Selection of Bactrocera tau (Walker) Reference Genes for Quantitative Real-Time PCR
Simple Summary
Abstract
1. Introduction
2. Materials and Method
2.1. Biological Samples
2.2. Total RNA Extraction and cDNA Synthesis
2.3. Primer Design and Sequence Accuracy Validation
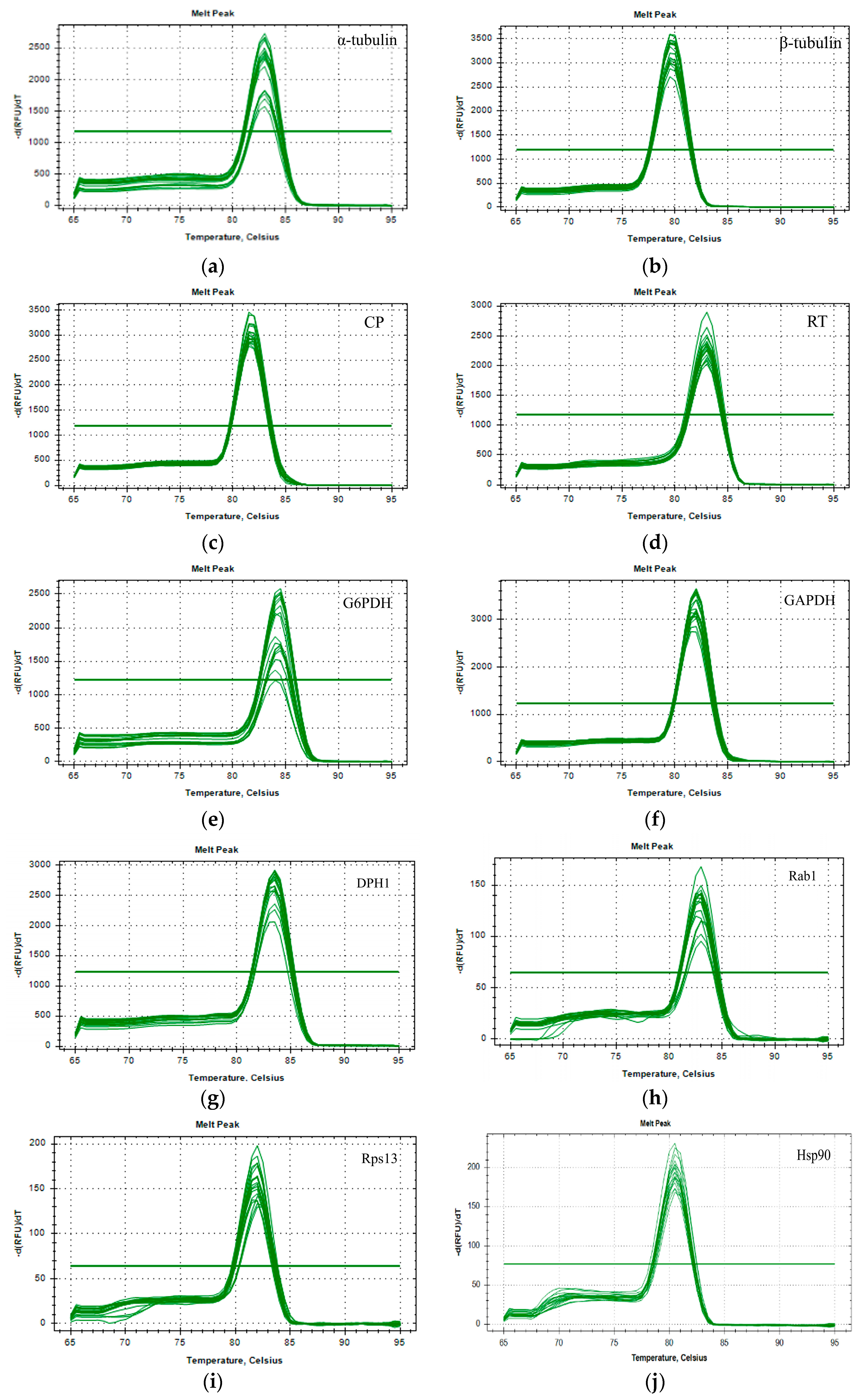
2.4. RT-qPCR and Amplification Efficiency Testing
2.5. Verification of the Stability of Reference Genes
2.6. Statistical Analysis
3. Results
3.1. Selection of Candidate Reference Genes
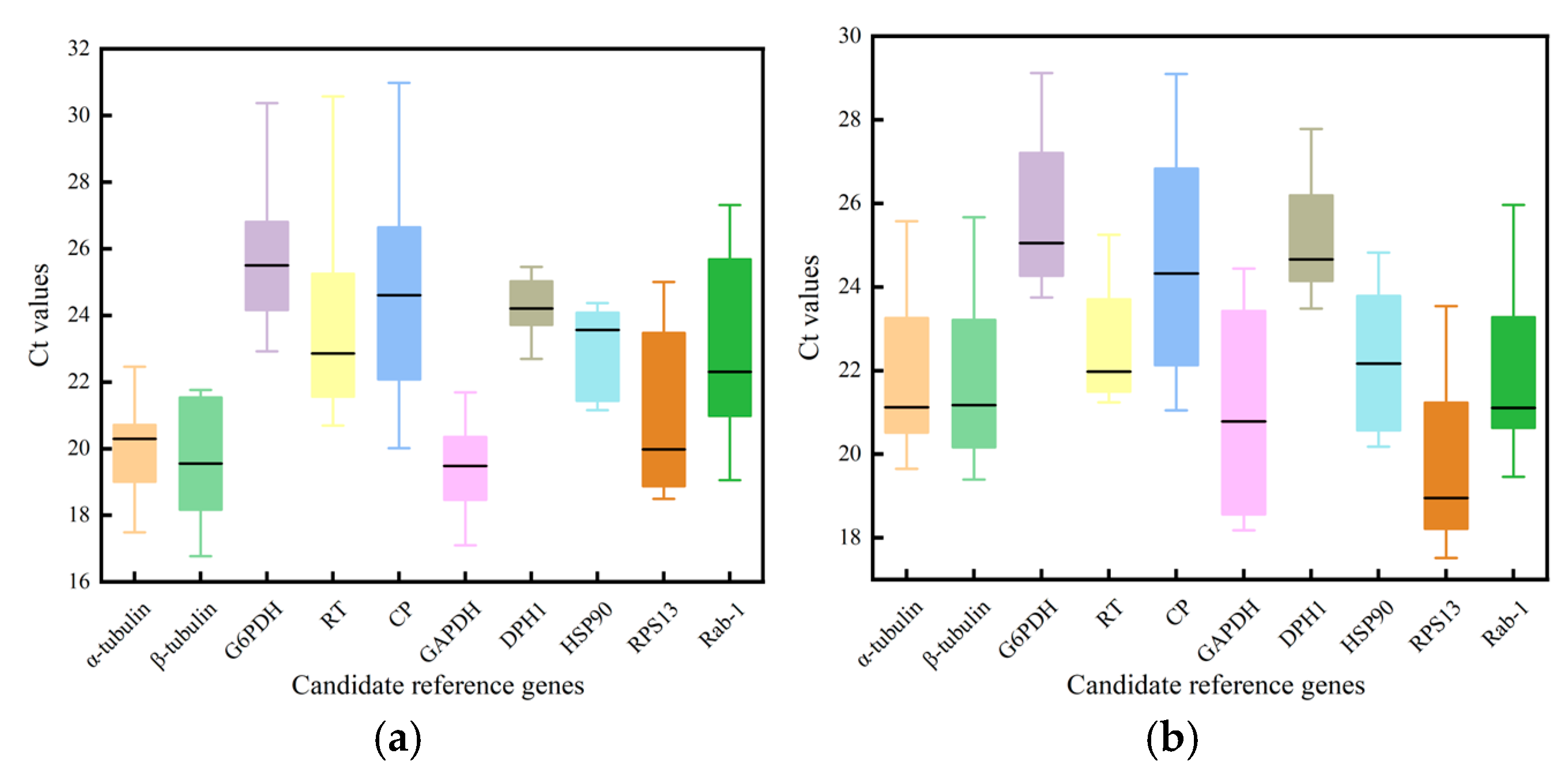
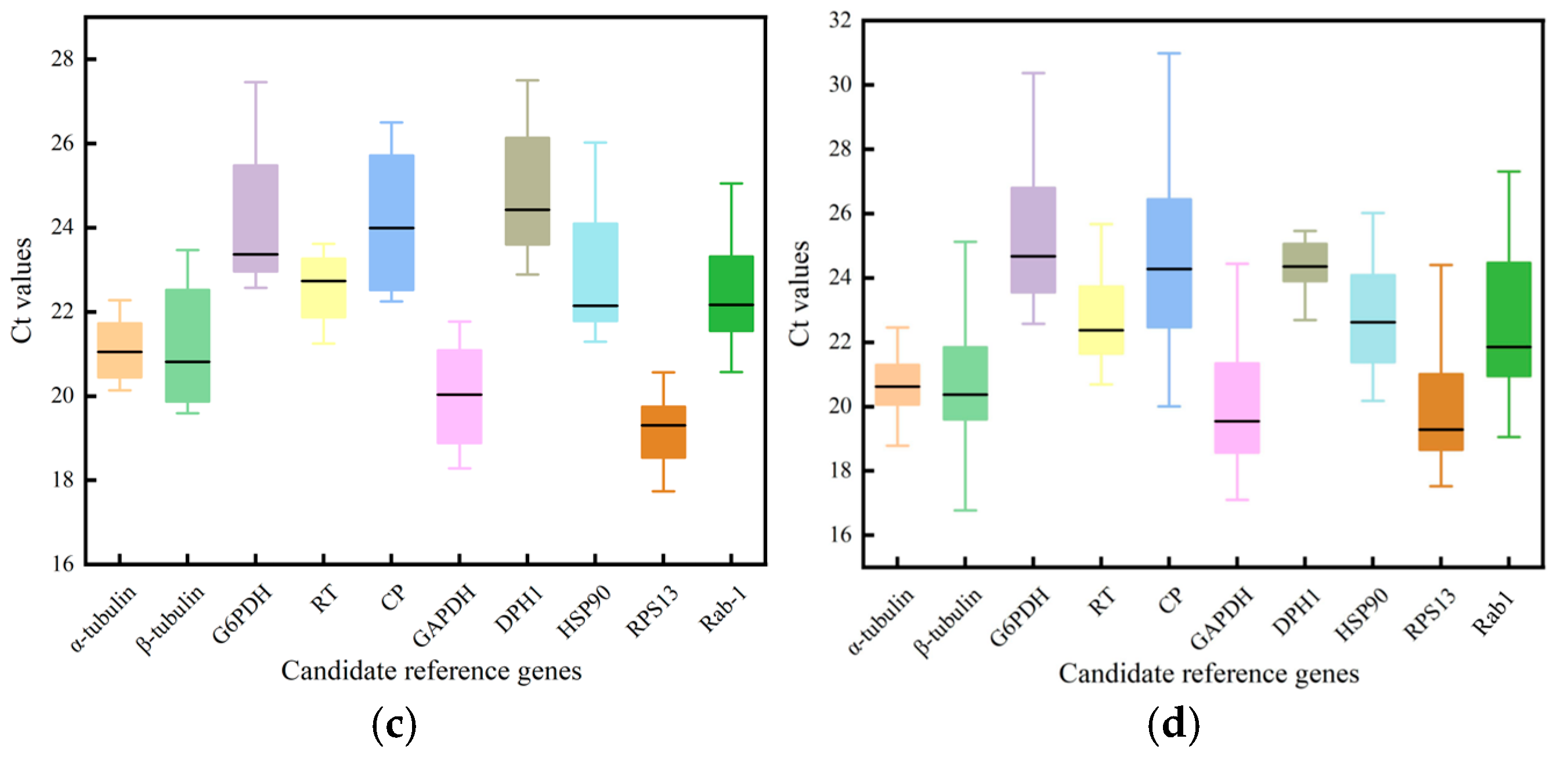
3.2. Variation in Expression of the Ten Reference Genes
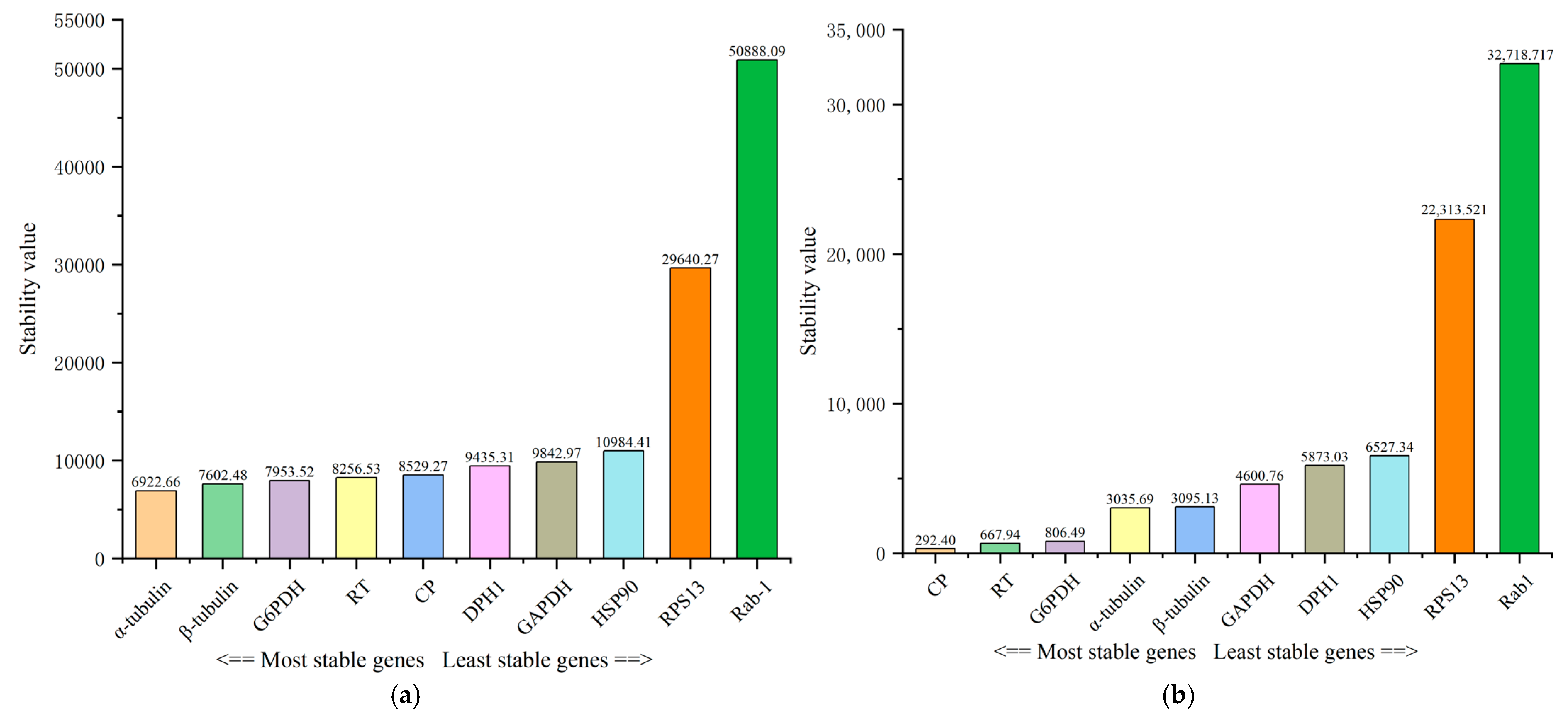
3.3. Stability of Expression of the Ten Reference Genes in Various Samples
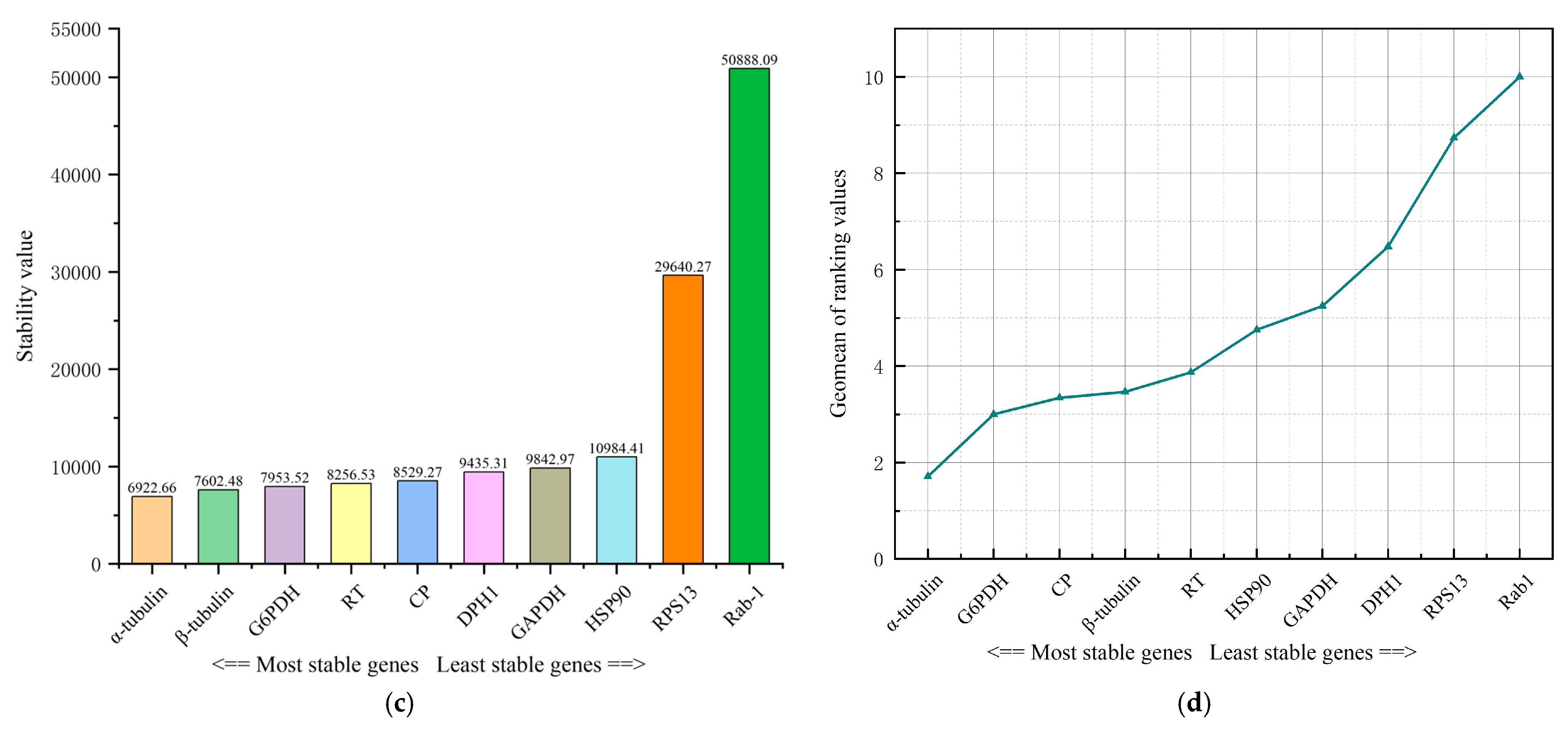
3.4. Empirical Verification of Reference Gene Stability
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| d | Day |
| α-tubulin | Tubulin alpha chain |
| G6PDH | Glucose-6-phosphate dehydrogenase |
| Rab1 | Rab1 |
| RT | Reverse transcriptase |
| RPS13 | 40s ribosomal protein s13 |
| β-tubulin | Beta-tubulin 1 chain |
| DPH1 | DnaJ protein homolog 1 |
| HSP90 | HSP90A |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase 2 |
| CP | Cysteine proteinase |
References
- Li, X.; Yang, H.; Hu, K.; Wang, J. Temporal dynamics of Bactrocera (Zeugodacus) tau (Diptera: Tephritidae) adults in north Jiangxi, a subtropical area of China revealed by eight years of trapping with cuelure. J. Asia-Pac. Entomol. 2020, 23, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.T.; Cao, L.J.; Chen, J.C.; Song, W.; Ma, W.H.; Yang, J.F.; Gao, X.Y.; Chen, H.S.; Zhang, Y.; Tian, Z.Y.; et al. Chromosome-level genome assembly of an agricultural pest Zeugodacus tau (Diptera: Tephritidae). Sci. Data 2023, 10, 848. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xu, Y.; Chen, X. Biology, Ecology and Management of Tephritid Fruit Flies in China: A Review. Insects 2023, 14, 196. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Chao, M.A.; Zhe, L.I.; Bang-Qin, Z.; Jiang, Z.; Chao-Liang, L.; Shuang-Xia, J.; Hull, J.J.; Li-Zhen, C. Assessment of suitable reference genes for qRT-PCR analysis in Adelphocoris suturalis. J. Integr. Agric. 2018, 17, 2745–2757. [Google Scholar]
- Liu, X.; Zhang, Q.; Xu, W.; Yang, Y.; Fan, Q.; Ji, Q. The Effect of Cuelure on Attracting and Feeding Behavior in Zeugodacus tau (Walker) (Diptera: Tephritidae). Insects 2023, 14, 836. [Google Scholar] [CrossRef]
- Shamshir, R.A.; Wee, S.L. Comparative Responses of Two Major Cucurbit Pests, Zeugodacus cucurbitae and Zeugodacus tau to Phenylbutanoid Male Lures. J. Chem. Ecol. 2024, 50, 947–954. [Google Scholar] [CrossRef]
- Liu, P.; Li, Z.; Zhang, Q.; Qiao, J.; Zheng, C.; Zheng, W.; Zhang, H. Identification of testis development-related genes by combining Iso-Seq and RNA-Seq in Zeugodacus tau. Front. Cell Dev. Biol. 2024, 12, 17. [Google Scholar] [CrossRef]
- Zhang, H.H.; Luo, M.J.; Zhang, Q.W.; Cai, P.M.; Idrees, A.; Ji, Q.E.; Yang, J.Q.; Chen, J.H. Molecular characterization of prophenoloxidase-1 (PPO1) and the inhibitory effect of kojic acid on phenoloxidase (PO) activity and on the development of Zeugodacus tau (Walker) (Diptera: Tephritidae). Bull. Entomol. Res. 2019, 109, 236–247. [Google Scholar] [CrossRef]
- Bachman, J. Reverse-transcription PCR (RT-PCR). Methods Enzym. 2013, 530, 67–74. [Google Scholar] [CrossRef]
- Donovan, N.J.; Chambers, G.A.; Cao, M. Detection of Viroids by RT-PCR. Methods Mol. Biol. 2022, 2316, 143–151. [Google Scholar] [CrossRef]
- Silveira, G.O.; Amaral, M.S.; Coelho, H.S.; Maciel, L.F.; Pereira, A.S.A.; Olberg, G.G.O.; Miyasato, P.A.; Nakano, E.; Verjovski-Almeida, S. Assessment of reference genes at six different developmental stages of Schistosoma mansoni for quantitative RT-PCR. Sci. Rep. 2021, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Nolan, T.; Pfaffl, M.W. Quantitative real-time RT-PCR—A perspective. J. Mol. Endocrinol. 2005, 34, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Vladimir, B.; Garson, J.A.; Jan, H.; Jim, H.; Mikael, K.; Reinhold, M.; Tania, N.; Pfaffl, M.W.; Shipley, G.L. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 611, 4611–4622. [Google Scholar] [CrossRef]
- Vandesompele, J.; Preter, K.D.; Pattyn, F.; Poppe, B.; Roy, N.V.; Paepe, A.D.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 00341. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.; Hands, R.E.; Bustin, S.A. Nature Protocols: Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 2006, 1, 1559–1582. [Google Scholar] [CrossRef]
- Derveaux, S.; Vandesompele, J.; Hellemans, J. How to do successful gene expression analysis using real-time PCR—ScienceDirect. Methods 2010, 50, 227–230. [Google Scholar] [CrossRef]
- LourençO, A.P.; Mackert, A.; Cristino, A.D.S.; Sim?es, Z.L.P. Validation of reference genes for gene expression studies in the honey bee, Apis mellifera, by quantitative real-time RT-PCR. Apidologie 2008, 39, 372–385. [Google Scholar] [CrossRef]
- Rumei, L.; Wen, X.; Shaoli, W.; Qingjun, W.; Nina, Y.; Xin, Y.; Huipeng, P.; Xiaomao, Z.; Lianyang, B.; Baoyun, X. Reference Gene Selection for qRT-PCR Analysis in the Sweetpotato Whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). PLoS ONE 2013, 8, e53006. [Google Scholar]
- Shi, X.Q.; Guo, W.C.; Wan, P.J.; Zhou, L.T.; Ren, X.L.; Ahmat, T.; Fu, K.Y.; Li, G.Q. Validation of reference genes for expression analysis by quantitative real-time PCR in Leptinotarsa decemlineata (Say). BMC Res. Notes 2013, 6, 93. [Google Scholar] [CrossRef]
- Miao, Y.; Yanhui, L.; Xun, Z.; Hu, W.; Muhammad, S.; Sha, Z.; Byung-Rae, J.; Jianhong, L.; Xiao-Wei, W. Selection and Evaluation of Potential Reference Genes for Gene Expression Analysis in the Brown Planthopper, Nilaparvata lugens (Hemiptera: Delphacidae) Using Reverse-Transcription Quantitative PCR. PLoS ONE 2014, 9, e86503. [Google Scholar]
- Volland, M.; Blasco, J.; Hampel, M. Validation of reference genes for RT-qPCR in marine bivalve ecotoxicology: Systematic review and case study using copper treated primary Ruditapes philippinarum hemocytes. Aquat. Toxicol. 2017, 185, 86–94. [Google Scholar] [CrossRef]
- Shen, C.H.; Peng, L.J.; Zhang, Y.X.; Zeng, H.R.; Yu, H.F.; Jin, L.; Li, G.Q. Reference Genes for Expression Analyses by qRT-PCR in Phthorimaea operculella (Lepidoptera: Gelechiidae). Insects 2022, 13, 140. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Rntoft, T.F. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Am. Assoc. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [PubMed]
- Ponton, F.; Chapuis, M.P.; Pernice, M.; Sword, G.A.; Simpson, S.J. Evaluation of potential reference genes for reverse transcription-qPCR studies of physiological responses in Drosophila melanogaster. J. Insect Physiol. 2011, 57, 840–850. [Google Scholar] [CrossRef]
- Shi, C.H.; Hu, J.R.; Li, C.R.; Wang, W.K. Progress in the study of reference genes in insect qRT-PCR. Jiangsu Agric. Sci. 2017, 45, 1–7. [Google Scholar]
- Chen, L.; Tian, Z.; Wang, X.Y.; Chen, X.M.; Lu, W.; Wang, X.P.; Zheng, X.L. Selection of reference genes for real-time fluorescent quantitative PCR in the red-shouldered moth, Euproctis chrysorrhoea. J. Environ. Entomol. 2021, 43, 15–24. [Google Scholar]
- Wolf, K.W.; Spanel-Borowski, K. Acetylation of α-tubilin in different bovine cell types: Implications for microtubule dynamics in interphase and mitosis. Blackwell Publ. Ltd. 1995, 19, 43–52. [Google Scholar] [CrossRef]
- McKenna, E.D.; Sarbanes, S.L.; Cummings, S.W.; Roll-Mecak, A. The Tubulin Code, from Molecules to Health and Disease. Annu. Rev. Cell Dev. Biol. 2023, 39, 331–361. [Google Scholar] [CrossRef]
- Moraes, B.; Martins, R.; Lopes, C.; Martins, R.; Arcanjo, A.; Nascimento, J.; Konnai, S.; Itabajara, D.S.V.J.; Logullo, C. G6PDH as a key immunometabolic and redox trigger in arthropods. Front. Physiol. 2023, 14, 11. [Google Scholar] [CrossRef]
- Liu, T. Evaluation of Reference Genes for Quantitative Reverse Transcription Polymerase Chain Reaction in Bactrocera dorsalis (Diptera: Tephritidae) Subjected to Various Phytosanitary Treatments. Insects 2021, 12, 945. [Google Scholar] [CrossRef]
- Zhou, X.; Liao, W.J.; Liao, J.M.; Liao, P.; Lu, H. Ribosomal proteins: Functions beyond the ribosome. J. Mol. Cell Biol. 2015, 7, 92–104. [Google Scholar] [CrossRef]
- Lü, J.; Yang, C.; Zhang, Y.; Pan, H. Selection of Reference Genes for the Normalization of RT-qPCR Data in Gene Expression Studies in Insects: A Systematic Review. Front. Physiol. 2018, 9, 1560. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.X.; Zhang, G.Q.; Zhao, Y.X.; Zhu, X.X.; Yu, X.F.; Yang, M.F.; Zhang, F. Selection and validation of optimal reference genes for RT-qPCR analyses in Aphidoletes aphidimyza Rondani (Diptera: Cecidomyiidae). Front. Physiol. 2023, 14, 1277942. [Google Scholar] [CrossRef] [PubMed]
- Neuman, S.D.; Lee, A.R.; Selegue, J.E.; Cavanagh, A.T.; Bashirullah, A. A novel function for Rab1 and Rab11 during secretory granule maturation. J. Cell Sci. 2021, 134, jcs259037. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Z.; Shi, A. Rab GTPases: The principal players in crafting the regulatory landscape of endosomal trafficking. Comput. Struct. Biotechnol. J. 2022, 20, 4464–4472. [Google Scholar] [CrossRef] [PubMed]
- Sechi, S.; Frappaolo, A.; Fraschini, R.; Capalbo, L.; Giansanti, M.G. Rab1 interacts with GOLPH3 and controls Golgi structure and contractile ring constriction during cytokinesis in Drosophila melanogaster. Open Biol. 2017, 7, 160257. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.H.; Kim, K.; Kim, D.; Lee, S.H.; Kim, S. Selection of stable reference genes for quantitative real-time PCR in the Varroa mite, Varroa destructor. Arch. Insect Biochem. Physiol. 2022, 110, e21905. [Google Scholar] [CrossRef]
- He, Q.Y.; Yan, J.Y.; Zhou, Y.T. Selection and Verification of the Referwnce Genes in lliberis pruni (Lepidoptera: Zygaenidae). J. Gansu Agric. Univ. 2024. Available online: https://link.cnki.net/urlid/62.1055.S.20240428.1040.031 (accessed on 1 February 2025). (In Chinese).
- Lü, Z.C.; Wang, L.H.; Dai, R.L.; Wan, F.H. Evaluation of Endogenous Reference Genes of Bactrocera (Tetradacus) minax by Gene Expression Profiling under Various Experimental Conditions. Fla. Entomol. Soc. 2014, 97, 597–604. [Google Scholar] [CrossRef]

| Samples | Quantity | |
|---|---|---|
| Development stages before eclosion | Eggs | 15 |
| 1st instar larvae | 15 | |
| 2nd instar larvae | 7 | |
| 3rd instar larvae | 3 | |
| 1-day pupae | 3 | |
| 7-day pupae | 3 | |
| Adults | Newly eclosed females | 2 |
| Newly eclosed males | 2 | |
| 13-day females | 2 | |
| 13-day males | 2 | |
| Body parts | 13-day adult heads | 5 |
| 13-day adult thoraxes | 3 | |
| 13-day female abdomens | 3 | |
| 13-day male abdomens | 3 | |
| Gene | Primers (5′–3′) | Amplicon Length (bp) |
|---|---|---|
| RPS13 | F: CTGTAAAGATGGGTCG | 931 |
| R: CTGCCACAAATTAACAAC | ||
| G6PDH | F: TGCCACTTGTAAGACCC | 1458 |
| R: ACACTTCGATTCCCATG | ||
| GAPDH | F: TAAGTAACCGGAGGCT | 1636 |
| R: GAAGGTCGTATCTAATGC | ||
| α-tubulin | F: ACGCTGCCAACAACTAT | 1074 |
| R: TTCATTTCGCCGTTTA | ||
| β-tubulin | F: CGATTTCGCCGTTAC | 1053 |
| R: GACTCTGTGCTCGATGTT | ||
| HSP90 | F: TGGTACAAAGGCATTCA | 1823 |
| R: AAGCATCCTCGGTGTC | ||
| CP | F: CTTCTATGCCTGGTATGAC | 1124 |
| R: AATCCTCTTCCGTTGG | ||
| DPH1 | F: TGCCAATGACGATGA | 1021 |
| R: GCCAGTGCCTCCTAT | ||
| Rab1 | F: TGCGTGGAAGTGAGA | 1039 |
| R: TATTCAGCAGCAACCT | ||
| RT | F: GTGCGTAGGGTTTGGG | 1428 |
| R: GCAGGTCGGAGAAGTGTT |
| Gene | Primers (5′–3′) | Amplicon Length (bp) | Slope | Correlation Coefficient (R2) | Amplification Efficiency (E) (%) |
|---|---|---|---|---|---|
| α-tubulin | F: ACGCCTGCTGGGAATTGTACTG | 90 | −3.126 | 0.991 | 108.880 |
| R: AATCATCACCACCGCCGACAG | |||||
| β-tubulin | F: GTGGAATATCGCAAACGGCAGTC | 120 | −3.157 | 0.997 | 107.375 |
| R: GTGGTCGCATGTCAATGAAGGAAG | |||||
| CP | F: TGAATGGCACCGCTGAGAAT | 140 | −3.035 | 0.991 | 113.545 |
| R: ACGCTTTTCACCGATCACCT | |||||
| RT | F: AGGCTGACATCACCGCAATCC | 123 | −3.042 | 0.994 | 113.173 |
| R: CCACGAATCCCACACCGAATTTG | |||||
| G6PDH | F: GCAAAGGTGGGCGTATTGGAATC | 127 | −3.245 | 0.987 | 103.314 |
| R: CAGCACTCACATTGGACGACATG | |||||
| GAPDH | F: ATAACCTTGCCAACGGCCTTA | 93 | −3.151 | 0.998 | 107.659 |
| R: TCCCTCCGGCAAACTGT | |||||
| DPH1 | F: CGGCGGTCCAGGAGAACATG | 102 | −3.494 | 0.985 | 93.286 |
| R: CCAAACGGATCTGACGAACCAAAG | |||||
| Rab1 | F: TGACGATGGCTGCCGAGATAAAG | 118 | −3.195 | 0.994 | 105.581 |
| R: CAGCAACCTGATTTGGTGTTCTCC | |||||
| RPS13 | F: TTTGAAACCCGACATCCCAGAGG | 141 | −3.179 | 0.988 | 106.331 |
| R: GCCAAACGGTGGATTCTTGACTC | |||||
| HSP90 | F: TCTTGCGTTACCACACCTCAGC | 121 | −3.155 | 0.988 | 107.471 |
| R: ACTTGTTCCTTCGACTCACCAGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Y.; Yu, Y.; Xu, P.; Zeng, X.; Long, X.; Wei, D.; He, Z.; Gao, X. Selection of Bactrocera tau (Walker) Reference Genes for Quantitative Real-Time PCR. Insects 2025, 16, 445. https://doi.org/10.3390/insects16050445
Zhai Y, Yu Y, Xu P, Zeng X, Long X, Wei D, He Z, Gao X. Selection of Bactrocera tau (Walker) Reference Genes for Quantitative Real-Time PCR. Insects. 2025; 16(5):445. https://doi.org/10.3390/insects16050445
Chicago/Turabian StyleZhai, Yutong, Yonghao Yu, Pengfei Xu, Xianru Zeng, Xiuzhen Long, Dewei Wei, Zhan He, and Xuyuan Gao. 2025. "Selection of Bactrocera tau (Walker) Reference Genes for Quantitative Real-Time PCR" Insects 16, no. 5: 445. https://doi.org/10.3390/insects16050445
APA StyleZhai, Y., Yu, Y., Xu, P., Zeng, X., Long, X., Wei, D., He, Z., & Gao, X. (2025). Selection of Bactrocera tau (Walker) Reference Genes for Quantitative Real-Time PCR. Insects, 16(5), 445. https://doi.org/10.3390/insects16050445




