More than Just Host Plant Preferences for the Two Main Vectors of Xylella fastidiosa in Europe: Two Insect Species and Two Different Behaviors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Plants
2.2. Oviposition Patterns Across Vector Species
2.3. Nymph Host Preferences
2.4. Nymph Survival and Development
2.5. Statistical Analyses
3. Results
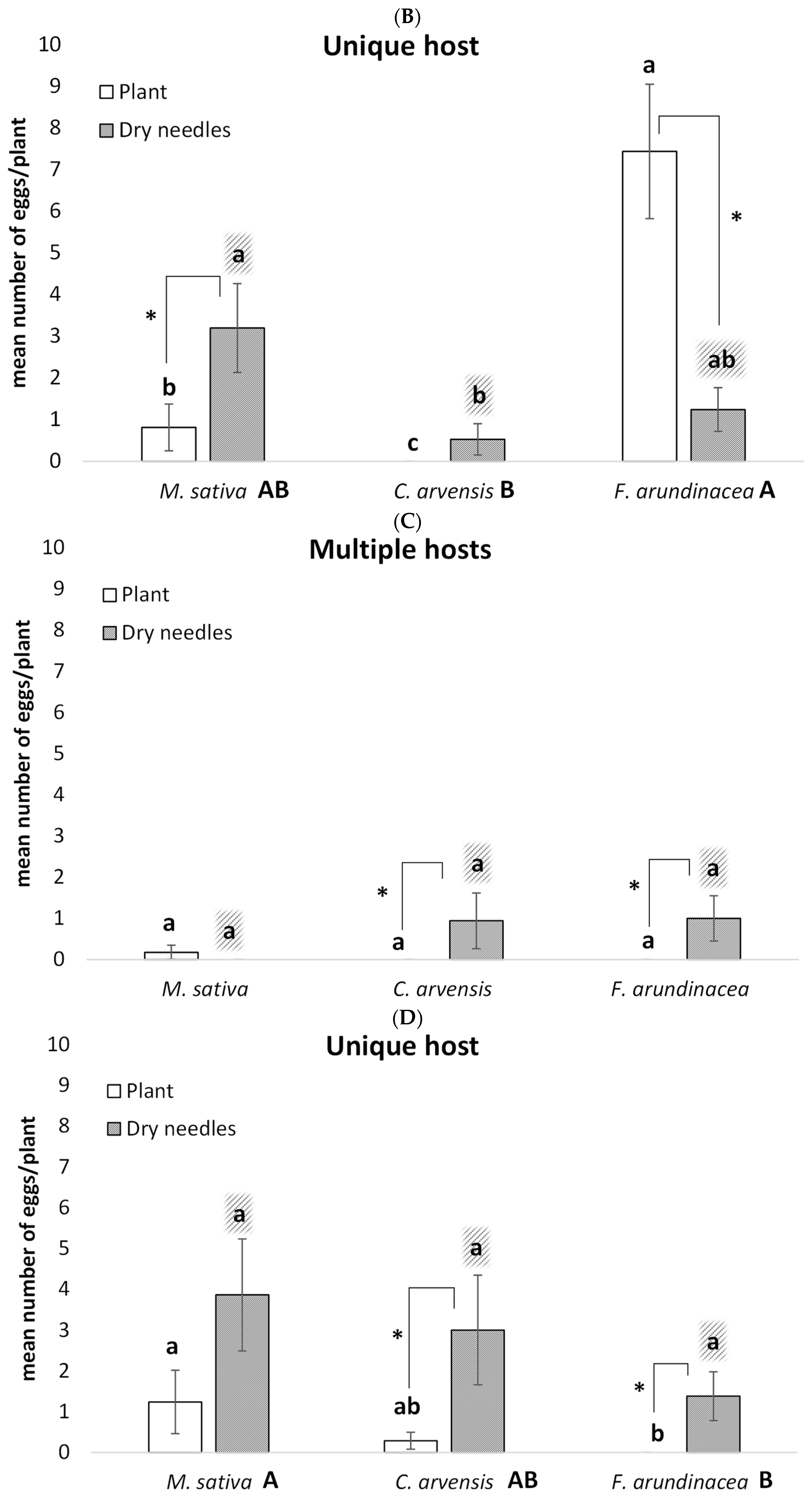
3.1. Oviposition Patterns Across Vector Species
Influence of Host Plant Diversity on Substrate Preference
- Neophilaenus campestris
- Philaenusspumarius
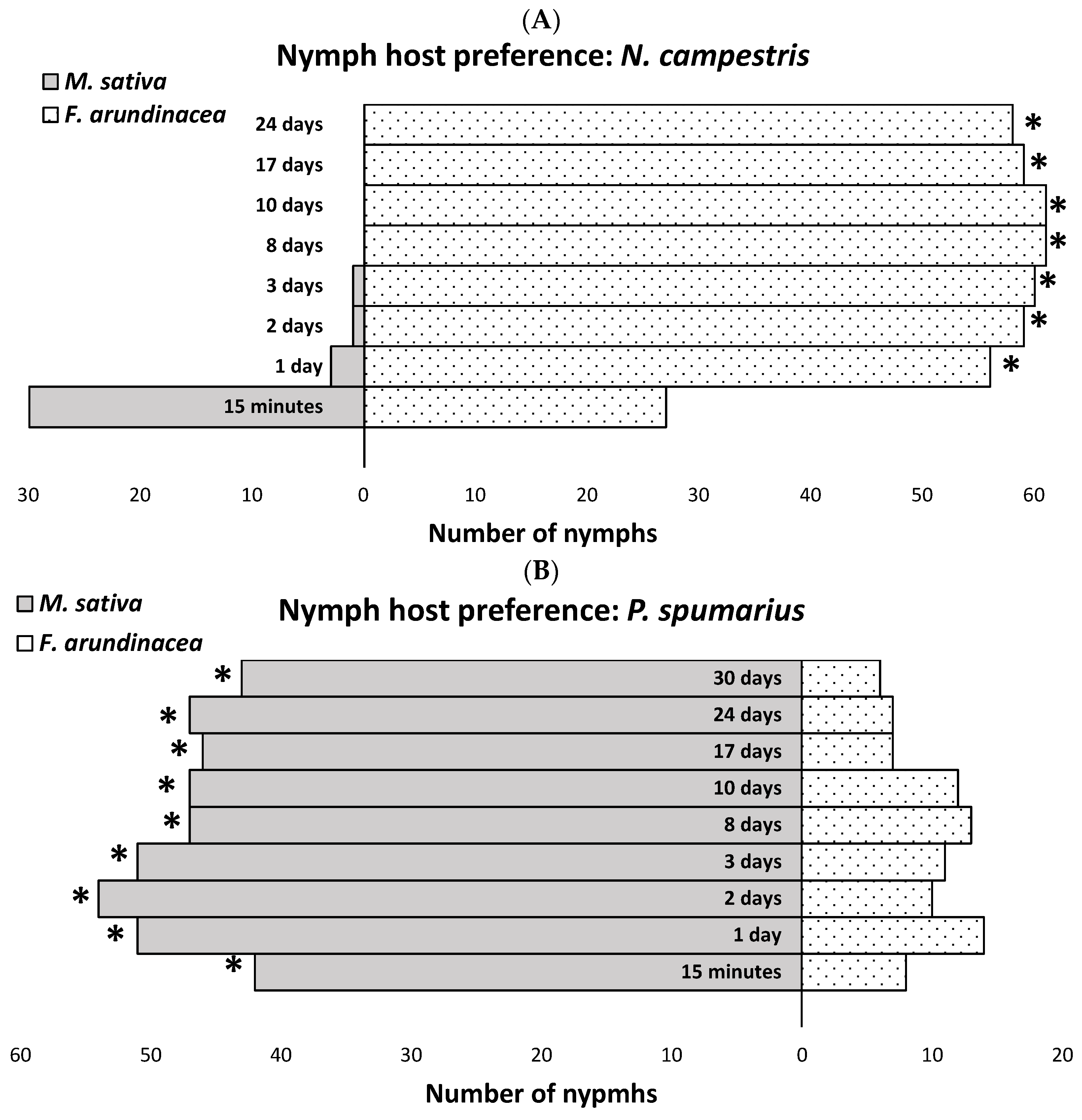
3.2. Nymph Host Preferences
3.3. Nymph Survival and Development
3.3.1. Nymph Survival
3.3.2. Nymph Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rapicavoli, J.; Ingel, B.; Blanco-Ulate, B.; Cantu, D.; Roper, C. Xylella fastidiosa: An examination of a re-emerging plant pathogen. Mol. Plant Pathol. 2018, 19, 786–800. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority); Cavalieri, V.; Fasanelli, E.; Gibin, D.; Gutierrez-Linares, A.; La Notte, P.; Pasinato, L.; Delbianco, A. Update of the Xylella spp. host plant database—Systematic literature search up to 31 December 2023. EFSA J. 2024, 22, e8898. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.; Machado, M.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Purcell, A.H. Paradigms: Examples from the bacterium Xylella fastidiosa. Annu. Rev. Phytopathol. 2013, 51, 339–356. [Google Scholar] [CrossRef]
- Rodrigues, I. Biological and Ecological Features of Xylem-Feeder Vectors Through Establishment of a Sustainable Strategy for Control of Xylella fastidiosa-Vectors. Ph.D. Thesis, University of Leon, Leon, Spain, 2023. [Google Scholar]
- Almeida, R.P.P.; Wistrom, C.; Hill, B.L.; Hashim, J.; Purcell, A.H. Vector transmission of Xylella fastidiosa to dormant grape. Plant Dis. 2005, 89, 1255–1259. [Google Scholar] [CrossRef]
- Redack, R.A.; Purcell, A.H.; Lopes, J.R.S.; Blua, M.J.; Mizel III, R.F.; Andersen, P.C. The biology of xylem fluis feeding insects vectors of Xylella fastidiosa and their relation to disease epidemiology. Annu. Rev. Entomol. 2004, 49, 243–270. [Google Scholar] [CrossRef]
- Chatterjee, S.; Almeida, R.P.; Lindow, S. Living in two worlds: The plant and insect lifestyles of Xylella fastidiosa. Annu. Rev. Phytopathol. 2008, 46, 243–271. [Google Scholar] [CrossRef]
- Almeida, R.P.P. Xylella fastidiosa vector transmission biology. In Vector-Mediated Transmission of Plant Pathogens; Brown, J.K., Ed.; PAS Press: Minneapolis, MN, USA, 2016; pp. 165–173. [Google Scholar]
- Lopes, J.R.; Daugherty, M.P.; Almeida, R.P.P. Context-dependent transmission of a generalist plant pathogen: Host species and pathogen strain mediate insect vector competence. Entomol. Exp. Appl. 2009, 131, 216–224. [Google Scholar] [CrossRef]
- Almeida, R.P.; Nunney, L. How do plant diseases caused by Xylella fastidiosa emerge? Plant Dis. 2015, 99, 1457–1467. [Google Scholar] [CrossRef]
- Sicard, A.; Zeilinger, A.R.; Vanhove, M.; Schartel, T.E.; Beal, D.J.; Daugherty, M.P.; Almeida, R.P.P. Xylella fastidiosa: Insights into an Emerging Plant Pathogen. Annu. Rev. Phytopathol. 2018, 56, 181–202. [Google Scholar] [CrossRef]
- Cornara, D.; Morente, M.; Markheiser, A.; Bodino, N.; Tsai, C.H.; Fereres, A.; Redak, R.A.; Perring, T.M.; Spotti-Lopes, J.R. An overview on the worldwide vectors of Xylella fastidiosa. Entomol. Gen. 2019, 39, 157–181. [Google Scholar] [CrossRef]
- Saponari, M.; Boscia, D.; Nigro, F.; Martelli, G.P. Identification of DNA sequences related to Xylella fastidiosa in oleander, almond and olive trees exhibiting leaf scorch symptoms in Apulia (southern Italy). J. Plant Pathol. 2013, 95, 659–668. [Google Scholar] [CrossRef]
- Villa, M.; Rodrigues, I.; Baptista, P.; Fereres, A.; Pereira, J.A. Populations and Host/Non-Host Plants of Spittlebugs Nymphs in Olive Orchards from Northeastern Portugal. Insects 2020, 11, 720. [Google Scholar] [CrossRef] [PubMed]
- López-Mercadal, J.; Delgado, S.; Mercadal, P.; Seguí, G.; Lalucat, J.; Busquets, A.; Gomila, M.; Lester, K.; Kenyon, D.M.; Ruíz-Pérez, M.; et al. Collection of data and information in Balearic Islands on biology of vectors and potential vectors of Xylella fastidiosa (GP/EFSA/ALPHA/017/01). EFSA Support. Publ. 2021, 18, 136. [Google Scholar] [CrossRef]
- Morente, M.; Fereres, A. Vectores de Xylella fastidiosa. In Enfermedades Causadas por la Bacteria Xylella fastidiosa; Landa, B., Marco-Noales, E., López, M.M., Eds.; Cajamar Caja Rural: Almeria, Spain, 2017; pp. 73–93. ISBN 978-84-95531-86-5. Available online: https://publicacionescajamar.es/wp-content/uploads/2023/03/enfermedades-causadas-por-la-bacteria-2.pdf (accessed on 20 December 2024).
- Herrero-Schell, J.; Aure, C.M.; Montoro, M.; Malagón, J.; Tormos, J.; Beitia, F. Presencia y evolución poblacional de potenciales vectores de Xylella fastidiosa en la Comunidad Valenciana. Phytoma-España 2020, 323, 26–32. [Google Scholar]
- Antonatos, S.; Papachristos, D.P.; Varikou, K.; Vahamidis, P.; Kapranas, A.; Milonas, P. Seasonal Appearance, Abundance, and Host Preference of Philaenus spumarius and Neophilaenus campestris (Hemiptera: Aphrophoridae) in Olive Groves in Greece. Environ. Entomol. 2021, 50, 1474–1482. [Google Scholar] [CrossRef]
- Dongiovanni, C.; Cavalieri, V.; Bodino, N.; Tauro, D.; Di Carolo, M.; Fumarola, G.; Altamura, G.; Lasorella, C.; Bosco, D. Plant Selection and Population Trend of Spittlebug Immatures (Hemiptera: Aphrophoridae) in Olive Groves of the Apulia Region of Italy. J. Econ. Entomol. 2019, 112, 67–74. [Google Scholar] [CrossRef]
- García-García, R.; Bernat-Ponce, S.; Beitia, F.; Nieves, L.; Aure, C.M. Incidencia de la espuma producida por ninfas de Philaenus spumarius (Hemiptera: Aphrophoridae), el principal insecto vector de Xylella fastidiosa en Europa, frente a la acción de enemigos naturales. Phytoma-España 2023, 352, 30–36. [Google Scholar]
- Szterk, A.; Flis, S.; Ofiara, K.; Strus, B. Chemical composition of the foam enfolding juveniles of Aphrophora alni (Hemiptera: Aphrophoridae). J. Asia-Pacific Entomol. 2024, 27, 102185. [Google Scholar] [CrossRef]
- Bodino, N.; Demichelis, S.; Simonetto, A.; Volani, S.; Saladini, M.A.; Gilioli, G.; Bosco, D. Phenology, Seasonal Abundance, and Host-Plant Association of Spittlebugs (Hemiptera: Aphrophoridae) in Vineyards of Northwestern Italy. Insects 2021, 12, 1012. [Google Scholar] [CrossRef]
- Morente, M.; Cornara, D.; Plaza, M.; Durán, J.M.; Capiscol, C.; Trillo, R.; Ruiz, M.; Ruz, C.; Sanjuan, S.; Pereira, J.A.; et al. Distribution and relative abundance of insect vectors of Xylella fastidiosa in olive groves of the Iberian Peninsula. Insects 2018, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Cornara, D.; Bosco, D.; Fereres, A. Philaenus spumarius: When an old acquaintance becomes a new threat to European agriculture. J. Pest Sci. 2018, 91, 957–972. [Google Scholar] [CrossRef]
- Stewart, A.; Bantock, T. Auchenorrhyncha Recording Scheme for Britain and Ireland. Leafhoppers, Planthoppers, Froghoppers, Treehoppers and Cicadas. Available online: www.ledra.co.uk (accessed on 20 December 2024).
- Monzó, C.; Calvo, M.; Belén-Arevalo, A.; Vila, E.; Herrero-Schell, J.; Casiraghi, A.; Gálvez, C.; González, M. Synergizing inoculative and conservation biological control strategies for pest management in subtropical Mediterranean woody crops: Plant species selection for ecological infrastructures. IOBC-WPRS Bulletin. 2024, 170, 52–56. [Google Scholar]
- Mockford, A.; Urbaneja, A.; Ashbrook, K.; Westbury, D.B. Developing perennial wildflower strips for use in Mediterranean orchard systems. Ecol. Evol. 2024, 13, e10285. [Google Scholar] [CrossRef]
- Aguilar-Fenollosa, E.; Ibáñez-Gual, M.V.; Pascual-Ruiz, S.; Hurtado, M.; Jacas, J.A. Effect of ground-cover management on spider mites and their phytoseiid natural enemies in clementine mandarin orchards (I): Bottom-up regulation mechanisms. Biol. Control 2011, 59, 158–170. [Google Scholar] [CrossRef]
- Gómez-Marco, F.; Urbaneja, A.; Tena, A. A sown grass cover enriched with wild forb plants improves the biological control of aphids in citrus. Basic Appl. Ecol. 2016, 17, 210–219. [Google Scholar] [CrossRef]
- Schmidt, N.P.; O’Neal, M.E.; Singer, J.W. Alfalfa living mulch advances biological control of soybean aphid. Environ. Entomol. 2007, 36, 416–424. [Google Scholar] [CrossRef]
- De Clavijo, E.R. The reproductive strategies of the heterocarpic annual Calendula arvensis (Asteraceae). Acta Oecol. 2005, 8, 119–126. [Google Scholar] [CrossRef]
- Lago, C. Dispersal Behaviour, Ecology and Control of Philaenus spumarius: The Main Vector of Xylella fastidiosa in Europe. Ph.D. Thesis, Polytechnic University of Madrid, Madrid, Spain, 2022. [Google Scholar]
- Cornara, D.; Panzarino, O.; Santoiemma, G.; Bodino, N.; Loverre, P.; Mastronardi, M.G.; Mattia, C.; de Lillo, E.; Addante, R. Natural areas as reservoir of candidate vectors of Xylella fastidiosa. Bull. Insectology 2021, 74, 173–180. [Google Scholar]
- Morente, M.; Ramírez, M.; Lago, C.; De Las Heras-Bravo, D.; Benito, A.; Moreno, A.; Fereres, A. Habitat manipulation for sustainable management of Philaenus spumarius, the main vector of Xylella fastidiosa in Europe. Pest Manag. Sci. 2022, 78, 4183–4194. [Google Scholar] [CrossRef]
- Aure, C.M.; Herrero-Schell, J.; Blanes-García, M.; Beitia, F. First Assays on the Response of Adults of Philaenus spumarius (Hemiptera: Aphrophoridae) to Different Host Plants. Available online: https://zenodo.org/search?page=1&size=20&q=4680075 (accessed on 12 December 2024).
- Avosani, R.N.; Mazzoni, V.; Anfora, G.; Hamouche, Z.; Zippari, C.; Vitale, M.L.; Verrastro, V.; Tarasco, E.; D’Isita, I.; Germinara, S.; et al. Intruding into a conversation: How behavioral manipulation could support management of Xylella fastidiosa and its insect vectors. J. Pest Sci. 2024, 97, 17–23. [Google Scholar] [CrossRef]
- Gallego, D.; Sabah, S.C.; Lencina, J.L.; Carrillo, A.F. Bioclimatic and Landscape Factors drive the Potential Distribution of Philaenus spumarius, Neophilaenus campestris and N. lineatus (Hemiptera, Aphrophoridae) in Southeastern Iberian Peninsula. Insects 2023, 14, 592. [Google Scholar] [CrossRef] [PubMed]
- Brodbeck, B.V.; Andersen, P.C.; Oden, S.; Mizell III, R.F. Preference–Performance Linkage of the Xylem Feeding Leafhopper, Homalodisca vitripennis (Hemiptera Cicadellidae). Environ. Entomol. 2007, 36, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Thompson, V.; Harkin, C.; Stewart, A.J.A. The most polyphagous insect herbivore? Host plant associations of the Meadow spittlebug, Philaenus spumarius (L.). PLoS ONE 2023, 18, e0291734. [Google Scholar] [CrossRef]
- Bodino, N.; Plazio, E.; Cavalieri, V.; Dongiovanni, E.; Ripamonti, M.; Volani, S.; Gilioli, G.; Fumarola, G.; Di Carolo, M.; Porcelli, F.; et al. Host-plant association and host-shifting of nymphs and adults of Philaenus spumarius L. in Italian olive orchards. In Proceedings of the 3rd Hemipteran-Plant Interactions Symposium (HPIS), Madrid, Spain, 4–8 June 2017. [Google Scholar]
- Panizzi, A.R. Suboptimal Nutrition and Feeding Behavior of Hemipterans on Less Preferred Plant Food Sources. Annu. Soc. Entomol. Brasil 2000, 29, 1–12. [Google Scholar] [CrossRef]
- Chuche, J.; Sauvion, N.; Thiéry, D. Mixed xylem and phloem sap ingestion in sheath-feeders as normal dietary behavior: Evidence from the leafhopper Scaphoideus titanus. J. Insect Physiol. 2017, 102, 62–72. [Google Scholar] [CrossRef]
- Tonkyn, D.W.; Whitcomb, R.F. Feeding Strategies and the Guild Concept Among Vascular Feeding Insects and Microorganisms. In Current Topics in Vector Research; Harris, K.F., Ed.; Springer: New York, NY, USA, 1987; Volume 4, pp. 179–199. [Google Scholar] [CrossRef]
- García-García, R. Presencia y Evolución Poblacional en Campo de Potenciales Vectores de Xylella fastidiosa Wells et al., 1987 (Xanthomonadales: Xanthomonadaceae). Master’s Thesis, Faculty of Biology, University of Valencia, Valencia, Spain, 2021. [Google Scholar]

| Diverse Host Plant Presence | Monospecific Host Plant Presence | |||||||
|---|---|---|---|---|---|---|---|---|
| M. sativa | C. arvensis | F. arundinacea | Mean | M. sativa | C. arvensis | F. arundinacea | Mean | |
| N. campestris | 1.94 ± 1.02 | 2.28 ± 0.96 * | 6.83 ± 1.4 | 3.69 ± 0.72 | 4 ± 1.32 | 0.52 ± 0.38 | 8.67 ± 1.59 | 4.4 ± 0.81 |
| P. spumarius | 0.18 ± 0.18 | 0.94 ± 0.69 | 1 ± 0.57 | 0.71 ± 0.3 | 5.1 ± 1.58 ** | 3.29 ± 1.37 * | 1.38 ± 0.6 | 3.25 ± 0.74 ** |
| Survival (%) | |||||
|---|---|---|---|---|---|
| Preferred Hosts | C. arvensis | M. sativa | P. dulcis | F. arundinacea | |
| N. campestris | 75.15 ± 7.58 | 0.00 ± 0.00b | 0.00 ± 0.00b | 0.00 ± 0.00b | 75.15 ± 7.58a |
| P. spumarius | 83.18 ± 5.02 | 82.41 ± 5.77a | 82.41 ± 5.77a | 27.61 ± 6.94b | 0.00 ± 0.00c |
| Developmental Time (days) | |||||
|---|---|---|---|---|---|
| Preferred Hosts | C. arvensis | M. sativa | P. dulcis | F. arundinacea | |
| N. campestris | 28.21 ± 0.88 | 0.00 ± 0.00b | 0.00 ± 0.00b | 0.00 ± 0.00b | 28.21 ± 0.88a |
| P. spumarius | 50.08 ± 0.75 | 48.37 ± 1.09a | 51.61 ± 1.90a | 50.64 ± 1.90a | 0.00 ± 0.00b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernat-Ponce, S.; García-García, R.; Aure, C.M.; Nieves, L.; Bouvet, J.P.; Beitia, F.J.; Monzó, C. More than Just Host Plant Preferences for the Two Main Vectors of Xylella fastidiosa in Europe: Two Insect Species and Two Different Behaviors. Insects 2025, 16, 416. https://doi.org/10.3390/insects16040416
Bernat-Ponce S, García-García R, Aure CM, Nieves L, Bouvet JP, Beitia FJ, Monzó C. More than Just Host Plant Preferences for the Two Main Vectors of Xylella fastidiosa in Europe: Two Insect Species and Two Different Behaviors. Insects. 2025; 16(4):416. https://doi.org/10.3390/insects16040416
Chicago/Turabian StyleBernat-Ponce, Saúl, Rosalía García-García, Cristina M. Aure, Lorena Nieves, Juan Pedro Bouvet, Francisco J. Beitia, and César Monzó. 2025. "More than Just Host Plant Preferences for the Two Main Vectors of Xylella fastidiosa in Europe: Two Insect Species and Two Different Behaviors" Insects 16, no. 4: 416. https://doi.org/10.3390/insects16040416
APA StyleBernat-Ponce, S., García-García, R., Aure, C. M., Nieves, L., Bouvet, J. P., Beitia, F. J., & Monzó, C. (2025). More than Just Host Plant Preferences for the Two Main Vectors of Xylella fastidiosa in Europe: Two Insect Species and Two Different Behaviors. Insects, 16(4), 416. https://doi.org/10.3390/insects16040416







