Diflubenzuron Did Not Affect the Abilities of the Backswimmer Buenoa tarsalis to Survive and Prey Upon Larvae of Aedes aegypti
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Insects
2.2. Survival Bioassays
2.3. Predation Bioassays with Bu. tarsalis Adults
2.4. Statistical Analysis
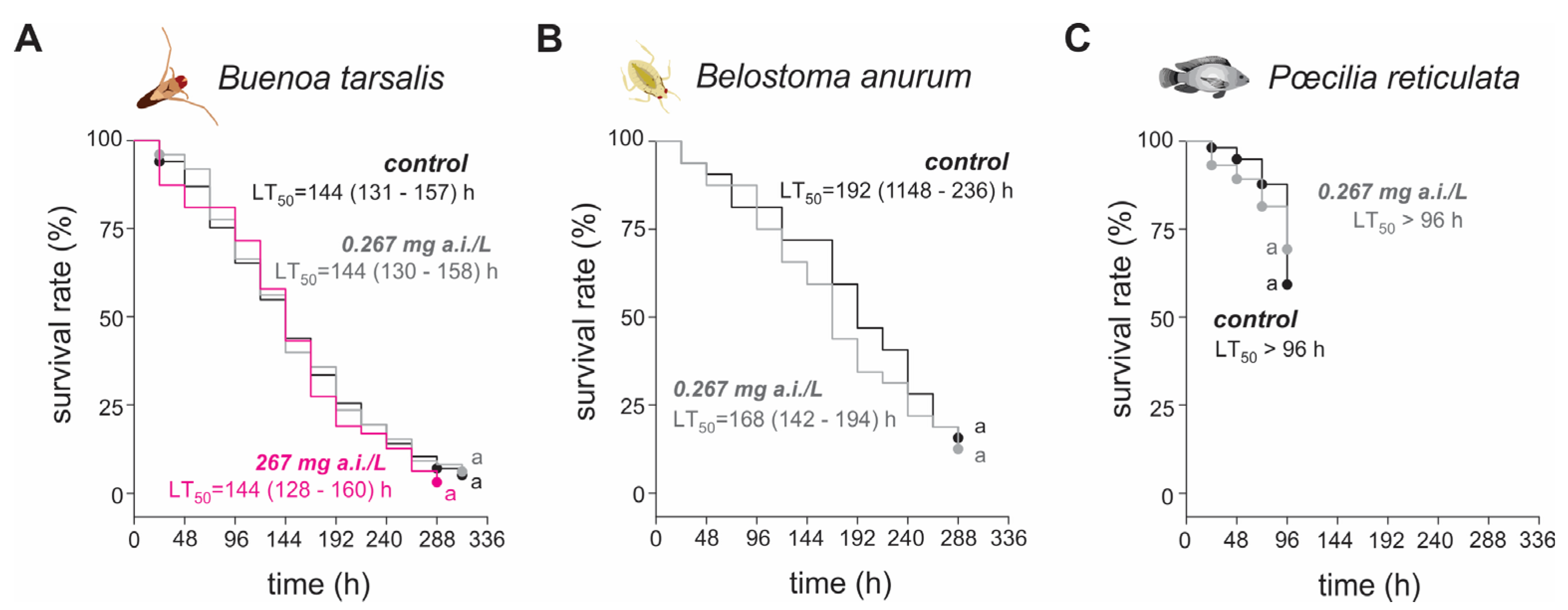
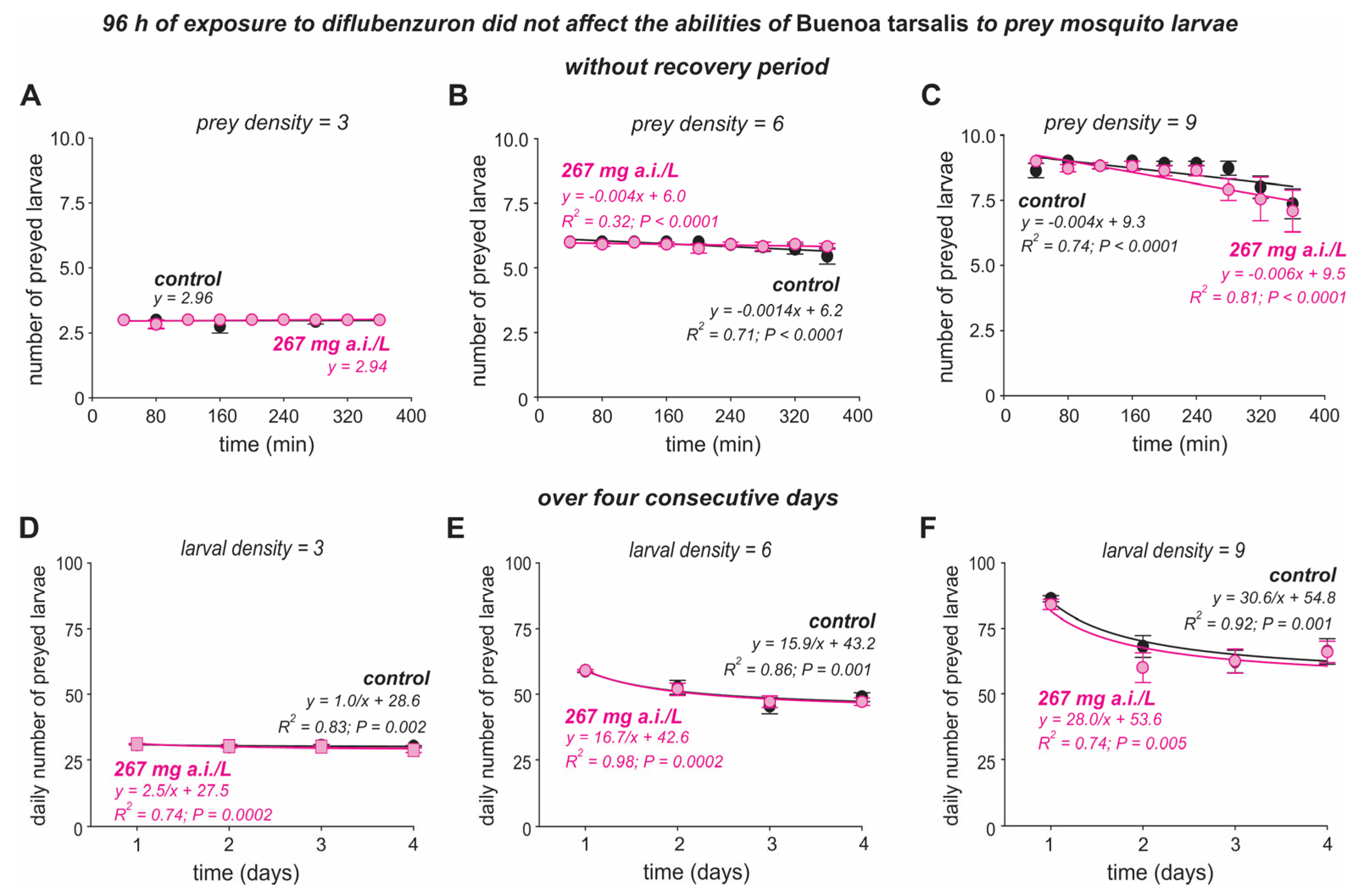
3. Results
3.1. Toxicity of Diflubenzuron to Three Naturally Occurring Mosquito Larvae Predators
3.2. Effect of Diflubenzuron on the Predatory Abilities of Bu. tarsalis Adults
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roiz, D.; Wilson, A.L.; Scott, T.W.; Fonseca, D.M.; Jourdain, F.; Müller, P.; Velayudhan, R.; Corbel, V. Integrated Aedes Management for the Control of Aedes-Borne Diseases. PLoS Negl. Trop. Dis. 2018, 12, e0006845. [Google Scholar] [CrossRef] [PubMed]
- WHO. Larval Source Management: A Supplementary Malaria Vector Control Measure: An Operational Manual; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Merzendorfer, H. Chitin Synthesis Inhibitors: Old Molecules and New Developments. Insect Sci. 2013, 20, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Douris, V.; Steinbach, D.; Panteleri, R.; Livadaras, I.; Pickett, J.A.; Van Leeuwen, T.; Nauen, R.; Vontas, J. Resistance Mutation Conserved between Insects and Mites Unravels the Benzoylurea Insecticide Mode of Action on Chitin Biosynthesis. Proc. Natl. Acad. Sci. USA 2016, 113, 14692–14697. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.A.; Aref, S.A.; Abdelhamid, A.A.; Elwassimy, M.M.; Abdel-Raheem, S.A.A. Biologically Active Organic Compounds as Insect Growth Regulators (IGRs): Introduction, Mode of Action, and Some Synthetic Methods. Curr. Chem. Lett. 2021, 10, 393–412. [Google Scholar] [CrossRef]
- Lahr, J.; Badji, A.; Marquenie, S.; Schuiling, E.; Ndour, K.B.; Diallo, A.O.; Everts, J.W. Acute Toxicity of Locust Insecticides to Two Indigenous Invertebrates from Sahelian Temporary Ponds. Ecotoxicol. Environ. Saf. 2001, 48, 66–75. [Google Scholar] [CrossRef]
- Abe, F.R.; Machado, A.A.; Coleone, A.C.; da Cruz, C.; Machado-Neto, J.G. Toxicity of Diflubenzuron and Temephos on Freshwater Fishes: Ecotoxicological Assays with Oreochromis niloticus and Hyphessobrycon eques. Water Air Soil. Pollut. 2019, 230, 77. [Google Scholar] [CrossRef]
- Ferreira, F.A.S.; Arcos, A.N.; Maia, N.S.G.; Sampaio, R.T.M.; Costa, F.M.; Rodrigues, I.B.; Tadei, W.P. Effects of Diflubenzuron on Associated Insect Fauna with Anopheles(Diptera: Culicidae) in Laboratory, Partial-Field, and Field Conditions in the Central Amazon. An. Acad. Bras. Cienc. 2020, 92, e20180590. [Google Scholar] [CrossRef]
- Junquera, P.; Hosking, B.; Gameiro, M.; Macdonald, A. Benzoylphenyl Ureas as Veterinary Antiparasitics. An Overview and Outlook with Emphasis on Efficacy, Usage and Resistance. Parasite 2019, 26, 26. [Google Scholar] [CrossRef]
- Luvizotto-Santos, R.; Eler, M.N.; Espíndola, E.L.G.; Vieira, E.M. The Use of Pesticides in Fish Farms and Fee Fishing Enterprises from Mogi-Guaçu Catchement. Bol. Do Inst. De Pesca 2009, 35, 343–358. [Google Scholar]
- Shaalan, E.A.-S.; Canyon, D. V Aquatic Insect Predators and Mosquito Control. Trop. Biomed. 2009, 26, 223–261. [Google Scholar]
- Relyea, R.A.; Hoverman, J.T. Interactive Effects of Predators and a Pesticide on Aquatic Communities. Oikos 2008, 117, 1647–1658. [Google Scholar] [CrossRef]
- Relyea, R.A.; Edwards, K. What Doesn’t Kill You Makes You Sluggish: How Sublethal Pesticides Alter Predator–Prey Interactions. Copeia 2010, 2010, 558–567. [Google Scholar] [CrossRef]
- Salaro, A.L.; Silva, S.B.; Ferraz, R.B.; Salinas Jiménez, L.G.; Carneiro, C.L.S.; Quadros, A.S.G.; Machado, J.P.; Freitas, M.B.; Oliveira, E.E. Acute Sublethal Exposure to Ethiprole Impairs Physiological and Oxidative Status in the Neotropical Fish Astyanax altiparanae. Environ. Pollut. 2023, 334, 122152. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.C.M.; Haddi, K.; Valbon, W.R.; Costa, L.T.M.; Ascêncio, S.D.; Santos, G.R.; Soares, I.M.; Barbosa, R.S.; Viana, K.F.; Silva, E.A.P.; et al. Methanolic Extracts of Chiococca alba in Aedes aegypti Biorational Management: Larvicidal and Repellent Potential, and Selectivity against Non-Target Organisms. Plants 2022, 11, 3298. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, Y.; Ramos, G.S.; Tomé, H.V.V.; Oliveira, E.E.; Salaro, A.L. Bti-Based Insecticide Enhances the Predatory Abilities of the Backswimmer Buenoa tarsalis (Hemiptera: Notonectidae). Ecotoxicology 2017, 26, 1147–1155. [Google Scholar] [CrossRef]
- Valbon, W.; Araújo, S.H.C.; Nery, R.S.; Barbosa, J.F.; Newland, P.L.; Oliveira, E.E. Sublethal Exposure to Pyriproxyfen Does Not Impair the Abilities of the Backswimmer Buenoa amnigenus to Prey upon Aedes aegypti Larvae. Ecotoxicology 2022, 31, 998–1008. [Google Scholar] [CrossRef]
- Sivagnaname, N. A Novel Method of Controlling a Dengue Mosquito Vector, Aedes aegypti (Diptera: Culicidae) Using an Aquatic Mosquito Predator, Diplonychus indicus (Hemiptera: Belostomatidae) in Tyres. Dengue Bulletim 2009, 33, 148–160. [Google Scholar]
- Saha, N.; Aditya, G.; Saha, G.K. Prey Preferences of Aquatic Insects: Potential Implications for the Regulation of Wetland Mosquitoes. Med. Vet. Entomol. 2014, 28, 1–9. [Google Scholar] [CrossRef]
- Valbon, W.R.; Haddi, K.; Gutiérrez, Y.; Cruz, F.M.; Azevedo, K.E.X.; Perez Campos, J.S.; Salaro, A.L.; Oliveira, E.E. Life History Traits and Predatory Performance of Belostoma anurum (Hemiptera: Belostomatidae), a Biological Control Agent of Disease Vector Mosquitoes. Neotrop. Entomol. 2019, 48, 899–908. [Google Scholar] [CrossRef]
- Valbon, W.R.; Cruz, F.M.; Ramos, G.S.; Tomé, H.V.V.; Oliveira, E.E. Sublethal Exposure to Deltamethrin Reduces the Abilities of Giant Water Bugs to Prey upon Aedes aegypti Larvae. Chemosphere 2018, 191, 350–356. [Google Scholar] [CrossRef]
- Cruz, F.M.; Carneiro, C.L.S.; Oliveira, J.M.; Valbon, W.R.; Lima, G.D.A.; Freitas, M.B.; Oliveira, E.E.; Salaro, A.L. “Fearing the Enemy”: Growth and Stress Biomarker Responses of Sexually Reversed Oreochromis niloticus in the Presence of Aquatic Predatory Insects. Physiol. Behav. 2021, 228, 113202. [Google Scholar] [CrossRef] [PubMed]
- Reegan, A.D.; Ben Bentrock, B.; Asharaja, A.C.; Tennyson, S.; Raveen, R. Toxicity and Sub-Lethal Effects of Temephos, Lambda-Cyhalothrin and Cypermethrin on Predatory Insect Diplonychus rusticus Fabricius (Hemiptera: Belostomatidae). Int. J. Trop. Insect Sci. 2021, 41, 841–848. [Google Scholar] [CrossRef]
- Fernando, G.K.A.W.; Jayakody, S.; Wijenayake, W.M.H.K.; Galappaththy, G.N.L.; Yatawara, M.; Harishchandra, J. A Comparison of the Larvivorous Habits of Exotic Poecilia reticulata and Native Aplocheilus parvus. BMC Ecol. 2018, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Deacon, A. Predatory Behaviour of Female Guppies (Poecilia reticulata) in a Mosquito Control Context: The Importance of Social and Habitat Factors. Aquat. Invasions 2019, 14, 478–489. [Google Scholar] [CrossRef]
- Sasanami, M.; Hustedt, J.; Alexander, N.; Horstick, O.; Bowman, L.; Hii, J.; Echaubard, P.; Braack, L.; Overgaard, H.J. Does Anthropogenic Introduction of Guppy Fish (Poecilia reticulata) Impact Faunal Species Diversity and Abundance in Natural Aquatic Habitats? A Systematic Review Protocol. Environ. Evid. 2021, 10, 33. [Google Scholar] [CrossRef]
- Medeiros, L.S.; Souza, J.P.; Winkaler, E.U.; Carraschi, S.P.; Cruz, C.; Souza-Júnior, S.C.; Machado-Neto, J.G. Acute Toxicity and Environmental Risk of Teflubenzuron to Daphnia magna, Poecilia reticulata and Lemna minor in the Absence and Presence of Sediment. J. Environ. Sci. Health Part B 2013, 48, 600–606. [Google Scholar] [CrossRef]
- Barbosa, J.F.; Nessimian, J.L. The Genus Buenoa Kirkaldy, 1904 (Hemiptera: Heteroptera: Nepomorpha: Notonectidae) in Northern Brazil: Inventory, New Records, and New Species. Zootaxa 2013, 3694, 101–130. [Google Scholar] [CrossRef]
- Santos, A.B.R.; Cossolin, J.F.S.; Barcellos, M.S.; Bozdoğan, H.; Lino-Neto, J.; Serrão, J.E. Spermatozoa Morphology of the Giant Water Bug Belostoma anurum (Herrich-Schäffer, 1848) (Heteroptera: Belostomatidae). Zool. Anz. 2020, 288, 103–106. [Google Scholar] [CrossRef]
- Haddi, K.; Tomé, H.V.V.; Du, Y.; Valbon, W.R.; Nomura, Y.; Martins, G.F.; Dong, K.; Oliveira, E.E. Detection of a New Pyrethroid Resistance Mutation (V410L) in the Sodium Channel of Aedes aegypti: A Potential Challenge for Mosquito Control. Sci. Rep. 2017, 7, 46549. [Google Scholar] [CrossRef]
- Salako, A.F.; Amaeze, N.H.; Shobajo, H.M.; Osuala, F.I. Comparative Acute Toxicity of Three Pyrethroids (Deltamethrin, Cypermethrin and Lambda-Cyhalothrin) on Guppy Fish (Poecilia reticulata Peters, 1859). Sci. Afr. 2020, 9, e00504. [Google Scholar] [CrossRef]
- Holling, C.S. Some Characteristics of Simple Types of Predation and Parasitism. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- von Ende, C.N. Repeated-Measures Analysis: Growth and Other Time-Dependent Measures. In Design and Analysis of Ecological Experiments; Chapman and Hall/CRC: Boca Raton, FL, USA, 1998; pp. 113–137. ISBN 9781003059813. [Google Scholar]
- Subrero, E.; Sforzini, S.; Viarengo, A.; Cucco, M. Exposure to Anti-Mosquito Insecticides Utilized in Rice Fields Affects Survival of Two Non-Target Species, Ischnura elegans and Daphnia magna. Paddy Water Environ. 2019, 17, 1–11. [Google Scholar] [CrossRef]
- Ser, O.; Cetin, H. Toxicity of Mosquito Larvicides on Non-Target Mosquito Predator Insect, Backswimmer (Notonecta sp.). Fresen Environ. Bull. 2015, 24, 311–316. [Google Scholar]
- Eba, K.; Duchateau, L.; Olkeba, B.K.; Boets, P.; Bedada, D.; Goethals, P.L.M.; Mereta, S.T.; Yewhalaw, D. Bio-Control of Anopheles Mosquito Larvae Using Invertebrate Predators to Support Human Health Programs in Ethiopia. Int. J. Environ. Res. Public Health 2021, 18, 1810. [Google Scholar] [CrossRef]
- Kweka, E.J.; Zhou, G.; Gilbreath, T.M.; Afrane, Y.; Nyindo, M.; Githeko, A.K.; Yan, G. Predation Efficiency of Anopheles gambiae Larvae by Aquatic Predators in Western Kenya Highlands. Parasites Vectors 2011, 4, 128. [Google Scholar] [CrossRef]
- Fischer, S.A.; Hall, L.W. Environmental Concentrations and Aquatic Toxicity Data on Diflubenzuron (Dimilin). Crit. Rev. Toxicol. 1992, 22, 45–79. [Google Scholar] [CrossRef]
- Soderlund, D.M.; Bloomquist, J.R. Neurotoxic Actions of Pyrethroid Insecticides. Annu. Rev. Entomol. 1989, 34, 77–96. [Google Scholar] [CrossRef]
- Mulla, M.S. The Future of Insect Growth Regulators in Vector Control. J. Am. Mosq. Control Assoc. 1995, 11, 269–273. [Google Scholar]
- Silva, A.L.N.; Rodrigues, R.A.; Siqueira, M.S.; Farias, K.N.N.; Kuibida, K.V.; Franco-Belussi, L.; Fernandes, C.E. Transaminase Profile and Hepatic Histopathological Traits in Piaractus mesopotamicus Exposed to Insecticide Diflubenzuron. Environ. Sci. Pollut. Res. 2021, 28, 22002–22010. [Google Scholar] [CrossRef]
- Leza, M.; Gonzalez-Ruiz, R.; Alemany, A. Short Term Effects of Bacillus thuringiensis and Diflubenzuron Aerial Applications on Non-Target Arthropods in a Mediterranean Forest. Int. J. Pest Manag. 2021, 67, 139–146. [Google Scholar] [CrossRef]
- Dai, P.; Jack, C.J.; Mortensen, A.N.; Bloomquist, J.R.; Ellis, J.D. The Impacts of Chlorothalonil and Diflubenzuron on Apis mellifera L. Larvae Reared in Vitro. Ecotoxicol. Environ. Saf. 2018, 164, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Camp, A.A.; Batres, M.A.; Williams, W.C.; Lehmann, D.M. Impact of Diflubenzuron on Bombus impatiens (Hymenoptera: Apidae) Microcolony Development. Environ. Entomol. 2020, 49, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Montaño-Reyes, A.; Llanderal-Cázares, C.; Valdez-Carrasco, J.; Miranda-Perkins, K.; Sánchez-Arroyo, H. Susceptibility and Alterations by Diflubenzuron in Larvae of Aedes aegypti. Arch. Insect Biochem. Physiol. 2019, 102, e21604. [Google Scholar] [CrossRef] [PubMed]
- Pasini, R.A.; Grützmacher, A.D.; de Bastos Pazini, J.; de Armas, F.S.; Bueno, F.A.; Pires, S.N. Side Effects of Insecticides Used in Wheat Crop on Eggs and Pupae of Chrysoperla externa and Eriopis connexa. Phytoparasitica 2018, 46, 115–125. [Google Scholar] [CrossRef]
- Gutiérrez, Y.; Tomé, H.V.V.; Guedes, R.N.C.; Oliveira, E.E. Deltamethrin Toxicity and Impaired Swimming Behavior of Two Backswimmer Species. Environ. Toxicol. Chem. 2016, 36, 1235–1242. [Google Scholar] [CrossRef]
- Giller, P.S.; McNeill, S. Predation Strategies, Resource Partitioning and Habitat Selection in Notonecta (Hemiptera/Heteroptera). J. Anim. Ecol. 1981, 50, 789–808. [Google Scholar] [CrossRef]
- Serradj, N.; Bendali-Saoudi, F.; Soltani, N. The effect of diflubenzuron (Dimilin® 25 WP) on some non-target aquatic insect and crustacean species. Pol. J. Entomol. 2022, 91, 174–183. [Google Scholar] [CrossRef]

| Sources of Variation | ||||
| df | F | p | ||
| Between Samples | ||||
| Insecticide (I) | 1 | 0.69 | 0.41 | |
| Density (D) | 2 | 157.9 | <0.0001 1 | |
| I × D | 2 | 0.38 | 0.69 | |
| Error | 51 | - | - | |
| dfden/dfnum | Wilks’ lambda | F | p | |
| Within Samples | ||||
| Time (T) | 44/8 | 0.44 | 6.95 | <0.0001 1 |
| T × I | 44/8 | 0.90 | 0.61 | 0.76 |
| T × D | 88/16 | 0.34 | 3.88 | <0.0001 1 |
| T × I × D | 88/16 | 0.80 | 0.63 | 0.85 |
| Sources of Variation | ||||
| df | F | p | ||
| Between Samples | ||||
| Insecticide (I) | 1 | 1.78 | 0.19 | |
| Density (D) | 2 | 63.7 | <0.0001 1 | |
| I × D | 2 | 1.19 | 0.31 | |
| Error | 51 | - | - | |
| dfden/dfnum | Wilks’ lambda | F | p | |
| Within Samples | ||||
| Time (T) | 49/3 | 0.52 | 15.5 | <0.0001 1 |
| T × I | 49/3 | 0.95 | 0.94 | 0.43 |
| T × D | 98/6 | 0.62 | 4.37 | 0.0006 1 |
| T × I × D | 98/6 | 0.91 | 0.75 | 0.61 |
| Sources of Variation | ||||
| df | F | p | ||
| Between Samples | ||||
| Insecticide (I) | 1 | 0.62 | 0.43 | |
| Density (D) | 2 | 1355.9 | <0.0001 1 | |
| I × D | 2 | 0.99 | 0.38 | |
| Error | 63 | - | - | |
| dfden/dfnum | Wilks’ lambda | F | p | |
| Within Samples | ||||
| Time (T) | 56/8 | 0.72 | 2.71 | 0.014 1 |
| T × I | 56/8 | 0.82 | 1.57 | 0.16 |
| T × D | 112/16 | 0.67 | 1.58 | 0.09 |
| T × I × D | 112/16 | 0.78 | 0.91 | 0.55 |
| Sources of Variation | ||||
| df | F | p | ||
| Between Samples | ||||
| Insecticide (I) | 1 | 0.41 | 0.52 | |
| Density (D) | 2 | 211.3 | <0.0001 1 | |
| I × D | 2 | 0.22 | 0.80 | |
| Error | 63 | - | - | |
| dfden/dfnum | Wilks’ lambda | F | p | |
| Within Samples | ||||
| Time (T) | 61/3 | 0.35 | 37.4 | <0.0001 1 |
| T × I | 61/3 | 0.95 | 1.05 | 0.38 |
| T × D | 122/6 | 0.45 | 10.1 | <0.0001 1 |
| T × I × D | 122/6 | 0.93 | 0.70 | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araujo, S.H.C.; Salinas Jimenez, L.G.; Corrêa, M.J.M.; Bohorquez Zapata, V.L.; Oliveira, M.S.S.; Fernandes, J.S.; Gomes, J.M.; Aguiar, R.W.S.; Santos, G.R.; Valbon, W.R.; et al. Diflubenzuron Did Not Affect the Abilities of the Backswimmer Buenoa tarsalis to Survive and Prey Upon Larvae of Aedes aegypti. Insects 2025, 16, 435. https://doi.org/10.3390/insects16040435
Araujo SHC, Salinas Jimenez LG, Corrêa MJM, Bohorquez Zapata VL, Oliveira MSS, Fernandes JS, Gomes JM, Aguiar RWS, Santos GR, Valbon WR, et al. Diflubenzuron Did Not Affect the Abilities of the Backswimmer Buenoa tarsalis to Survive and Prey Upon Larvae of Aedes aegypti. Insects. 2025; 16(4):435. https://doi.org/10.3390/insects16040435
Chicago/Turabian StyleAraujo, Sabrina H. C., Luis G. Salinas Jimenez, Maria J. M. Corrêa, Viviana L. Bohorquez Zapata, Monalisa S. S. Oliveira, Joshua S. Fernandes, Jônatas M. Gomes, Raimundo W. S. Aguiar, Gil R. Santos, Wilson R. Valbon, and et al. 2025. "Diflubenzuron Did Not Affect the Abilities of the Backswimmer Buenoa tarsalis to Survive and Prey Upon Larvae of Aedes aegypti" Insects 16, no. 4: 435. https://doi.org/10.3390/insects16040435
APA StyleAraujo, S. H. C., Salinas Jimenez, L. G., Corrêa, M. J. M., Bohorquez Zapata, V. L., Oliveira, M. S. S., Fernandes, J. S., Gomes, J. M., Aguiar, R. W. S., Santos, G. R., Valbon, W. R., & Oliveira, E. E. (2025). Diflubenzuron Did Not Affect the Abilities of the Backswimmer Buenoa tarsalis to Survive and Prey Upon Larvae of Aedes aegypti. Insects, 16(4), 435. https://doi.org/10.3390/insects16040435








