The Post-Invasion Population Dynamics and Damage Caused by Globose Scale in Central Eurasia: Destiny of Wild Apricot Still at Stake
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.1.1. Survey Site
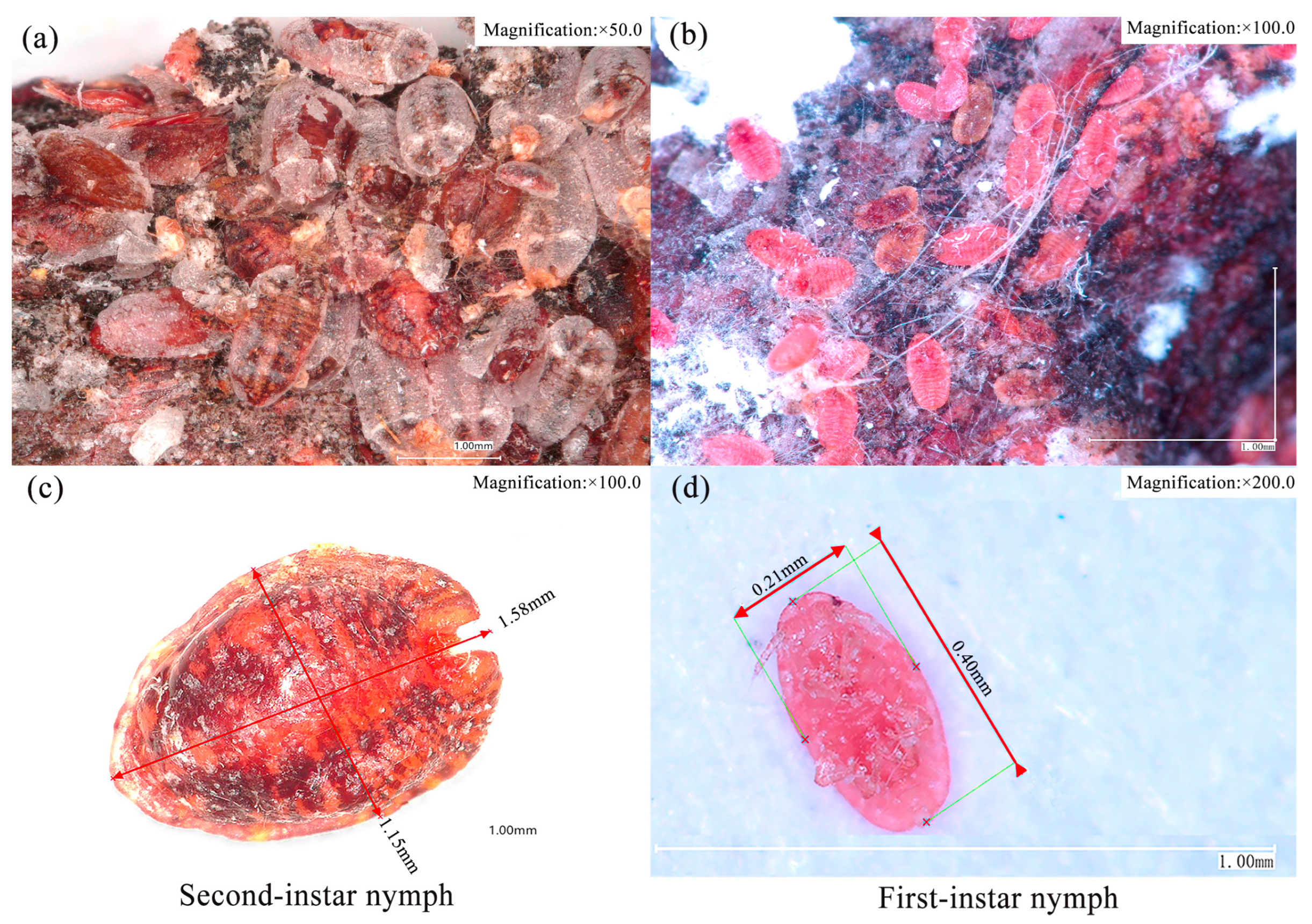
2.1.2. Density Survey of Sphaerolecanium prunastri
2.1.3. Damage Ranking of Wild Apricots
2.1.4. The Growth of Wild Apricots
2.1.5. Assessment of Flower Buds and Fruit Yield
2.2. Data Analysis
3. Results
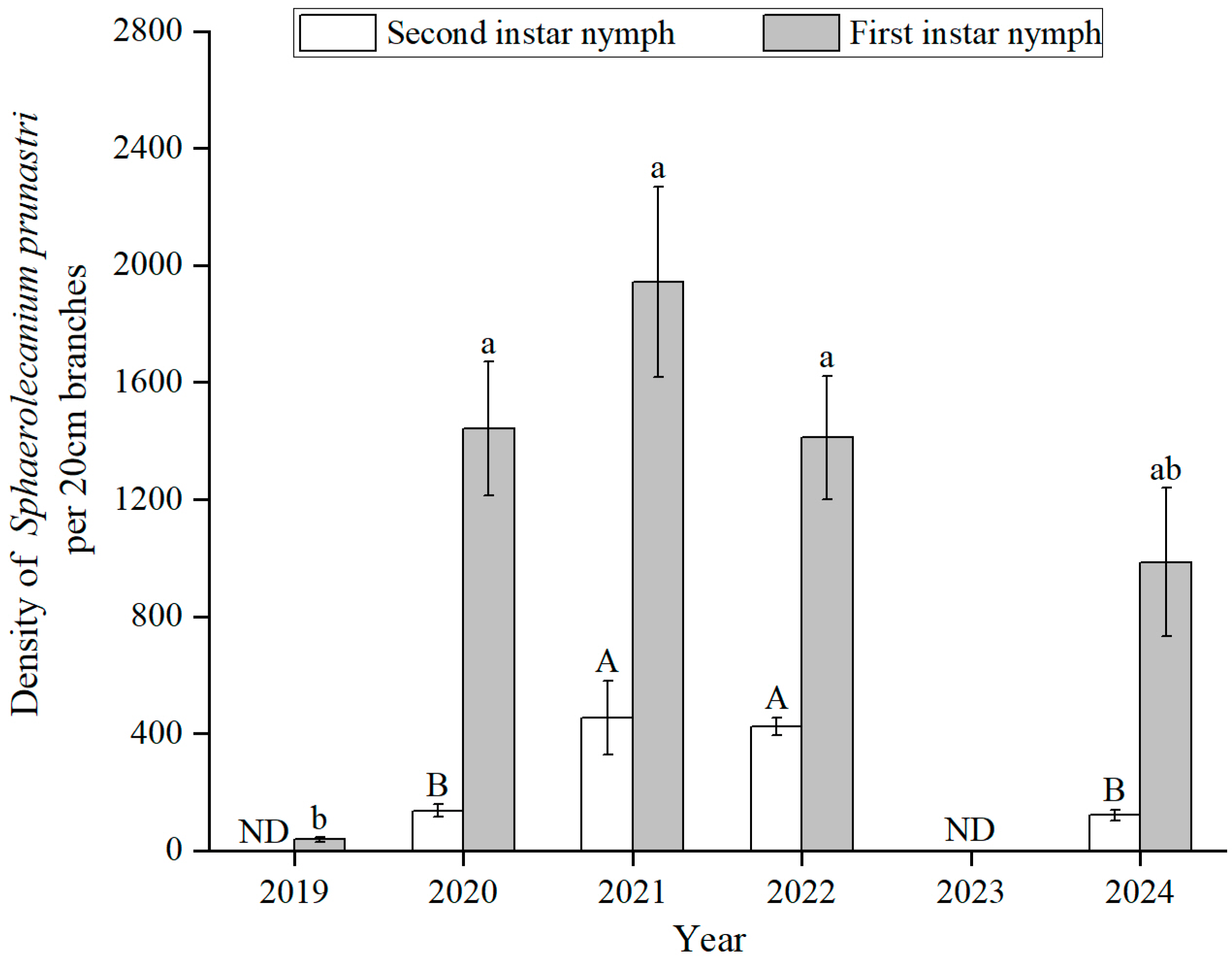
3.1. Population Dynamics of GS Nymphs over Years
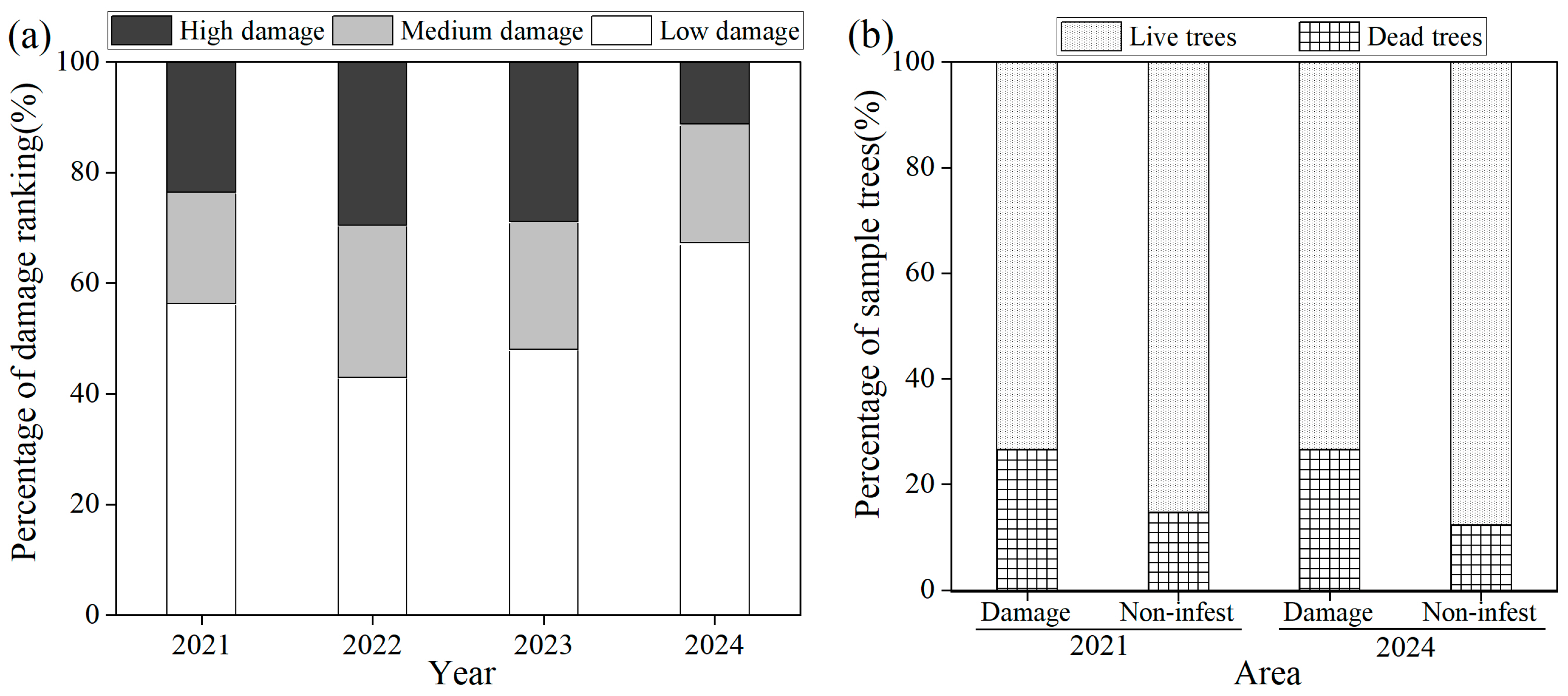
3.2. Damage Rankings of Wild Apricots
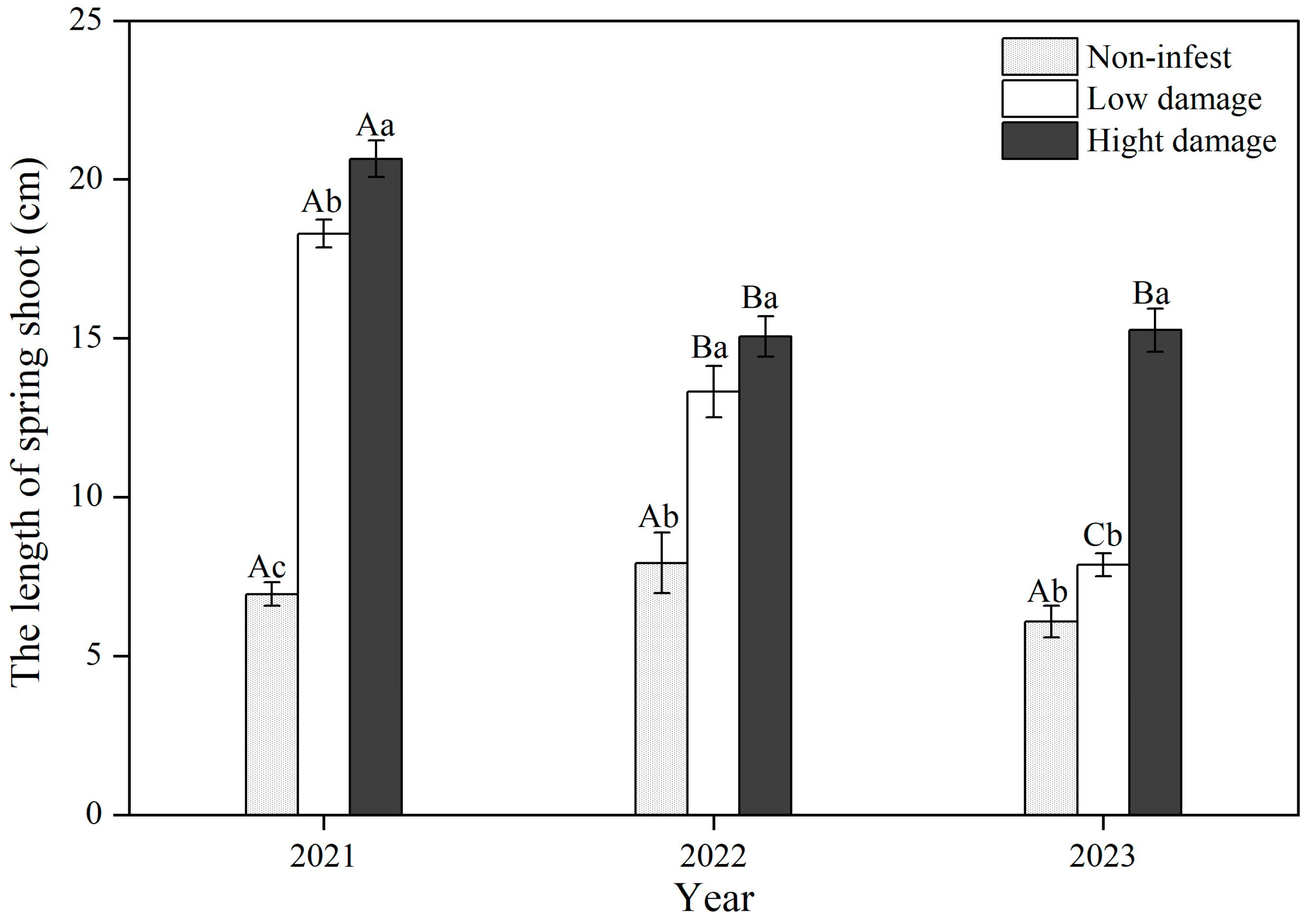
3.3. Length of Spring Shoots Among Damage Rankings
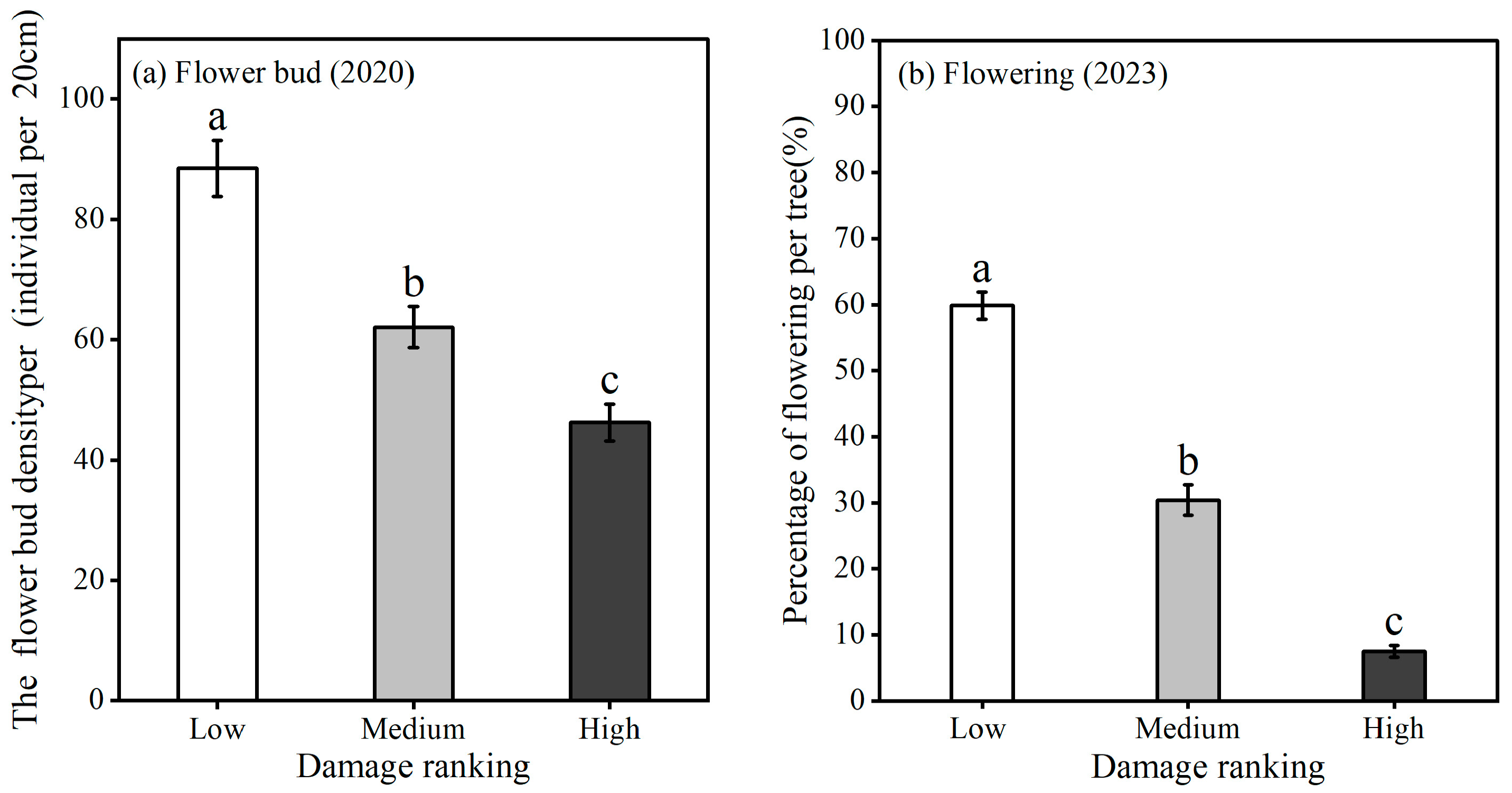
3.4. Flower Buds and Flowering
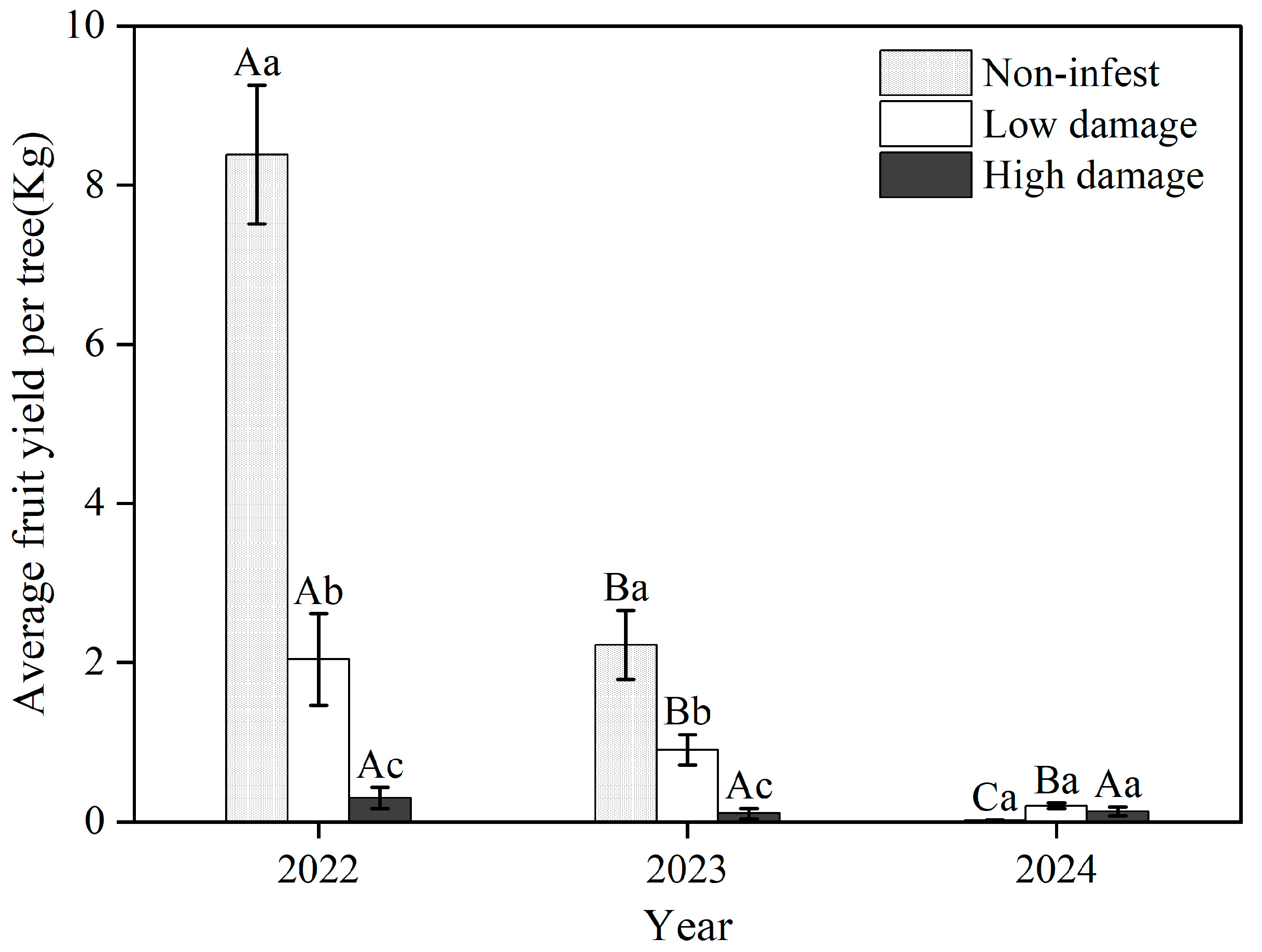
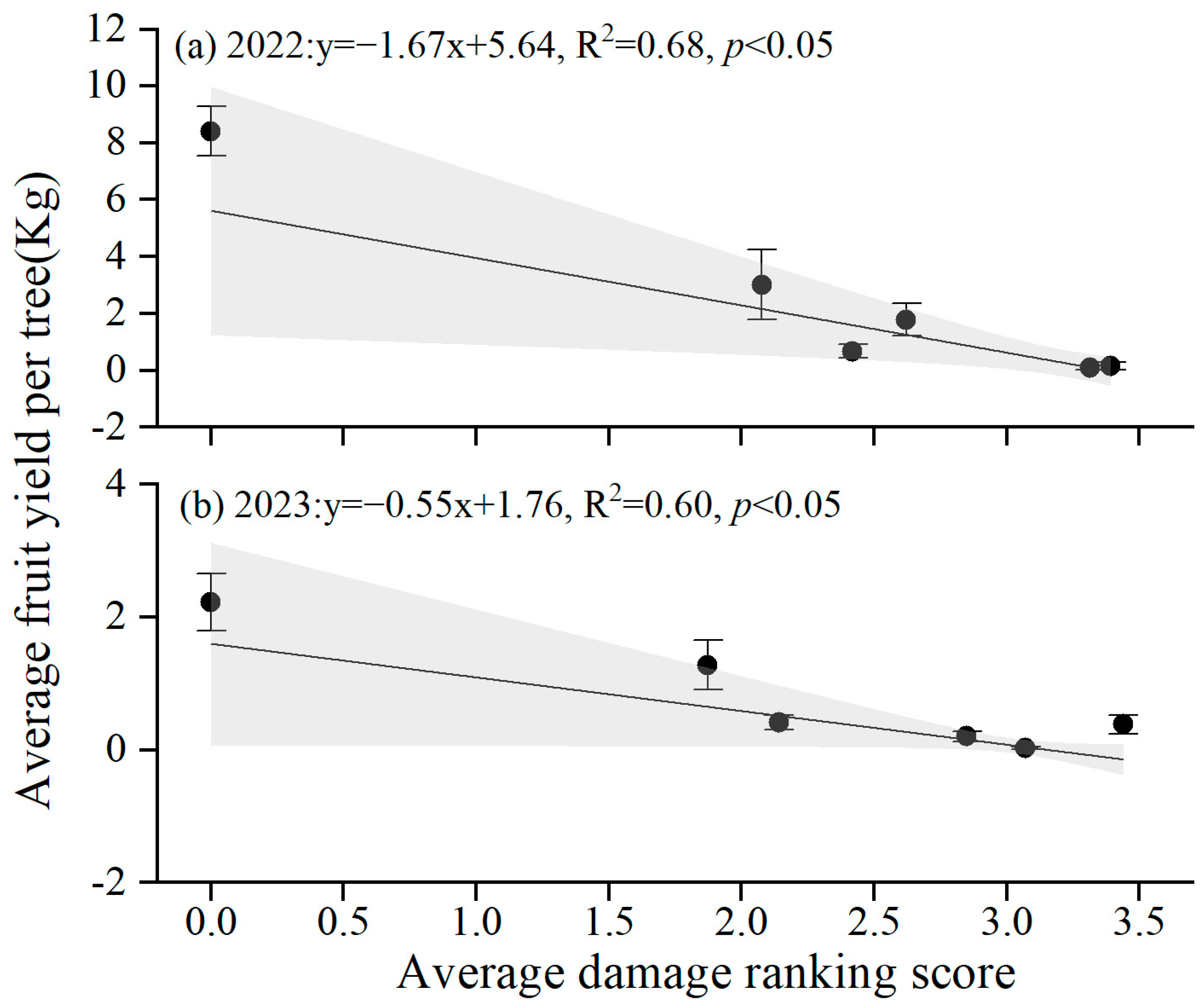
3.5. Fruit Yield Loss
4. Discussion
4.1. GS Population Development and Damage in Wild Apricot Forests
4.2. The Effect of GS on the Growth of Wild Apricots
4.3. The Effect of GS on the Reproductive Capacity of Wild Apricots
4.4. Integrated Management Approach to GS
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, D.; Miller, G.; Hodges, G.S.; Davidson, J.A. Introduced scale insects (Hemiptera: Coccoidea) of the United States and their impact on U.S. agriculture. Proc. Entomol. Soc. Wash. 2004, 107, 123–158. [Google Scholar]
- Morales, M.; Denno, B.; Miller, D.; Miller, G.; Ben-Dov, Y.; Hardy, N. ScaleNet: A literature-based model of scale insect biology and systematics. Database J. Biol. Databases Curation 2016, 2016, bav118. [Google Scholar]
- Amouroux, P.; Crochard, D.; Germain, J.F.; Correa, M.; Ampuero, J.; Groussier, G.; Kreiter, P.; Malausa, T.; Zaviezo, T. Genetic diversity of armored scales (Hemiptera: Diaspididae) and soft scales (Hemiptera: Coccidae) in Chile. Sci. Rep. 2017, 7, 2014. [Google Scholar] [CrossRef]
- Malumphy, C.; Hamilton, M.; Manco, B.N.; Green, P.; Sanchez, M.; Corcoran, M.; Salamanca, E. Toumeyella parvicornis (Hemiptera: Coccidae), Causing Severe Decline of Pinus caribaea var. Bahamensis in the Turks and Caicos Islands. Fla. Entomol. 2012, 95, 113–119. [Google Scholar] [CrossRef]
- Di Sora, N.; Contarini, M.; Rossini, L.; Turco, S.; Brugneti, F.; Metaliaj, R.; Vejsiu, I.; Peri, L.; Speranza, S. First report of Toumeyella parvicornis (Cockerell) (Hemiptera: Coccidae) in Albania and its potential spread in the coastal area of the Balkans. EPPO Bull. 2024, 54, 160–165. [Google Scholar] [CrossRef]
- Nicoletti, R.; De Masi, L.; Migliozzi, A.; Calandrelli, M.M. Analysis of Dieback in a Coastal Pinewood in Campania, Southern Italy, through High-Resolution Remote Sensing. Plants 2024, 13, 182. [Google Scholar] [CrossRef] [PubMed]
- Knight, I.A.; Wilson, B.E.; Gill, M.; Aviles, L.; Cronin, J.T.; Nyman, J.A.; Schneider, S.A.; Diaz, R. Invasion of Nipponaclerda biwakoensis (Hemiptera: Aclerdidae) and Phragmites australis die-back in southern Louisiana, USA. Biol. Invasions 2018, 20, 2739–2744. [Google Scholar] [CrossRef]
- Schneider, S.; Broadley, H.; Andersen, J.; Elkinton, J.; Hwang, S.-Y.; Liu, C.; Noriyuki, S.; Park, J.-S.; Dao, H.; Lewis, M.; et al. An invasive population of Roseau Cane Scale in the Mississippi River Delta, USA originated from northeastern China. Biol. Invasions 2022, 24, 2735–2755. [Google Scholar] [CrossRef]
- Knight, I.A.; Cronin, J.T.; Gill, M.; Nyman, J.A.; Wilson, B.E.; Diaz, R. Investigating Plant Phenotype, Salinity, and Infestation by the Roseau Cane Scale as Factors in the Die-Back of Phragmites australis in the Mississippi River Delta, USA. Wetlands 2020, 40, 1327–1337. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Z.; Linghu, W.; Gao, G. Sphaerolecanium prunastri (Hemiptera: Coccoidea: Coccidae), A New Pest in Wild Fruit Forests, Xinjiang. For. Res. 2021, 34, 152–158. [Google Scholar]
- Çiftçi, Ü.; Bolu, H. First Records of Coccomorpha (Hemiptera) Species in Diyarbakır, Turkey. J. Entomol. Sci. 2021, 56, 235–245. [Google Scholar] [CrossRef]
- Gülmez, M.; Ülgentürk, S.; Ulusoy, M. Soft scale (Hemiptera: Coccomorpha: Coccidae) species on fruit orchards of Diyarbakır and Elazığ provinces in TürkiyeDiyarbakır ve Elazığ illeri meyve bahçelerindeki Koşnil (Hemiptera:Coccomorpha: Coccidae) türleri. Turk. J. Entomol. 2023, 47, 199–213. [Google Scholar] [CrossRef]
- Karaca, I.; Japoshvili, G.; Demirozer, O. The chalcid parasitoid complex (Hymenoptera: Chalcidoidea) associated with the globose scale (Sphaerolecanium prunastri Fonscolombe) (Hemiptera: Coccoidea) in Isparta Province, Turkey and some east European countries. J. Plant Dis. Prot. 2003, 110, 505–511. [Google Scholar] [CrossRef]
- Özgen, i.; Bolu, H. Determination of Sphaerolecanium prunastri (Boyer de Fonscolombe, 1834) (Hemiptera: Coccidae) Plum scale, the distribution, infestations and natural enemies in Malatya province in Turkey. Turk. J. Entomol. 2009, 33, 83–91. [Google Scholar]
- Bulak, Y.; Yildirim, E. Evaluation of hosts and distribution of Hyalopterus pruni (Geoffroy, 1762) and Sphaerolecanium prunastri (Boyer de Fonscolombe, 1834), which are pests of fruit trees in Iğdır. In Proceedings of the International Conference on Food, Agriculture and Animal Sciences, Sivas, Turkey, 27–29 April 2023. [Google Scholar]
- Japoshvili, G.; Ay, R.; Karaca, I.; Gabroshvili, N.; Barjadze, S.; Chaladze, G. Studies On the Parasitoid Complex Attacking the Globose Scale Sphaerolecanium prunastri (Fonscolombe) (Hemiptera: Coccoidea) on Prunus Species in Turkey. J. Kans. Entomol. Soc. 2009, 81, 339–344. [Google Scholar] [CrossRef]
- Li, L.; Hai, Y.; Mohammat, A.; Tang, Z.; Fang, J. Community structure and conservation of wild fruit forests in the IIi Valley, Xinjiang. Arid Zone Res. 2011, 28, 60–66. [Google Scholar] [CrossRef]
- Linghu, W.; Lu, Z.; Wang, Y.; Gao, G. The Effects of Globose Scale (Sphaerolecanium prunastri) Infestation on the Growth of Wild Apricot (Prunus armeniaca) Trees. Forests 2023, 14, 2032. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Z.; Linghu, W.; Wang, Q.; Gao, G. Occurrence and harm of Sphaerolecanium prunastri in wild fruit forests in Xinjiang. Xinjiang Agric. Sci. 2022, 59, 1741–1747. [Google Scholar]
- Diao, Y. Primary Research on Generative Biological Characteristics of Wild Apricot (Armeniaca vulgaris Lam.) in Xinjiang. Master’s Thesis, Xinjiang Agriculture University, Urumqi, China, 2009. [Google Scholar]
- You, L.; Chen, G.; Liu, L.; Jie, G.; Liao, K. Dynamics and correlation analysis of growth and development of root system and above -ground part of Armeniaca vulgaris Lam. Xinyuan wild fruit forest. J. Xinjiang Agric. Univ. 2019, 42, 9–14. [Google Scholar]
- Durovic, G.; Ülgentürk, S. Description of Plum Scale Immatures Stages (Sphaerolecanium prunastri (Boyer de Fonscolombe) (Hemiptera: Coccidae). Yüzüncü Yıl Üniversitesi Tarım Bilim. Derg. 2021, 31, 982–994. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Y.; Deng, B.; Ahmad, M.; Zalucki, M.P.; Gao, G.; Lu, Z. Harmonia axyridis (Boyer de Fonscolombe) (Hemiptera: Coccoidea) as a potential biological control agent of the invasive soft scale, Sphaerolecanium prunastri (Boyer de Fonscolombe) (Hemiptera: Coccoidea) in native wild apricot forests. Egypt. J. Biol. Pest Control 2024, 34, 28. [Google Scholar] [CrossRef]
- Zirao, L.; Huang, L.; Zhao, W.; Yao, Y. A new species of the genus Coccophagus (Hymenoptera: Aphelinidae) associated with Sphaerolecanium prunastri (Hemiptera: Coccoidea) from the Tianshan Mountains, Xinjiang. Entomotaxonomia 2022, 4, 228–239. [Google Scholar]
- Cao, Z.; Zhang, Z.; Kang, N.; ZHao, Q.; Hu, H. Research of parasitoids of Sphaerolecanium prunastri fonscolombe in the western Tianshan wild fruit forest. Xinjiang Agric. Sci. 2024, 61, 971–983. [Google Scholar]
- Wang, Y. Biology of Sphaerolecanium prunastri and Its Control by Dominant Natural Enemy in the Wild Fruit Forest in Yili. Master’s Thesis, Xinjiang Agriculture University, Urumqi, China, 2021. [Google Scholar]
- Park, Y.S.; Chung, Y.J. Hazard rating of pine trees from a forest insect pest using artificial neural networks. For. Ecol. Manag. 2006, 222, 222–233. [Google Scholar] [CrossRef]
- Polat, A.A.; Çalışkan, O. Fruit Set and Yield of Apricot Cultivars under Subtropical Climate Conditions of Hatay, Turkey. J. Agric. Sci. Technol. 2014, 16, 863–872. [Google Scholar]
- Tarantino, A.; Lops, F.; Disciglio, G.; Lopriore, G. Effects of plant biostimulants on fruit set, growth, yield and fruit quality attributes of ‘Orange rubis’ apricot (Prunus armeniaca L.) cultivar in two consecutive years. Sci. Hortic. 2018, 239, 26–34. [Google Scholar] [CrossRef]
- Ouyang, Y.; Gong, X.; Duan, C.; Zhang, C.; Chenyang, M.; Han, P.; Zhang, Y.; Hao, G. Water- and carbon-related physiological mechanisms underlying the decline of wild apricot trees in Ili, Xinjiang, China. Chin. J. Plant Ecol. 2024, 48, 1192–1201. [Google Scholar]
- Tiffin, P. Mechanisms of tolerance to herbivore damage:what do we know? Evol. Ecol. 2000, 14, 523–536. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Sullivan, J.J. The impact of herbivory on plants in different resource conditions: A meta-analysis. Ecology 2001, 82, 2045–2058. [Google Scholar] [CrossRef]
- Stevens, M.T.; Kruger, E.L.; Lindroth, R.L. Variation in tolerance to herbivory is mediated by differences in biomass allocation in aspen. Funct. Ecol. 2008, 22, 40–47. [Google Scholar] [CrossRef]
- Zvereva, E.L.; Lanta, V.; Kozlov, M.V. Effects of sap-feeding insect herbivores on growth and reproduction of woody plants: A meta-analysis of experimental studies. Oecologia 2010, 163, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Dilley, B.; Davies, D.; Glass, T.; Bond, A.; Ryan, P. Severe impact of introduced scale insects on Island Trees threatens endemic finches at the Tristan da Cunha archipelago. Biol. Conserv. 2020, 251, 108761. [Google Scholar] [CrossRef]
- Larson, K.C.; Whitham, T.G. Competition between gall aphids and natural plant sinks: Plant architecture affects resistance to galling. Oecologia 1997, 109, 575–582. [Google Scholar] [CrossRef]
- Gonda-King, L.; Gómez, S.; Martin, J.L.; Orians, C.M.; Preisser, E.L. Tree responses to an invasive sap-feeding insect. Plant Ecol. 2014, 215, 297–304. [Google Scholar] [CrossRef]
- Shi, D.; Guo, C.; Jiang, N.; Tang, Y.; Zhen, F.; Wang, J.; Liao, K.; Liu, L. Characteristics and spatial distribution pattern of natural regeneration young plants of Prunus armeniaca in Xinjiang, China. Chin. J. Plant Ecol. 2023, 47, 515–529. [Google Scholar] [CrossRef]
- Linghu, W.; Lu, Z.; Wang, Y.; Wang, Q.; Gao, G. Effects of pruning time and intensity on the population density of Sphaerolecanium prunastri (Boyer de Fonscolombe)and growth of Armeniaca vulgaris Lamarck. North. Hortic. 2021, 20, 34–41. [Google Scholar]
- Zhang, P.; Li, Y.; Wang, T.; Zhang, X.; Yanlong, Z.; Xu, H.; Jashenko, R.; Dong, Z.; Zalucki, M.; Lu, Z. Pruning can recover the health of wild apple forests attacked by the wood borer Agrilus mali in central Eurasia. Entomol. Gen. 2024, 44, 545–552. [Google Scholar] [CrossRef]
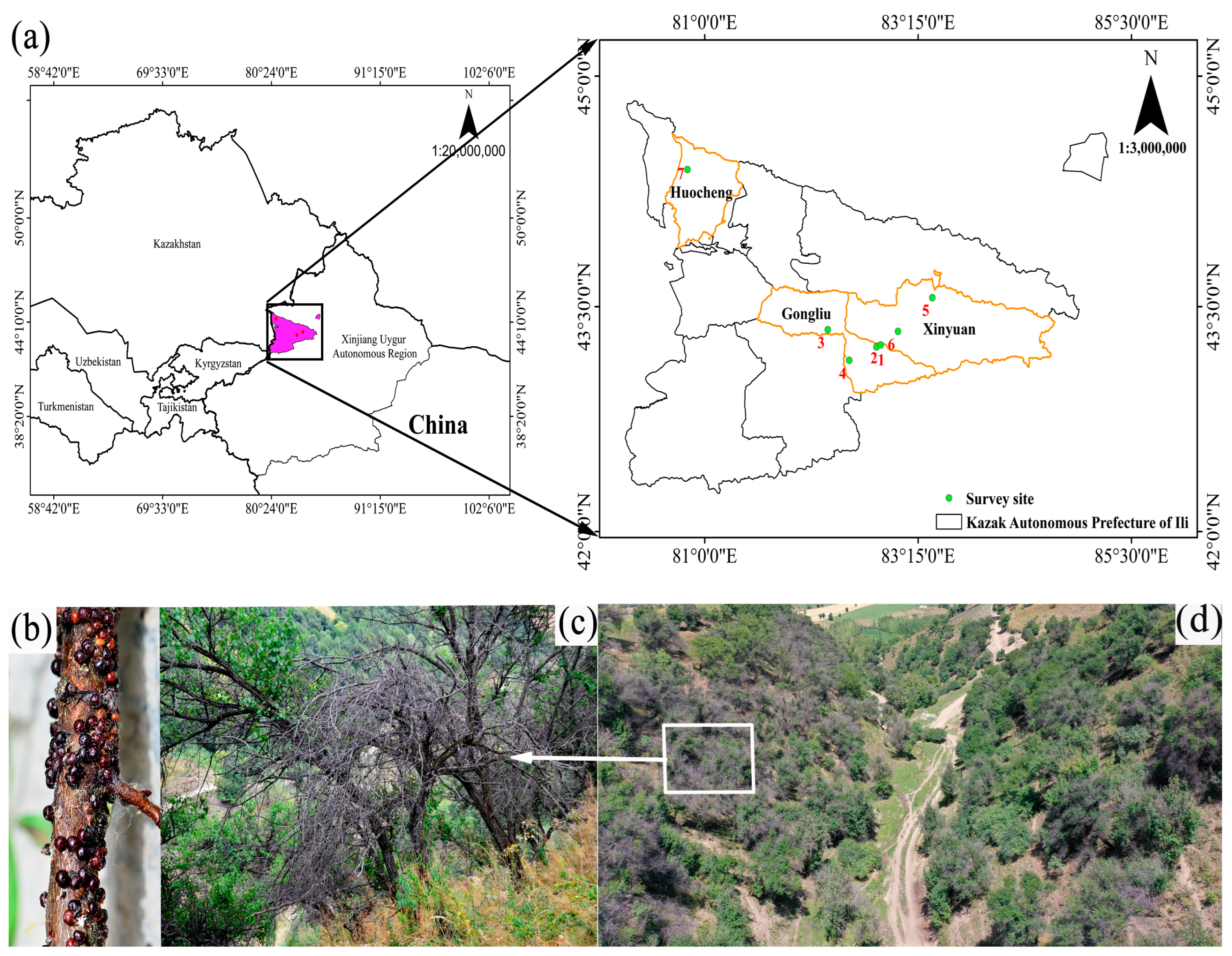


| County | Survey Site | Plot Number | Latitude (N) | Longitude (E) | Altitude (m) | Height (m) | Diameter at Breast Height (cm) | Wild Apricot Tree Density (Number/ha) |
|---|---|---|---|---|---|---|---|---|
| Gongliu | Kuolesai | 1 | 43.234 | 82.811 | 1248.10 | 7.93 ± 0.35 | 21.81 ± 1.48 | 224 |
| 2 | 43.259 | 82.857 | 1343.23 | 7.28 ± 0.40 | 13.57 ±1.68 | 248 | ||
| 3 | 43.248 | 82.697 | 1190.32 | 8.28 ± 0.24 | 23.78 ± 1.73 | 264 | ||
| Jinqikesai | 1 | 43.248 | 82.857 | 1321.14 | 7.79 ± 0.37 | 22.2 ± 2.52 | 288 | |
| 2 | 43.246 | 82.857 | 1343.23 | 7.94 ± 0.43 | 21.96 ± 1.5 | 240 | ||
| 3 | 43.248 | 82.858 | 1319.34 | 7.25 ± 0.44 | 15.57 ± 1.56 | 312 | ||
| Keersenbu lake | 1 | 43.363 | 82.120 | 1252.33 | 6.30 ± 0.41 | 18.26 ± 1.36 | 264 | |
| 2 | 43.349 | 82.298 | 1237.30 | 8.31 ± 0.51 | 17.50 ± 1.71 | 232 | ||
| 3 | 43.349 | 82.299 | 1211.59 | 8.05 ± 0.70 | 15.92 ± 0.98 | 240 | ||
| Xiaomohuer | 1 | 43.178 | 82.733 | 1376.60 | 8.02 ± 0.26 | 27.99 ± 0.88 | 176 | |
| 2 | 43.141 | 82.442 | 1245.00 | 9.49 ± 0.45 | 22.25 ± 1.29 | 264 | ||
| 3 | 43.177 | 82.733 | 1356.96 | 6.33 ± 0.57 | 27.52 ± 1.35 | 152 | ||
| Xinyuan | Tuergen | 1 | 43.561 | 83.482 | 1026.33 | 6.05 ± 0.56 | 19.98 ± 2.02 | 360 |
| 2 | 43.560 | 83.401 | 1110.72 | 6.24 ± 0.61 | 26.6 ± 3.37 | 288 | ||
| 3 | 43.539 | 83.485 | 1055.23 | 7.54 ± 0.95 | 19.92 ± 2.90 | 264 | ||
| Talede | 4 | 43.338 | 83.039 | 1165.06 | 6.77 ± 0.51 | 20.94 ± 1.37 | 240 | |
| Huocheng | Xiaoxigou | 1 | 44.380 | 80.818 | 1084.52 | 5.72 ± 0.47 | 27.17 ± 1.74 | 208 |
| 2 | 44.429 | 80.832 | 1160.95 | 5.62 ± 0.22 | 25.37 ± 1.70 | 216 | ||
| 3 | 44.394 | 80.814 | 1051.36 | 6.39 ± 0.22 | 18.57 ± 1.25 | 232 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Li, Y.; Li, C.; Gao, G.; Dong, Z.; Rostami, E.; Lu, Z.; Zalucki, M.P. The Post-Invasion Population Dynamics and Damage Caused by Globose Scale in Central Eurasia: Destiny of Wild Apricot Still at Stake. Insects 2025, 16, 409. https://doi.org/10.3390/insects16040409
Zhang P, Li Y, Li C, Gao G, Dong Z, Rostami E, Lu Z, Zalucki MP. The Post-Invasion Population Dynamics and Damage Caused by Globose Scale in Central Eurasia: Destiny of Wild Apricot Still at Stake. Insects. 2025; 16(4):409. https://doi.org/10.3390/insects16040409
Chicago/Turabian StyleZhang, Ping, Yifan Li, Cuihong Li, Guizhen Gao, Zhaoke Dong, Elahe Rostami, Zhaozhi Lu, and Myron P. Zalucki. 2025. "The Post-Invasion Population Dynamics and Damage Caused by Globose Scale in Central Eurasia: Destiny of Wild Apricot Still at Stake" Insects 16, no. 4: 409. https://doi.org/10.3390/insects16040409
APA StyleZhang, P., Li, Y., Li, C., Gao, G., Dong, Z., Rostami, E., Lu, Z., & Zalucki, M. P. (2025). The Post-Invasion Population Dynamics and Damage Caused by Globose Scale in Central Eurasia: Destiny of Wild Apricot Still at Stake. Insects, 16(4), 409. https://doi.org/10.3390/insects16040409





