Know Where You Go: Infestation Dynamics and Potential Distribution of Two Bed Bug Species (Hemiptera: Cimicidae) in Africa
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Evaluation of C. hemipterus and C. lectularius Infestation Dynamics in Different Counties in Kenya
2.1.1. Interview and Data Collection for Infestation Dynamics
2.1.2. Infestation Dynamics Model Development
2.2. Prediction of the Potential Distribution of C. hemipterus and C. lectularius and Their Co-Occurrence Across the African Continent
2.2.1. Sample Collection for Potential Distribution
2.2.2. Environmental Data
2.2.3. Ecological Niche Model Development Calibration
2.2.4. Co-Suitability Habitat of Cimex hemipterus and Cimex lectularius
3. Results
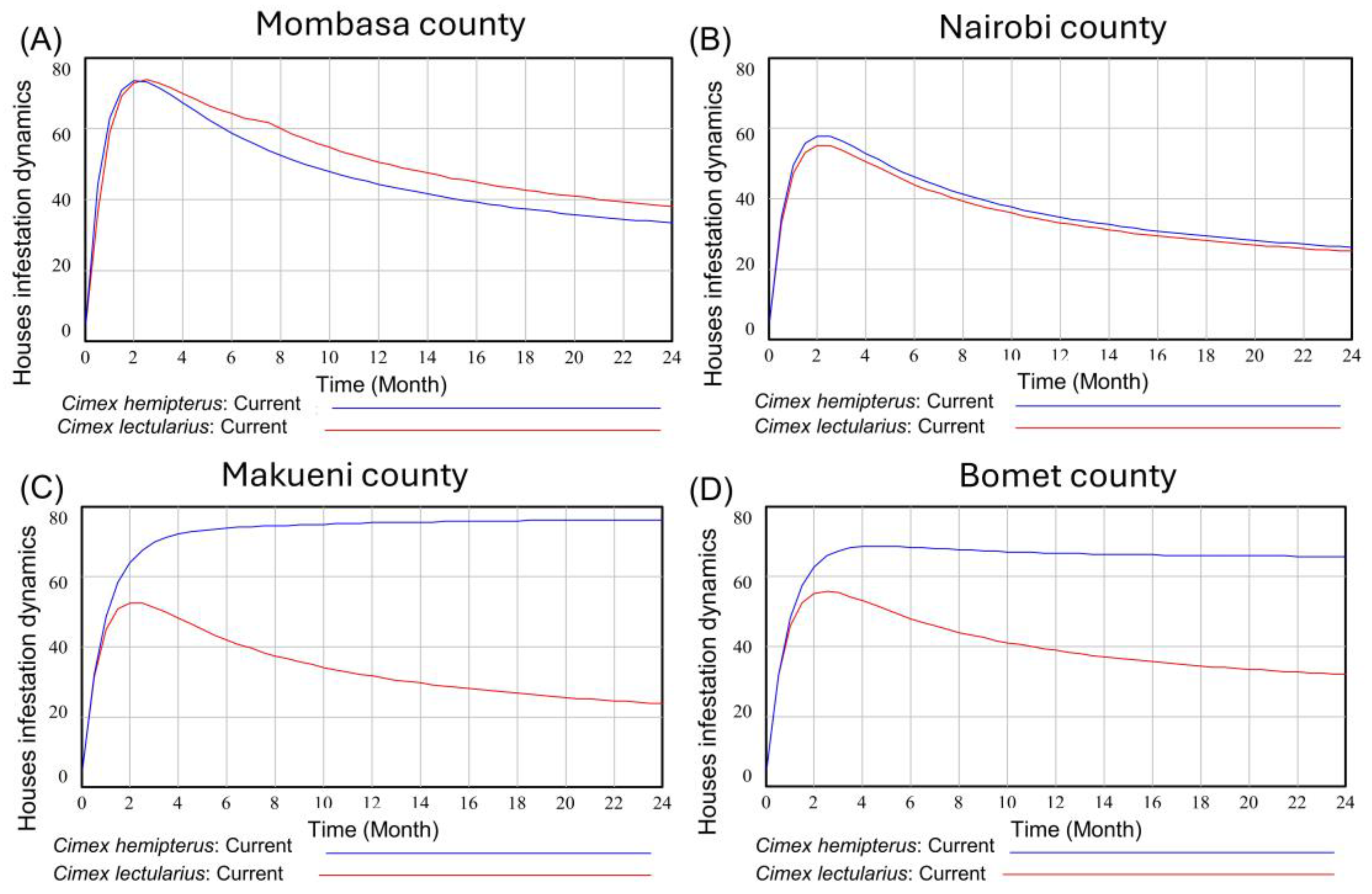
3.1. The Infestation Dynamics of C. hemipterus and C. lectularius in Different Counties of Kenya
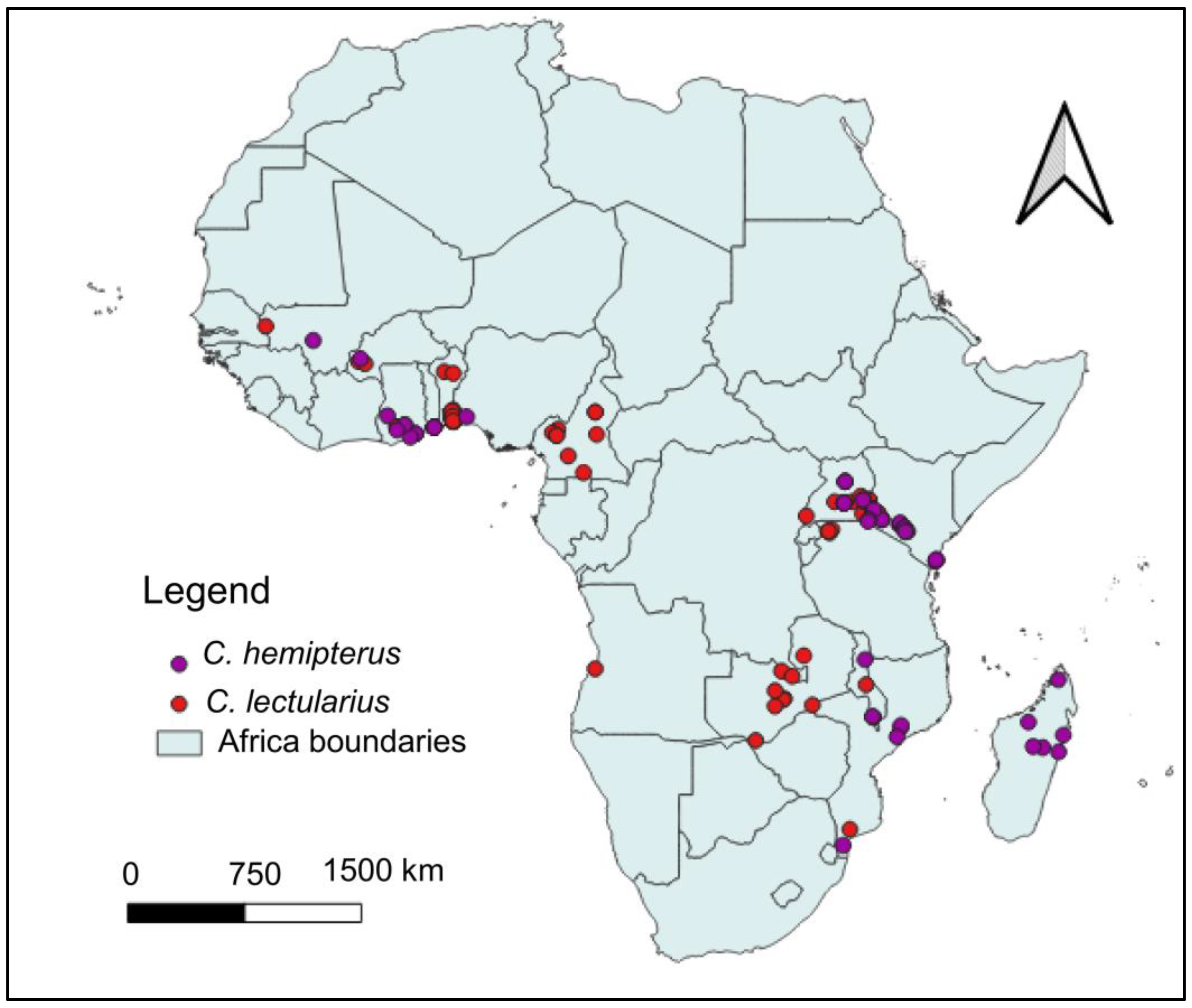
3.2. Current Distribution of C. hemipterus and C. lectularius
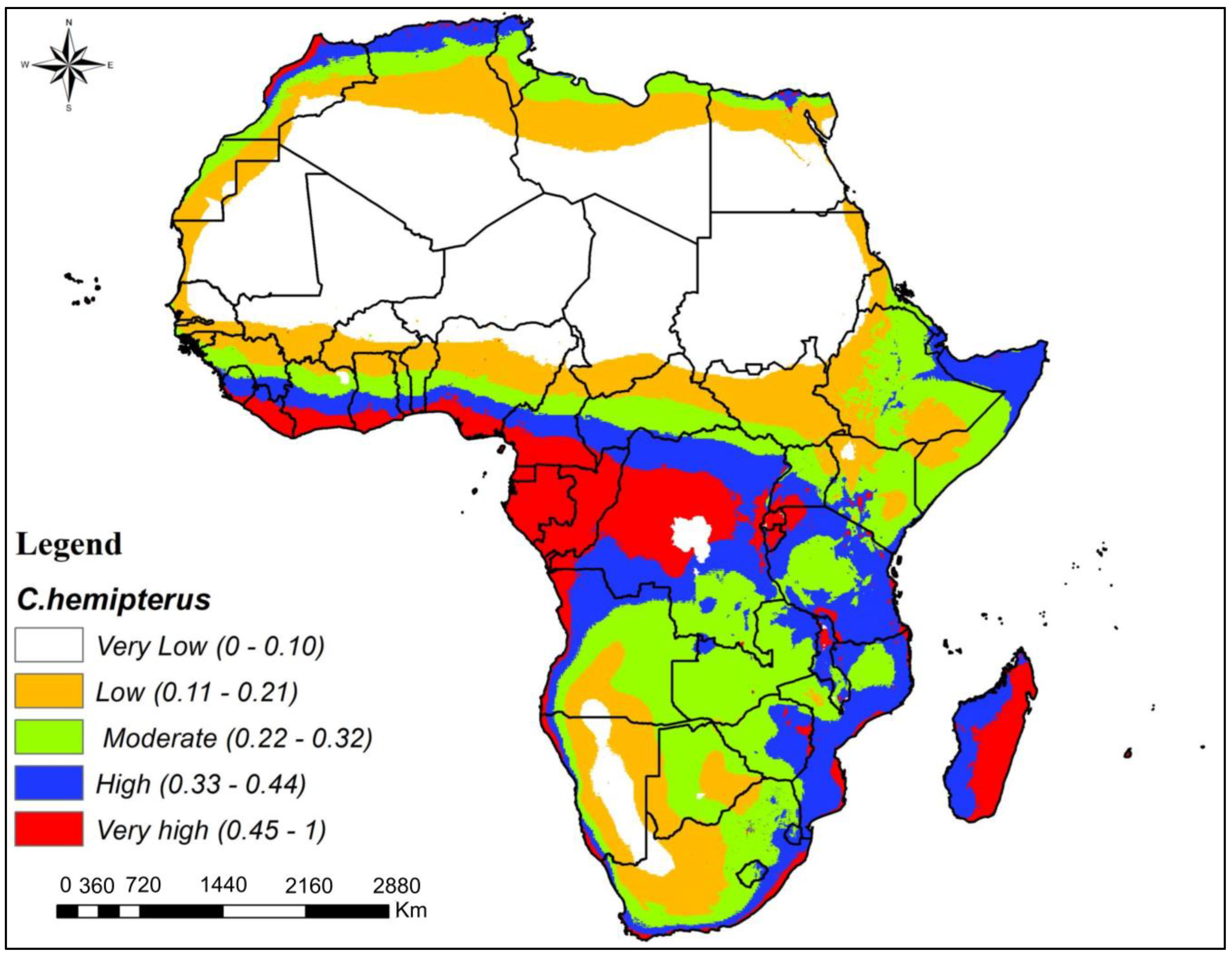
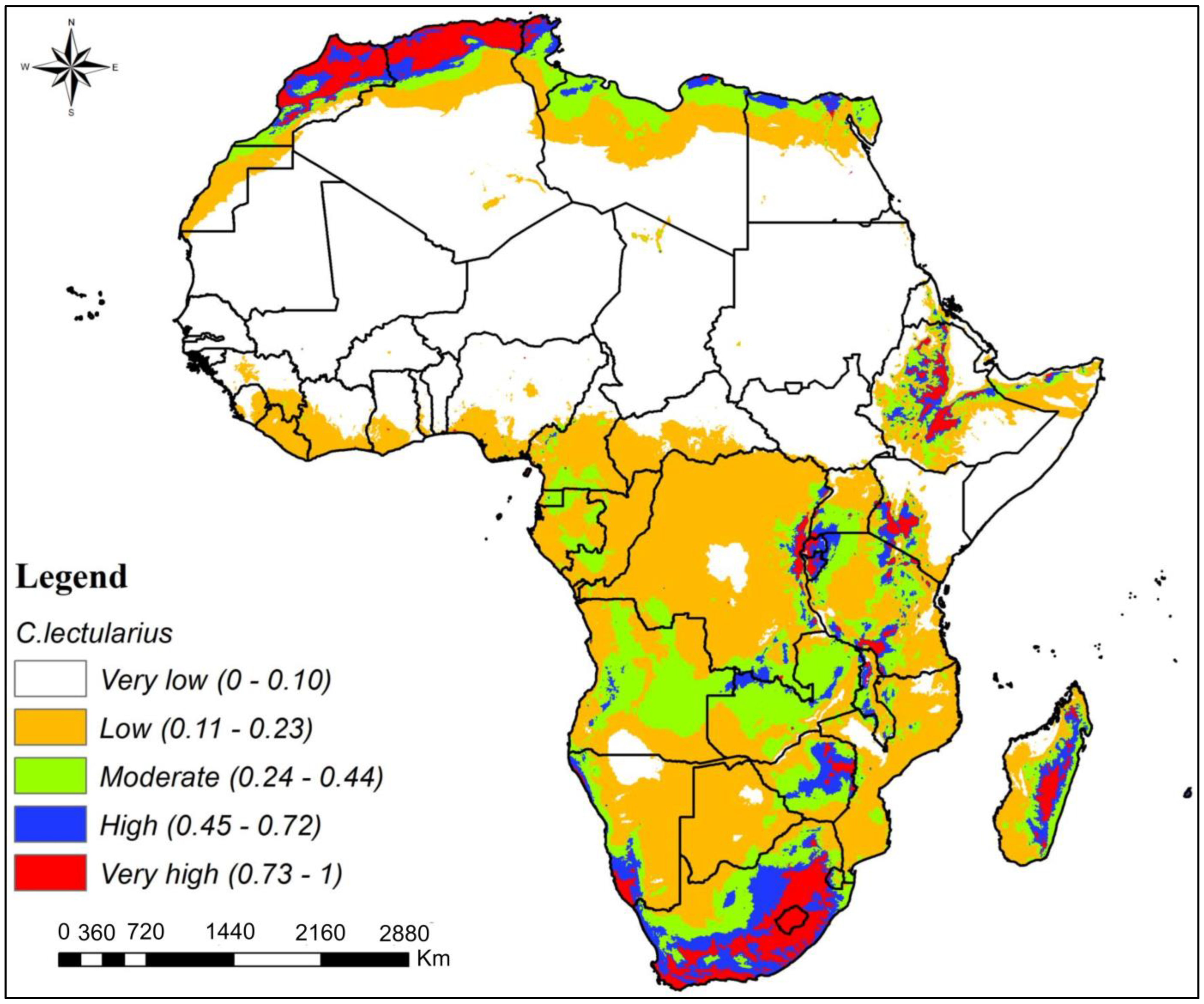
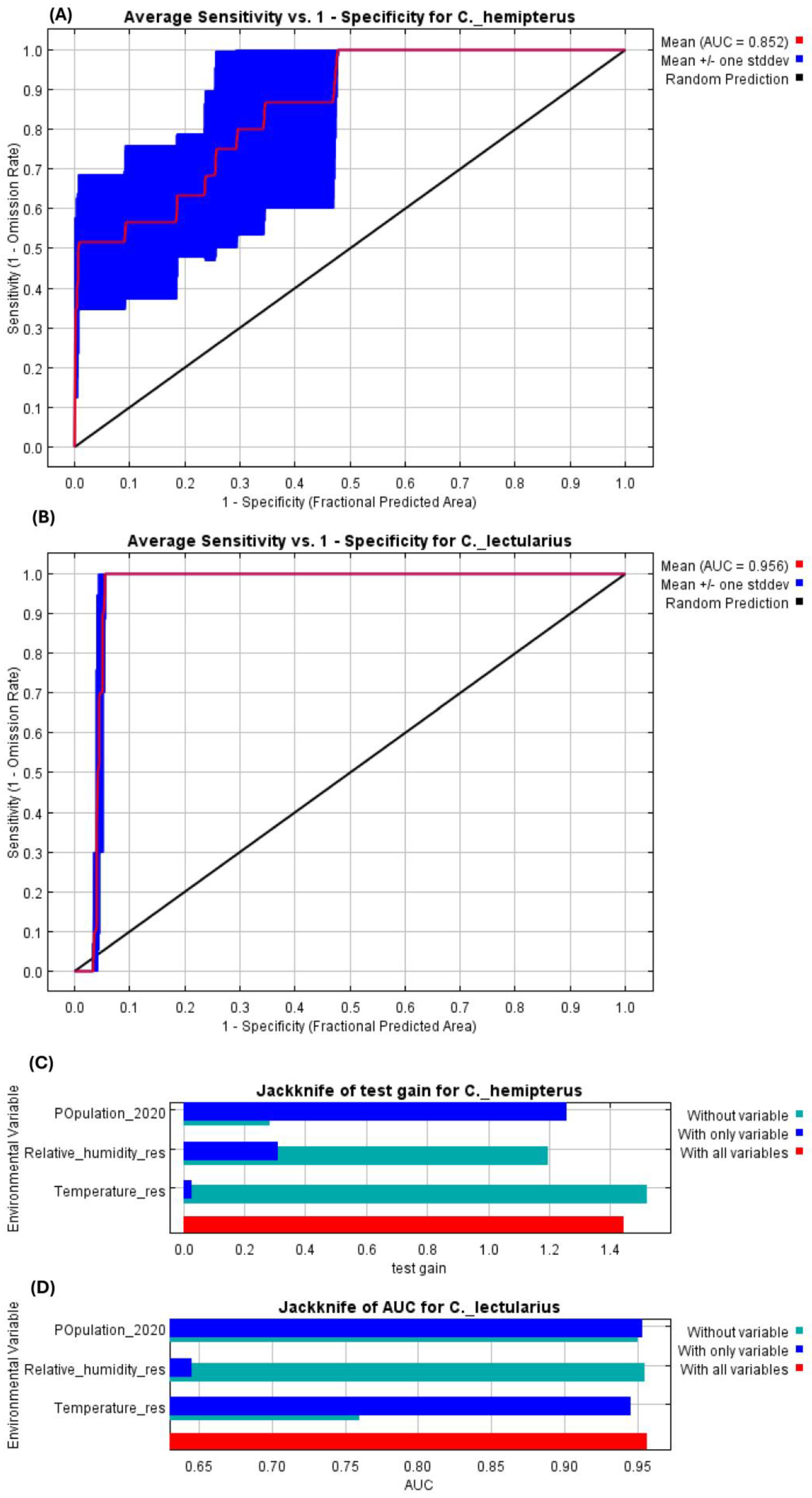
3.3. Potential Habitat Suitability of C. hemipterus and C. lectularius Bed Bug Species in Africa
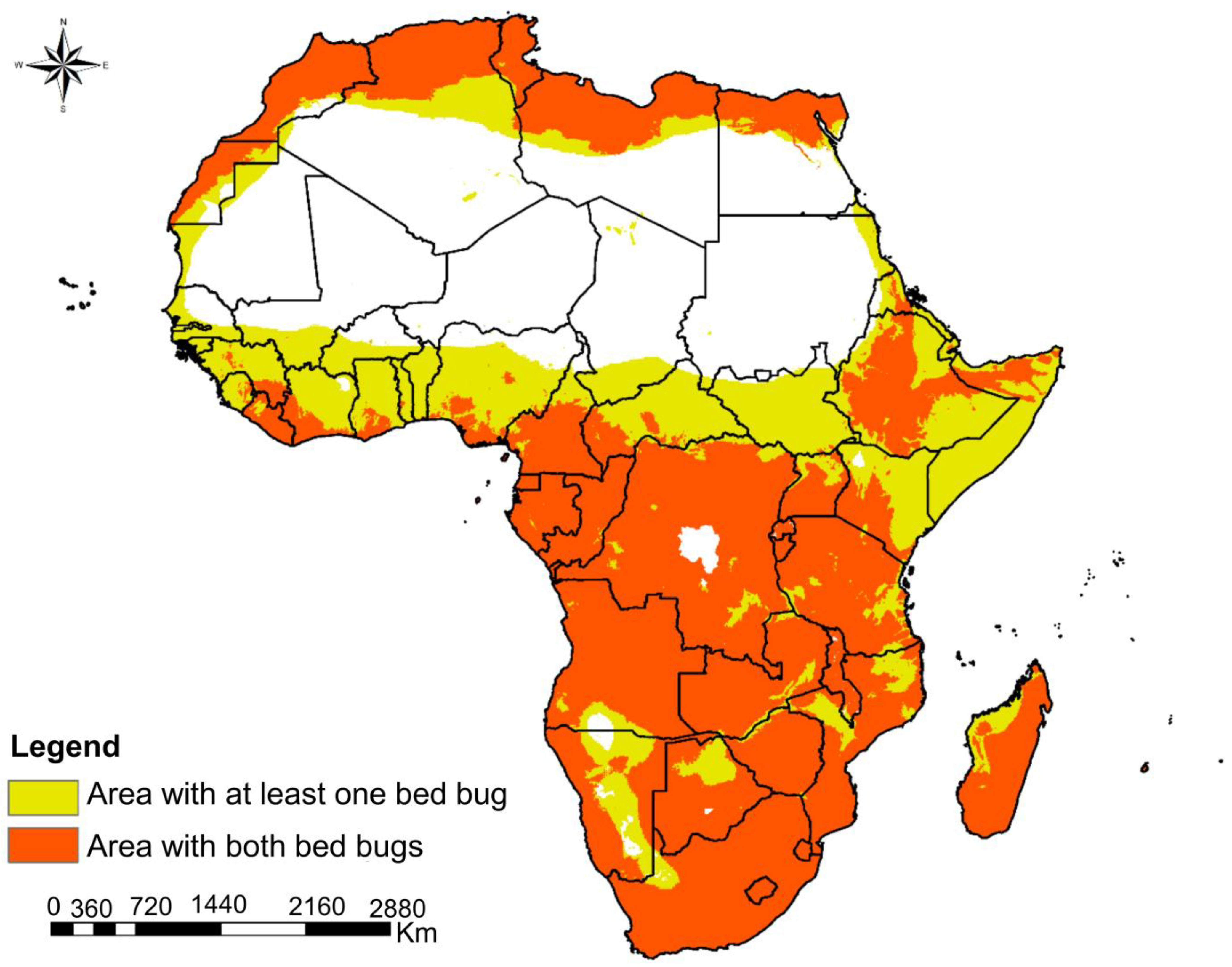
3.4. Co-Suitability Habitat of C. hemipterus and C. lectularius in Africa
3.5. Cimex hemipterus and C. lectularius Bed Bug Species Habitat Suitability Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mbuta, D.M.; Khamis, F.M.; Sokame, B.M.; Ng’ong’a, F.; Akutse, K.S. Household perception and infestation dynamics of bedbugs among residential communities and its potential distribution in Africa. Sci. Rep. 2022, 12, 19900. [Google Scholar] [CrossRef] [PubMed]
- Doggett, S.L.; Miller, D.; Lee, C.-Y. Advances in the Biology and Management of Modern Bedbugs; John Wiley & Sons: Hoboken, NJ, USA, 2018; Available online: https://www.researchgate.net/publication/323726822_Advances_in_the_Biology_and_Management_of_Modern_Bed_Bugs (accessed on 25 May 2024).
- Humphreys, G. Public health round-up. Bull. World Health Organ. 2025, 103, 3–4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- US Environmental Protection Agency (EPA). Bed Bugs: A Public Health Issue. 2023. Available online: https://www.epa.gov/bedbugs/bed-bugs-public-health-issue (accessed on 22 June 2024).
- Bern, C.; Kjos, S.; Yabsley, M.J.; Montgomery, S.P. Trypanosoma cruzi and Chagas’ disease in the United States. Clin. Microbiol. Rev. 2011, 24, 655. [Google Scholar] [CrossRef]
- Amiri, P.; Rahimi, B.; Khalkhali, H.R. Electronic physician. Electron. Physician 2018, 10, 6201–6207. [Google Scholar] [CrossRef] [PubMed]
- Siva-Jothy, M.T.; Zhong, W.; Naylor, R.; Heaton, L.; Hentley, W.; Harney, E. Female bedbugs (Cimex lectularius L.) anticipate the immunological consequences of traumatic insemination via feeding cues. Proc. Natl. Acad. Sci. USA 2019, 116, 14682–14687. [Google Scholar] [CrossRef] [PubMed]
- Tielemans, E.; Rautenbach, C.; Besselaar, J.F.; Beugnet, F. Efficacy of a topical product combining esafoxolaner, eprinomectin and praziquantel against bedbug (Cimex lectularius) experimental infestations in cats. Parasite 2022, 29, 59. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.C.; Schal, C. Behavioral interactions of bedbugs with long-lasting pyrethroid-treated bed nets: Challenges for vector control. Parasites Vectors 2022, 15, 488. [Google Scholar] [CrossRef]
- Strickland, J.; Larson, N.R.; Feldlaufer, M.; Zhang, A. Characterizing repellencies of Methyl benzoate and its analogs against the common bedbug, Cimex lectularius. Insects 2022, 13, 1060. [Google Scholar] [CrossRef]
- Akhoundi, M.; Sereno, D.; Durand, R.; Mirzaei, A.; Bruel, C.; Delaunay, P.; Marty, P.; Izri, A. Bedbugs (Hemiptera: Cimicidae): Overview of classification, evolution and dispersion. Int. J. Environ. Health Res. 2020, 17, 4576. [Google Scholar] [CrossRef]
- Hamlili, F.Z.; Bérenger, J.M.; Parola, P. Cimicids of medical and veterinary importance. Insects 2023, 14, 392. [Google Scholar] [CrossRef]
- Doggett, S.L.; Lee, C.Y. Historical and contemporary control options against bedbugs, Cimex spp. Ann. Rev. Entom. 2023, 68, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Koganemaru, R.; Miller, D.M. The bedbug problem: Past, present, and future control methods. Pestic. Biochem. Physiol. 2013, 106, 177–189. [Google Scholar] [CrossRef]
- Deku, G.; Combey, R.; Doggett, S.L. Morphometrics of the tropical bedbug (Hemiptera: Cimicidae) from Cape Coast, Ghana. J. Med. Entomol. 2022, 59, 1534. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla-Vaca, A.; Silva-Medina, M.M.; Escandón-Vargas, K. Bedbugs, Cimex spp.: Their current world resurgence and healthcare impact. Asian Pac. J. Trop. Dis. 2015, 5, 342–352. [Google Scholar] [CrossRef]
- Akhoundi, M.; Zumelzu, C.; Sereno, D.; Marteau, A.; Brun, S.; Jan, J.; Izri, A. Bedbugs (Hemiptera: Cimicidae): A Global challenge for public health and control management. Diagnostics 2023, 13, 2281. [Google Scholar] [CrossRef]
- Chebbah, D.; Elissa, N.; Sereno, D.; Hamarsheh, O.; Marteau, A.; Jan, J.; Izri, A.; Akhoundi, M. Bed bugs (Hemiptera: Cimicidae) population diversity and first record of Cimex hemipterus in Paris. Insects 2021, 12, 578. [Google Scholar] [CrossRef]
- Fourie, J.; Crafford, D. The bed bug resurgence in Africa. In Advances in the Biology and Management of Modern Bed Bugs; Wiley: Hoboken, NJ, USA, 2018; pp. 87–94. [Google Scholar]
- Kermack, W.O.; McKendrick, A.G. A contribution to the mathematical theory of epidemics. Proc. R. Soc. Lond. Ser. A Contain. Pap. A Math. Phys. Character 1927, 115, 700–721. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, G.; Jarvis, A. Very high-resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Wang, R.; Jiang, C.; Guo, X.; Chen, D.; You, C.; Zhang, Y.; Wang, M.; Li, Q. Potential distribution of Spodoptera frugiperda (J.E. Smith) in China and the major factors influencing distribution. Glob. Ecol. Con. 2020, 21, e00865. [Google Scholar] [CrossRef]
- Leroy, B.; Meynard, C.N.; Bellard, C.; Courchamp, F. Virtual species, an R package to generate virtual species distributions. Ecography 2016, 39, 599–607. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- How, Y.-F.; Lee, C.-Y. Effects of temperature and humidity on the survival and water loss of Cimex hemipterus (Hemiptera: Cimicidae). J. Med. Entomol. 2014, 47, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Rukke, B.A.; Sivasubramaniam, R.; Birkemoe, T.; Aak, A. Temperature stress deteriorates bedbug (Cimex lectularius) populations through decreased survival, fecundity and offspring success. PLoS ONE 2018, 13, e0193788. [Google Scholar] [CrossRef]
- Booth, T.H. Why understanding the pioneering and continuing contributions of BIOCLIM to species distribution modelling is important. Austral Ecol. 2018, 43, 852–860. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Webber, B.L.; Leriche, A.; Ota, N.; Macadam, I.; Bathols, J.; Scott, J.K. CliMond: Global high-resolution historical and future scenario climate surfaces for bioclimatic modelling. Methods Ecol. Evol. 2012, 3, 53–64. [Google Scholar] [CrossRef]
- Lloyd, C.T. High resolution global gridded data for use in population studies. ISPRS-Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2017, XLII-4/W2, 117–120. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Li, J.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Marchioro, C.A.; Krechemer, F.S. Potential global distribution of Diabrotica species and the risks for agricultural production. Pest Manag. Sci. 2018, 74, 2100–2109. [Google Scholar] [CrossRef]
- Phillips, S.B.; Aneja, V.P.; Kang, D.; Arya, S.P. Modelling and analysis of the atmospheric nitrogen deposition in North Carolina. Int. J. Glob. Environ. Issues 2006, 6, 231–252. [Google Scholar] [CrossRef]
- Araújo, M.B.; Rozenfeld, A. The geographic scaling of biotic interactions. Ecography 2014, 37, 406–415. [Google Scholar] [CrossRef]
- Chase, J.M.; Myers, J.A. Disentangling the importance of ecological niches from stochastic processes across scales. Philos. Trans. R. Soc. B 2011, 366, 2351–2363. [Google Scholar] [CrossRef] [PubMed]
- Farnon Ellwood, M.D.; Manica, A.; Foster, W.A. Stochastic and deterministic processes jointly structure tropical arthropod communities. Ecol. Lett. 2009, 12, 277–284. [Google Scholar] [CrossRef]
- Fridley, J.D.; Vandermast, D.B.; Kuppinger, D.M.; Manthey, M.; Peet, R.K. Co-occurrence based assessment of habitat generalists and specialists: A new approach for the measurement of niche width. J. Ecol. 2007, 95, 707–722. [Google Scholar] [CrossRef]
- Yu, J.; Xu, J.; Chen, Y.; Li, W.; Wang, Q.; Yoo, B.; Han, J.J. Learning generalized intersection over union for dense pixelwise prediction. In Proceedings of the 38th International Conference on Machine Learning, Virtual, 18–24 July 2021; pp. 12198–12207. [Google Scholar]
- Verma, V.; Aggarwal, R.K. A comparative analysis of similarity measures akin to the Jaccard index in collaborative recommendations: Empirical and theoretical perspective. Soc. Netw. Anal. Min. 2020, 10, 43. [Google Scholar] [CrossRef]
- Delaunay, P.; Pharm, D. Human travel and traveling bedbugs. J. Travel Med. 2012, 19, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Fung HC, E.; Wong, H.; Chiu, S.W.; Hui JH, L.; Lam, H.M.; Chun, R.Y.; Wrong, S.Y.; Chan, S. Risk factors associated with bedbug (Cimex spp.) infestations among Hong Kong households: A cross-sectional study. J. Hous. Built. Environ. 2020, 37, 1411–1429. [Google Scholar] [CrossRef]
- Wang, C.; Singh, N.; Zha, C.; Cooper, R. Bedbugs: Prevalence in low-income communities, resident’s reactions, and implementation of a low-cost inspection protocol. J. Med. Entomol. 2016, 53, 639–646. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, Q.; Miller, P.A.; Zhang, Q.; Berntell, E.; Smith, B. Impacts of large-scale Sahara solar farms on global climate and vegetation cover. Geophys. Res. Lett. 2021, 48, e2020GL090789. [Google Scholar] [CrossRef]
- Thompson, A. Death Valley Could Set a World Record Hot Temperature—Scientific American. 2023. Available online: https://www.scientificamerican.com/article/death-valley-could-set-a-world-record-hot-temperature/ (accessed on 4 May 2024).
- Barreca, A.I. Climate change, humidity, and mortality in the United States. J. Environ. Econ. Manag. 2012, 63, 19–34. [Google Scholar] [CrossRef]
- Stern, N. The economics of climate change. Am. Econ. Rev. 2008, 98, 1–37. [Google Scholar] [CrossRef]
- Nicholson, S.E. Deserts. In Encyclopedia of Hydrology and Lakes; Springer: Dordrecht, The Netherlands, 1998; pp. 186–197. [Google Scholar] [CrossRef]
- Kells, S.A.; Goblirsch, M.J. Temperature and time requirements for controlling bedbugs (Cimex lectularius) under commercial heat treatment conditions. Insects 2011, 2, 412. [Google Scholar] [CrossRef] [PubMed]
- Wan Mohammad, W.N.F.; Soh, L.-S.; Wan Ismail, W.N.; Veera Singham, G. Infestation pattern and population dynamics of the tropical bedbug, Cimex hemipterus (F.) (Hemiptera: Cimicidae) based on novel microsatellites and mtDNA markers. Insects 2020, 11, 472. [Google Scholar] [CrossRef]
- Benoit, J.B.; Adelman, Z.N.; Reinhardt, K.; Dolan, A.; Poelchau, M.; Jennings, E.C.; Szuter, E.M.; Hagan, R.W.; Gujar, H.; Shukla, J.N.; et al. Unique features of a global human ectoparasite identified through sequencing of the bedbug genome. Nat. Commun. 2016, 7, 10165. [Google Scholar] [CrossRef]
- Benkacimi, L.; Gazelle, G.; El Hamzaoui, B.; Bérenger, J.M.; Parola, P.; Laroche, M. MALDI-TOF MS identification of Cimex lectularius and Cimex hemipterus bedbugs. Infect. Genet. Evol. 2020, 85, 104536. [Google Scholar] [CrossRef]
- Balvín, O.; Sasínková, M.; Martinů, J.; Nazarizadeh, M.; Bubová, T.; Booth, W.; Vargo, E.L.; Štefka, J. Early evidence of establishment of the tropical bedbug (Cimex hemipterus) in central Europe. Med. Vet. Entomol. 2021, 35, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Ashbrook, A.R.; Scharf, M.E.; Bennett, G.W.; Gondhalekar, A.D. Bedbugs (Cimex lectularius L.) exhibit limited ability to develop heat resistance. PLoS ONE 2019, 14, e0211677. [Google Scholar] [CrossRef]
- Benoit, J.B. Stress tolerance of bedbugs: A review of factors that cause trauma to Cimex lectularius and C. hemipterus. Insects 2011, 2, 151. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbuta, D.M.; Sokame, B.M.; Khamis, F.M.; Akutse, K.S. Know Where You Go: Infestation Dynamics and Potential Distribution of Two Bed Bug Species (Hemiptera: Cimicidae) in Africa. Insects 2025, 16, 395. https://doi.org/10.3390/insects16040395
Mbuta DM, Sokame BM, Khamis FM, Akutse KS. Know Where You Go: Infestation Dynamics and Potential Distribution of Two Bed Bug Species (Hemiptera: Cimicidae) in Africa. Insects. 2025; 16(4):395. https://doi.org/10.3390/insects16040395
Chicago/Turabian StyleMbuta, Dennis M., Bonoukpoè M. Sokame, Fathiya M. Khamis, and Komivi S. Akutse. 2025. "Know Where You Go: Infestation Dynamics and Potential Distribution of Two Bed Bug Species (Hemiptera: Cimicidae) in Africa" Insects 16, no. 4: 395. https://doi.org/10.3390/insects16040395
APA StyleMbuta, D. M., Sokame, B. M., Khamis, F. M., & Akutse, K. S. (2025). Know Where You Go: Infestation Dynamics and Potential Distribution of Two Bed Bug Species (Hemiptera: Cimicidae) in Africa. Insects, 16(4), 395. https://doi.org/10.3390/insects16040395





