and suppressed immunity in honey bees (Apis mellifera) treated with antibiotics. J. Exp. Biol. 2019, 222, 202077. [Google Scholar] [CrossRef]
- Kim, H.; Frunze, O.; Lee, J.H.; Kwon, H.W. Enhancing honey bee health: Evaluating pollen substitute diets in field and cage experiments. Insects 2024, 15, 361. [Google Scholar] [CrossRef]
- Frunze, O.; Kim, H.; Lee, J.-H.; Kwon, H.-W. The effects of artificial diets on the expression of molecular marker genes related to honey bee health. Int. J. Mol. Sci. 2024, 25, 4271. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Chen, Y.; Huang, E.; Huang, M.H. The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honey bees (Apis mellifera L.). J. Insect Physiol. 2010, 56, 1184–1191. [Google Scholar] [CrossRef]
- Branchiccela, B.; Castelli, L.; Corona, M.; Díaz-Cetti, S.; Invernizzi, C.; Martínez de la Escalera, G.; Mendoza, Y.; Santos, E.; Silva, C.; Zunino, P.; et al. Impact of nutritional stress on the honeybee colony health. Sci. Rep. 2019, 9, 10156. [Google Scholar] [CrossRef]
- De Jong, D.; Roma, D.D.; Gonçalves, L.S. A comparative analysis of shaking solutions for the detection of Varroa jacobsoni on adult honeybees. Apidologie 1982, 13, 297–306. [Google Scholar] [CrossRef]
- Palmer-Young, E.C.; Markowitz, L.M.; Grubbs, K.; Zhang, Y.; Corona, M.; Schwarz, R.; Chen, Y.; Evans, J.D. Antiparasitic effects of three floral volatiles on trypanosomatid infection in honey bees. J. Invertebr. Pathol. 2022, 194, 107830. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Plaza, P.; Michałowski, T.; Navas, M.J.; Asuero, A.G.; Wybraniec, S. An overview of the Kjeldahl method of nitrogen determination. Part I. Early history, chemistry of the procedure, and titrimetric finish. Crit. Rev. Anal. Chem. 2013, 43, 178–223. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Priego-Capote, F. Soxhlet extraction: Past and present panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef]
- Koleoglu, G.; Goodwin, P.H.; Reyes-Quintana, M.; Hamiduzzaman, M.M.; Guzman-Novoa, E. Varroa destructor parasitism reduces hemocyte concentrations and prophenol oxidase gene expression in bees from two populations. Parasitol. Res. 2018, 117, 1175–1183. [Google Scholar] [CrossRef]
- Gregorc, A.; Evans, J.D.; Scharf, M.; Ellis, J.D. Gene expression in honey bee (Apis mellifera) larvae exposed to pesticides and Varroa mites (Varroa destructor). J. Insect Physiol. 2012, 58, 1042–1049. [Google Scholar] [CrossRef]
- Corona, M.; Velarde, R.A.; Remolina, S.; Moran-Lauter, A.; Wang, Y.; Hughes, K.A.; Robinson, G.E. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Natl. Acad. Sci. USA 2007, 104, 7128–7133. [Google Scholar] [CrossRef]
- Allaire, J.J. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 1 October 2023).
- Ruedenauer, F.A.; Biewer, N.W.; Nebauer, C.A.; Scheiner, M.; Spaethe, J.; Leonhardt, S.D. Honey bees can taste amino and fatty acids in pollen, but not sterols. Front. Ecol. Evol. 2021, 9, 684175. [Google Scholar] [CrossRef]
- Lavine, M.D.; Strand, M.R. Insect hem ocytes and their role in immunity. Insect. Biochem. Mol. Biol. 2002, 32, 1295–1309. [Google Scholar] [CrossRef]
- Bryś, M.S.; Skowronek, P.; Strachecka, A. Pollen diet-properties and impact on a bee colony. Insects 2021, 12, 798. [Google Scholar] [CrossRef]
- Szymaś, B.; Jędruszuk, A. The influence of different diets on haemocytes of adult worker honey bees, Apis mellifera. Apidologie 2003, 34, 97–102. [Google Scholar] [CrossRef]
- Amdam, G.V.; Aase, A.L.; Seehuus, S.C.; Kim Fondrk, M.; Norberg, K.; Hartfelder, K. Social reversal of immunosenescence in honey bee workers. Exp. Gerontol. 2005, 40, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.R.; Brockmann, A.; Pirk, C.W.; Stanley, D.W.; Tautz, J. Adult honeybees (Apis mellifera L.) abandon hemocytic, but not phenoloxidase-based immunity. J. Insect Physiol. 2008, 54, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.E. Regulation of division of labor in insect societies. Annu. Rev. Entomol. 1992, 37, 637–665. [Google Scholar] [CrossRef]
- Amdam, G.V.; Omholt, S.W. The regulatory anatomy of honeybee lifespan. J. Theor. Biol. 2002, 216, 209–228. [Google Scholar] [CrossRef]
- de Miranda, J.R.; Genersch, E. Deformed wing virus. J. Invertebr. Pathol. 2010, 103 (Suppl. S1), S48–S61. [Google Scholar] [CrossRef]
- Locke, B.; Semberg, E.; Forsgren, E.; de Miranda, J.R. Persistence of subclinical deformed wing virus infections in honeybees following Varroa mite removal and a bee population turnover. PLoS ONE 2017, 12, e0180910. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tapia-Rivera, J.C.; Tapia-González, J.M.; Alburaki, M.; Chan, P.; Sánchez-Cordova, R.; Macías-Macías, J.O.; Corona, M. The Effects of Artificial Diets Containing Free Amino Acids Versus Intact Proteins on Biomarkers of Nutrition and Deformed Wing Virus Levels in the Honey Bee. Insects 2025, 16, 375. https://doi.org/10.3390/insects16040375
Tapia-Rivera JC, Tapia-González JM, Alburaki M, Chan P, Sánchez-Cordova R, Macías-Macías JO, Corona M. The Effects of Artificial Diets Containing Free Amino Acids Versus Intact Proteins on Biomarkers of Nutrition and Deformed Wing Virus Levels in the Honey Bee. Insects. 2025; 16(4):375. https://doi.org/10.3390/insects16040375
Chicago/Turabian StyleTapia-Rivera, José Carlos, José María Tapia-González, Mohamed Alburaki, Philene Chan, Rogelio Sánchez-Cordova, José Octavio Macías-Macías, and Miguel Corona. 2025. "The Effects of Artificial Diets Containing Free Amino Acids Versus Intact Proteins on Biomarkers of Nutrition and Deformed Wing Virus Levels in the Honey Bee" Insects 16, no. 4: 375. https://doi.org/10.3390/insects16040375
APA StyleTapia-Rivera, J. C., Tapia-González, J. M., Alburaki, M., Chan, P., Sánchez-Cordova, R., Macías-Macías, J. O., & Corona, M. (2025). The Effects of Artificial Diets Containing Free Amino Acids Versus Intact Proteins on Biomarkers of Nutrition and Deformed Wing Virus Levels in the Honey Bee. Insects, 16(4), 375. https://doi.org/10.3390/insects16040375






