Simple Summary
Tomatoes (Solanum lycopersicum) are highly vulnerable to the whitefly-transmitted tomato yellow leaf curl disease (TYLCD). This study evaluates multi-disease and insect-resistant tomato lines incorporating Ty-1/Ty-3 genes (for virus resistance) and WF2-10 and WF3-09 genes (for whitefly resistance). Multi-disease and insect-resistant lines exhibit significantly higher acylsugar levels, which contribute to whitefly deterrence. These lines displayed reduced tomato yellow leaf curl Thailand virus (TYLCTHV) accumulation and milder disease symptoms over time. It was found that lines combining virus and vector resistance performed better than those with only Ty-resistance, whitefly resistance, or the susceptible control.
Abstract
Tomato (Solanum lycopersicum) is an essential vegetable crop cultivated worldwide, but its production is highly vulnerable to tomato yellow leaf curl disease (TYLCD), which is transmitted by whiteflies (Bemisia tabaci). Management strategies typically focus on controlling either the virus or its vector. This study evaluates the effectiveness of multi-disease and insect-resistant tomato lines, developed by the World Vegetable Center (WorldVeg), which integrate Ty-1/Ty-3 genes for virus resistance and WF2-10 and WF3-09 genes for whitefly resistance. Virus accumulation, whitefly settling behavior, and adult mortality were assessed among multi-resistant lines, a Ty-resistant line, a whitefly-resistant line, and a susceptible check using preference bioassays, controlled inoculation experiments, and acylsugar quantification. Multi-resistant lines exhibited significantly higher acylsugar concentrations, reduced whitefly preference for settling, and increased whitefly adult mortality. Additionally, these lines displayed less severe disease symptoms and lower virus accumulation over time than Ty-resistant, whitefly-resistant, and susceptible controls. These findings highlight the superior efficacy of combined virus and vector resistance in mitigating tomato yellow leaf curl Thailand virus (TYLCTHV) transmission. This research underscores the importance of integrated genetic resistance as a key element of sustainable integrated pest management strategies, offering an environmentally friendly solution for safeguarding global tomato production.
1. Introduction
Tomato, a globally important vegetable crop, is highly valued for its nutritional richness and culinary versatility. Tomato cultivation spans an area of approximately 5 million hectares worldwide, yielding a production of nearly 186.82 million tonnes with an average productivity of 36.97 tonnes per hectare [1].
Biotic stress in plants includes pests and diseases, which account for significant losses threatening crop yield and productivity [2]. The whitefly, Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae), causes damage to the phloem and physiological disorders such as irregular fruit ripening, which reduces fruit quality and increases the number of unmarketable fruits [3]. Whiteflies excrete honeydew, which supports the growth of sooty mold fungus. However, its role as a plant virus vector causes the most serious damage [4]. Virus species belonging to five different genera (Begomovirus, Crinivirus, Closterovirus, Ipomovirus, and Carlavirus) are transmitted by whiteflies [5]. Among the viruses that can infect tomatoes, tomato yellow leaf curl virus (TYLCV) ranks third after tobacco mosaic virus and tomato spotted wilt virus on the list of the most important plant viruses worldwide [6]. TYLCV and at least 12 TYLCV-like Begomovirus species form a complex of viruses responsible for causing tomato yellow leaf curl disease (TYLCD) in various parts of the world. Tomato yellow leaf curl Thailand virus (TYLCTHV) is the predominant Begomovirus species responsible for TYLCD in Taiwan. TYLCTHV is mainly restricted to the tomato phloem tissues and is transmitted in a persistent circulative manner [7]. TYLCD causes leaf yellowing, curling, stunting, and, in severe cases, premature abscission of flowers and fruits, followed by cessation of plant growth [8]. Early tomato infection can cause up to 100% yield loss [9].
For profitable tomato production, TYLCD management is very important. Possible measures to control the spread and effects of the virus are breeding cultivars resistant to the virus and resistant to the vector. Resistance to TYLCD was found in several wild relatives of tomato, from which six virus resistance genes (Ty-1 to Ty-6) have been identified. These genes have been mapped in wild tomato species including S. chilense (Ty-1, Ty-3, Ty-4 and Ty-6), S. habrochaites syn. L. hirsutum (Ty-2), and S. peruvianum (ty-5). Ty-1 and Ty-2 genes express complete or nearly complete dominance, while Ty-3 shows partial dominance [9]. The ty-5 gene is recessively inherited and results from a loss-of-function mutation [8]. Ty-1 and Ty-3 are the primary resistance genes widely used in tomato breeding programs. The Ty-1/Ty-3 gene combination conferred the highest level of resistance based on disease incidence and severity [9]. Ty-2 is also used in breeding, either alone or in combination with other Ty-genes [8]. Ty genes have been successful in generating TYLCD-resistant commercial tomato cultivars, but generally have only reduced symptom severity, and the resistance achieved has never been 100% [10]. Plant co-infection by multiple Begomovirus strains offers opportunities to recombine and evolve new virulent, resistance-breaking forms of the virus. Another disadvantage of relying solely on Ty genes is that they constitute virus sources for susceptible genotypes [11].
While chemical vector control is ineffective, host plant resistance to insect vectors is suitable for managing circulative vector-borne viral diseases. Durable whitefly resistance, especially in the field, is more likely if tomato cultivars mount resistance based on a combination of antixenosis and antibiosis factors, thus forcing whiteflies to surmount a wide range of plant defenses [12]. Physically, plants deploy trichome and acylsugar-based strategies to restrain whiteflies from feeding. There are two groups of trichomes: glandular trichomes (Type I, IV, VI, and VII trichomes), which have “heads” containing various sticky and/or toxic exudates and secrete acylsugars, and non-glandular trichomes (Type II, III, and V trichomes) that do not secrete acylsugars. Acylsugars confer resistance to a wide range of important pests [12,13,14]. Natural resistance to multiple pests based on secretions by the different types of glandular trichomes present on stems and leaves of tomato and its wild relatives has been described [15]. Resistance is primarily through feeding deterrence, although oviposition deterrence also plays a role for certain pests. The capacity to repel and avoid whitefly landing, probing, and feeding is important to thwart whiteflies as vectors of plant viruses, especially Begomoviruses.
However, vector resistance alone would only lead to reduced virus spread, while the virus can still accumulate in plants without any virus resistance mechanism to slow down its replication. Thus, a holistic approach involving integrated pest management (IPM) is required for the management of TYLCD. IPM can include, along with other measures, host plant resistance to both the whitefly and the virus. Dual resistance in tomato cultivars would be valuable in repelling whiteflies and inhibiting virus replication in the plant, thus helping preserve the durability of virus resistance genes and possibly contributing to slowing Begomovirus evolution.
WorldVeg has developed BC4F5 breeding lines that are multi-disease and insect-resistant. The accession VI007099 of S. galapagense, a close wild relative of the cultivated tomato, is resistant to the whitefly because it possesses type IV glandular trichomes that produce acylsucroses [12]. This resistance trait was introgressed into the multi-disease resistant elite line CLN3682C, and after four steps of recurrent backcrossing with selection for two whitefly resistance markers and acylsugar quantification, breeding lines AVTO2428, AVTO2432, AVTO2437, and AVTO2436 were selected from CLN4636BC4F5. These lines contain Ty-1/Ty-3 genes for virus resistance and WF2-10 and WF3-09 genes for whitefly resistance. The tomato lines carry resistance that confers protection against a broad spectrum of viruses, including TYLCTHV, which is predominant in Taiwan.
So far, limited research has examined the response of insect-resistant lines to the accumulation and spread of viruses causing TYLCD [16,17,18,19]. The reaction of dual-resistant lines to the virus accumulation has not been studied extensively. It remains unclear whether dual resistance against the insect vector and the virus limits Begomovirus accumulation because vector resistance can, in some cases, increase virus transmission [20,21]. However, vector-resistant cultivars have helped reduce the spread of other plant viruses [22]. The specific virus–vector interactions that determine Begomovirus transmission are complex, involving the virus and vector, the host plant, and the environment.
Therefore, this study was conducted to compare the TYLCTHV accumulation in plants combining virus and insect resistance with those resistant to either the insect or the virus alone and susceptible plants.
2. Materials and Methods
The experiment was performed under greenhouse conditions (26.7 ± 3.4 °C temperature; 69.1 ± 3.9% relative humidity) at WorldVeg in Tainan, Taiwan, from 23 February to 22 May 2024.
2.1. Tomato Plants, Virus Isolate, and Whitefly Population
Four multi-disease and insect-resistant lines, AVTO2428, AVTO2432, AVTO2437, and AVTO2436 (S. lycopersicum); one virus-resistant line, AVTO2445 (S. lycopersicum) (stable and advanced line of the recurrent female parent of the multi-disease and insect-resistant lines); one whitefly-resistant check, AVTO2446 (S. galapagense); and one susceptible check, AVTO9304 (S. lycopersicum), were used. The four multi-disease and insect-resistant lines are BC4F5 lines developed by transferring insect resistance from S. galapagense accession into an elite multiple disease-resistant line (including TYLCD resistance). The characteristics of the lines used in the study are summarized in Table 1.

Table 1.
Characteristics of lines used in the study.
Tomato plants infected with TYLCTHV were obtained from the virology department of WorldVeg. In this experiment, we used the LJ3-5 isolate of TYLCTHV, originally collected from Kaohsiung, Taiwan. The complete genomic sequences of this isolate, including both DNA-A and DNA-B components, are publicly available in GenBank under accession numbers EF577266 and EF577267. Healthy B. tabaci individuals (both Q and B biotypes) were obtained from the WorldVeg virology department and reared on cabbage plants (Brassica oleracea var. capitata; “Green Tide” variety) in insect cages in an insect-proof plastic house. Custom-built insect cages (100 cm × 60 cm × 70 cm) enclosed with 50-mesh insect-proof netting were used. Viruliferous whiteflies were obtained by releasing healthy adults on TYLCTHV-infected tomato plants and allowing them to feed for four days.
2.2. Whitefly Preference, Adult Mortality Bioassay, and TYLCTHV Control Inoculation
A choice bioassay was conducted to study the whitefly preference. The photographs depicting the choice assay experimental setup used to evaluate plant preference by adult whiteflies is present in Figures S1 and S2. The 28-day-old seedlings of three plants of each of the 7 genotypes were placed randomly in a circle within insect cages, and the setup was replicated thrice. A total of 70 B. tabaci adult mating pairs were released into the cage, resulting in 10 mating pairs per genotype. The viruliferous whiteflies were released in the center of the circle. The inoculation access period (IAP) was 8 days (192 h). After the IAP, the whitefly adults were counted on the whole plants. Adult mortality (%) was calculated by taking the percentage of the number of dead whiteflies found on the plant to the total number of whiteflies on the plant. The plants were then treated with Confidor insecticide.
2.3. TYLCTHV Accumulation
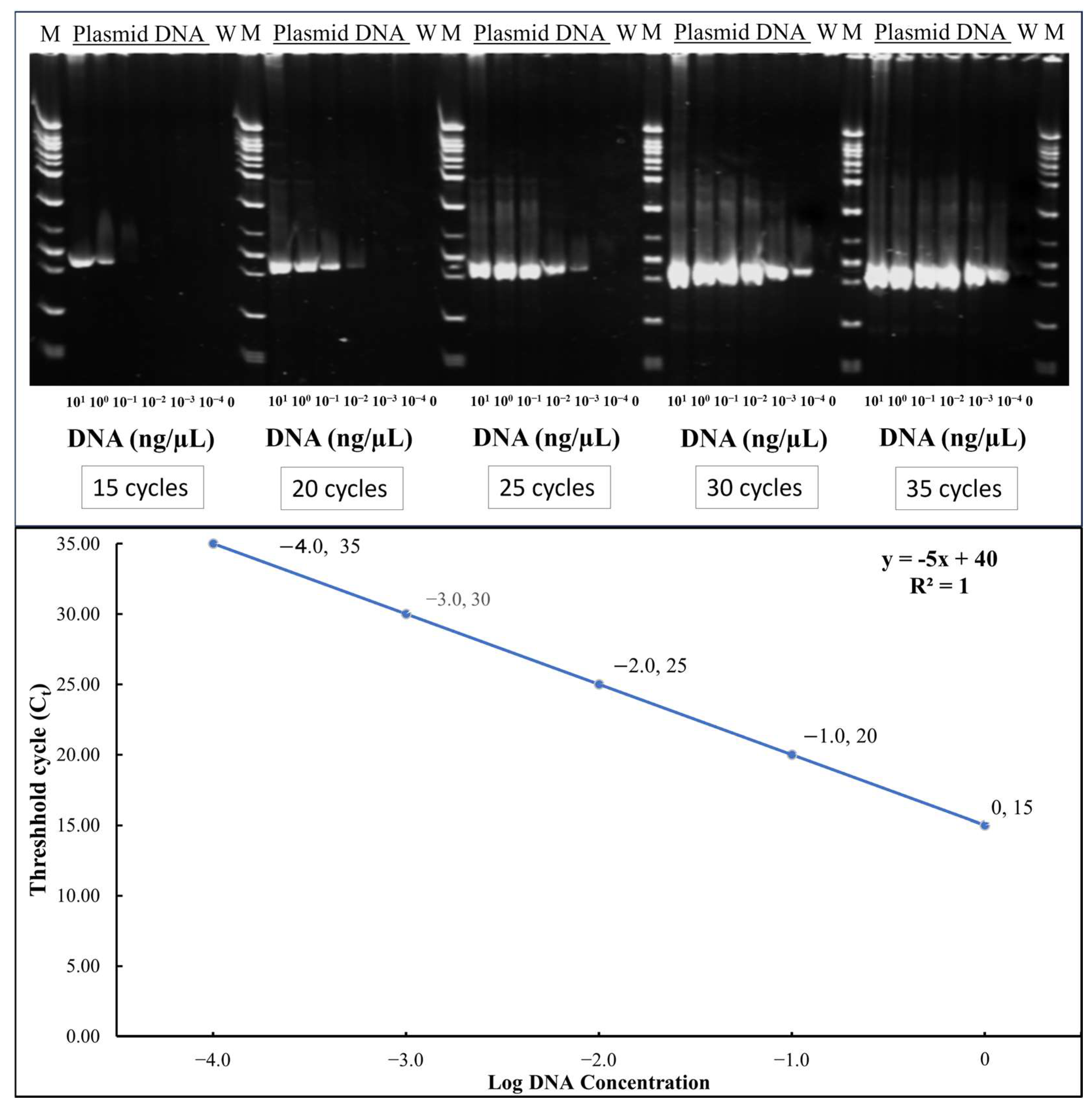
TYLCTHV was detected by semi-quantitative Polymerase Chain Reaction (PCR). The DNA extraction procedure was performed according to Fulton et al. with modifications [23]. A detailed step-by-step protocol for DNA extraction from tomato leaf samples is provided in Text S1. The genomic DNA was quantified through Nanodrop (Thermo Scientific NanoDrop spectrometer, Waltham, MA, USA) and then diluted to 10 ng/μL. The diluted sample was subjected to PCR (Biorad CFX96 Real Time PCR System, Bio-Rad Laboratories, Inc., Hercules, CA, USA). Each plant was sampled and tested for the presence and amount of TYLCTHV in the plants (3-, 5- and 20-day post-inoculation) by comparing with the standard curve developed using serially diluted plasmid DNA at different cycles as shown in Figure 1.
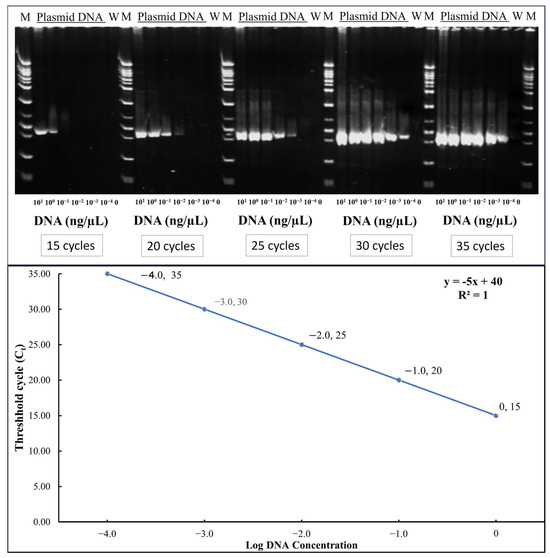
Figure 1.
Gel electrophoresis of PCR products of serially diluted plasmid DNA at different cycles and standard curve: threshold cycles(Ct) vs. log DNA concentration (y = −5x + 40). The values shown represent the log-transformed DNA concentration with conversions as follows: Log −4.0 = 0.0001 ng/μL; Log −3.0 = 0.001 ng/μL; Log −2.0 = 0.01 ng/μL; Log −1.0 = 0.1 ng/μL; Log 0.0 = 1 ng/μL.
To assess relative viral load, PCR was conducted for each sample at four different cycle numbers: 15, 20, 25, and 30. Amplified products were run through agarose gel electrophoresis, and band presence or absence was assessed. This was compared against a standard curve generated using serial dilutions of plasmid DNA (1, 10−1, 10−2, 10−3, and 10−4 ng/μL). Scored electrophoresis data is in File S1 and raw gel images are available in File S2. As shown in Figure 1, plasmid DNA at 1 ng/μL yielded visible bands at all four cycle numbers, while lower concentrations showed amplification at progressively higher cycles. Samples showing bands at all four cycles were interpreted to contain at least 1 ng/μL of viral DNA. PCR amplification was carried out in a 10 μL reaction volume containing 6.0 μL of double-distilled water, 1.0 μL of 10× PCR buffer, 0.6 μL of dNTPs (Premix dNTP, 2.5mM; Protech Technology Enterprise Co., Ltd., Taipei City, Taiwan), 0.1 μL each of forward (GGACATGCAGGTGAGGAGTCC) and reverse primers (TTATACGGATGGCCGCTTT), 0.2 μL of Taq DNA polymerase (Super-Therm Gold DNA Polymerase; JMR Holdings Inc., West Midlands, UK), and 2.0 μL of DNA template. The thermal cycling conditions consisted of an initial denaturation at 95 °C for 10 min, followed by 15, 20, 25, or 30 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 45 s, and extension at 72 °C for 45 s. A final extension step was performed at 72 °C for 5 min.
2.4. Disease Scoring
The plants were phenotypically evaluated and scored based on the severity of the disease symptoms using a 1–6 scale as shown in Figure 2.

Figure 2.
Visual representation of the 1–6 phenotyping scale for scoring disease severity in plants. 1 = Healthy; 2 = Very mild; 3 = Mild; 4 = Moderate; 5 = Severe; 6 = Very severe.
2.5. Acylsugar Assay
A standard peroxidase/glucose oxidase (PGO)-based acylsugar assay was performed following the methodology described by Savory [24]. The protocol for acylsugar quantification in plant samples is provided in Text S2.
2.6. Statistical Analysis
Data were subjected to statistical analysis using SPSS for Windows, version 25.0 (SPSS Inc., Chicago, IL, USA). The data were checked for normality and variance homogeneity using Shapiro–Wilk and Levene’s tests, respectively. A Sqrt(x) transformation was applied to normalize the adult whitefly data, whereas an arcsine(x) transformation was applied to adult mortality (%). Analysis of variance (ANOVA) was performed, and the means were compared through Tukey’s Honestly Significant Difference (HSD) test (p < 0.05). The Kruskal–Wallis test was performed for acylsugar content, virus accumulation, and disease severity index, followed by Dunn’s test (p < 0.05) for pairwise comparison wherever statistically significant differences were found.
3. Results
A series of controlled greenhouse experiments were conducted to assess the response of multi-disease and insect-resistant tomato lines to the accumulation of TYLCTHV. Summary of the experimental results are provided in File S3. The results are summarized below.
3.1. Acylsugar Content in Leaves
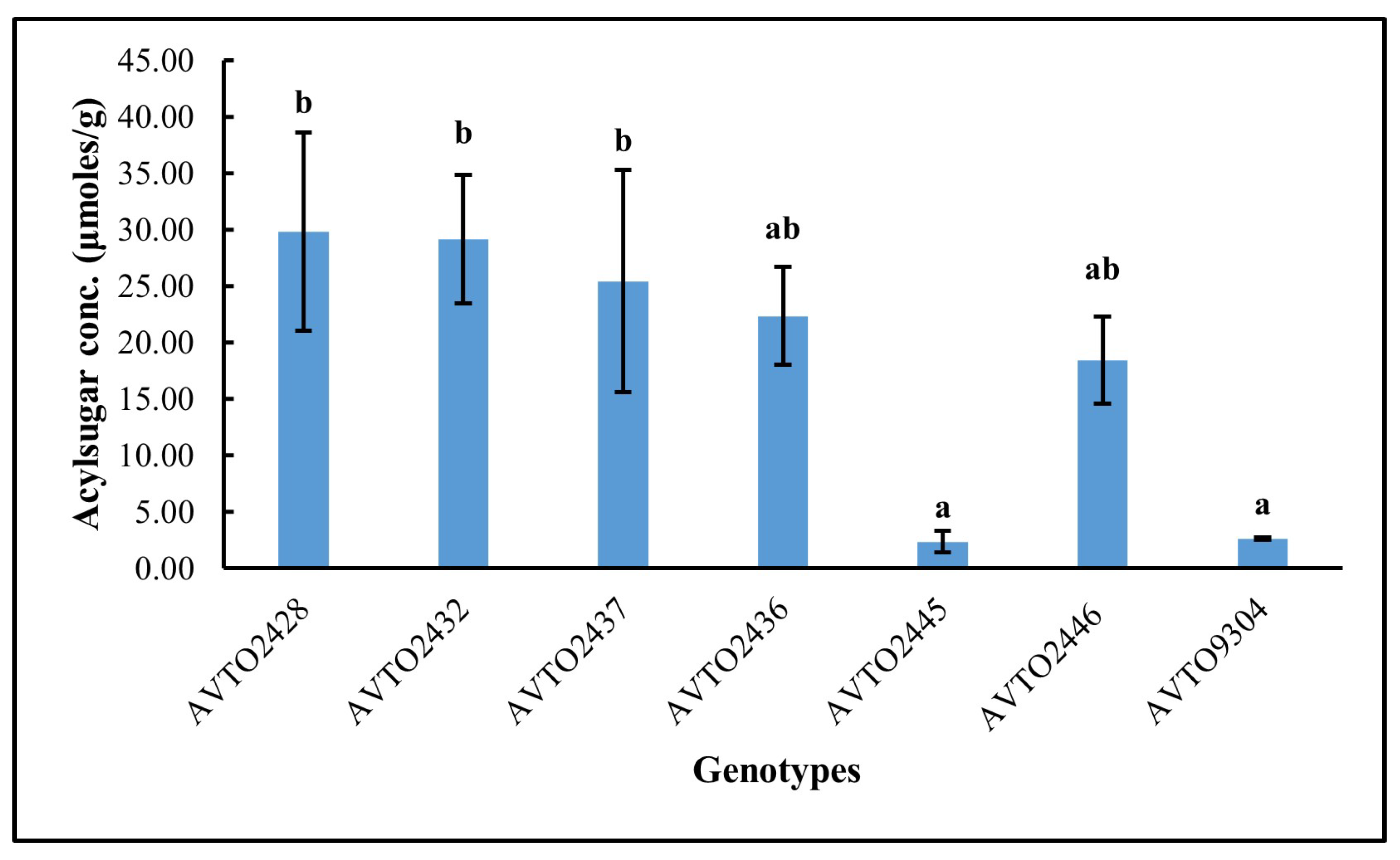
The total acylsugar concentration in different genotypes is presented in Figure 3. The total acylsugar concentration was lower in the genotypes AVTO2445 and AVTO9304. It was highest in AVTO2428, followed by AVTO2432, AVTO2437, AVTO2436, and AVTO2446. A Kruskal–Wallis test indicated a significant difference in acylsugar content across the seven genotypes (df = 6, N = 21; test statistic χ2 = 14.892, p = 0.021). The acylsugar concentrations of AVTO2445 and AVTO9304 were not significantly different from each other but were significantly lower than the multi-disease and insect-resistant lines, viz., AVTO2428, AVTO2432, and AVTO2437.
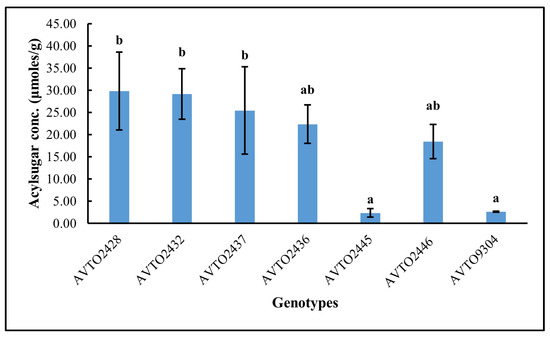
Figure 3.
Total acylsugar concentration (μmoles/g) across genotypes. Different letters indicate statistical differences according to Dunn’s post hoc tests (p < 0.05). Error bars represent standard deviation.
3.2. Whitefly Preference Assay
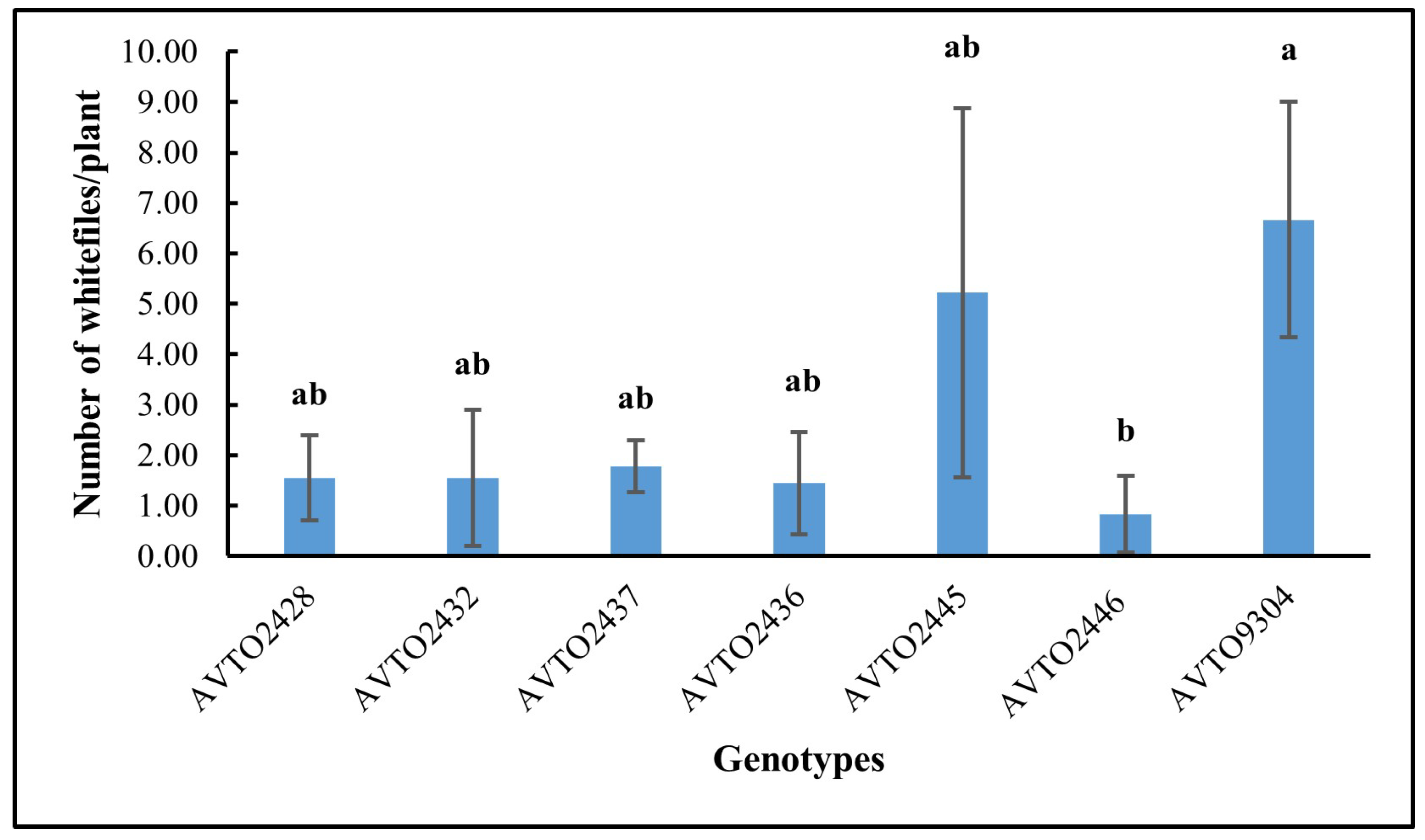
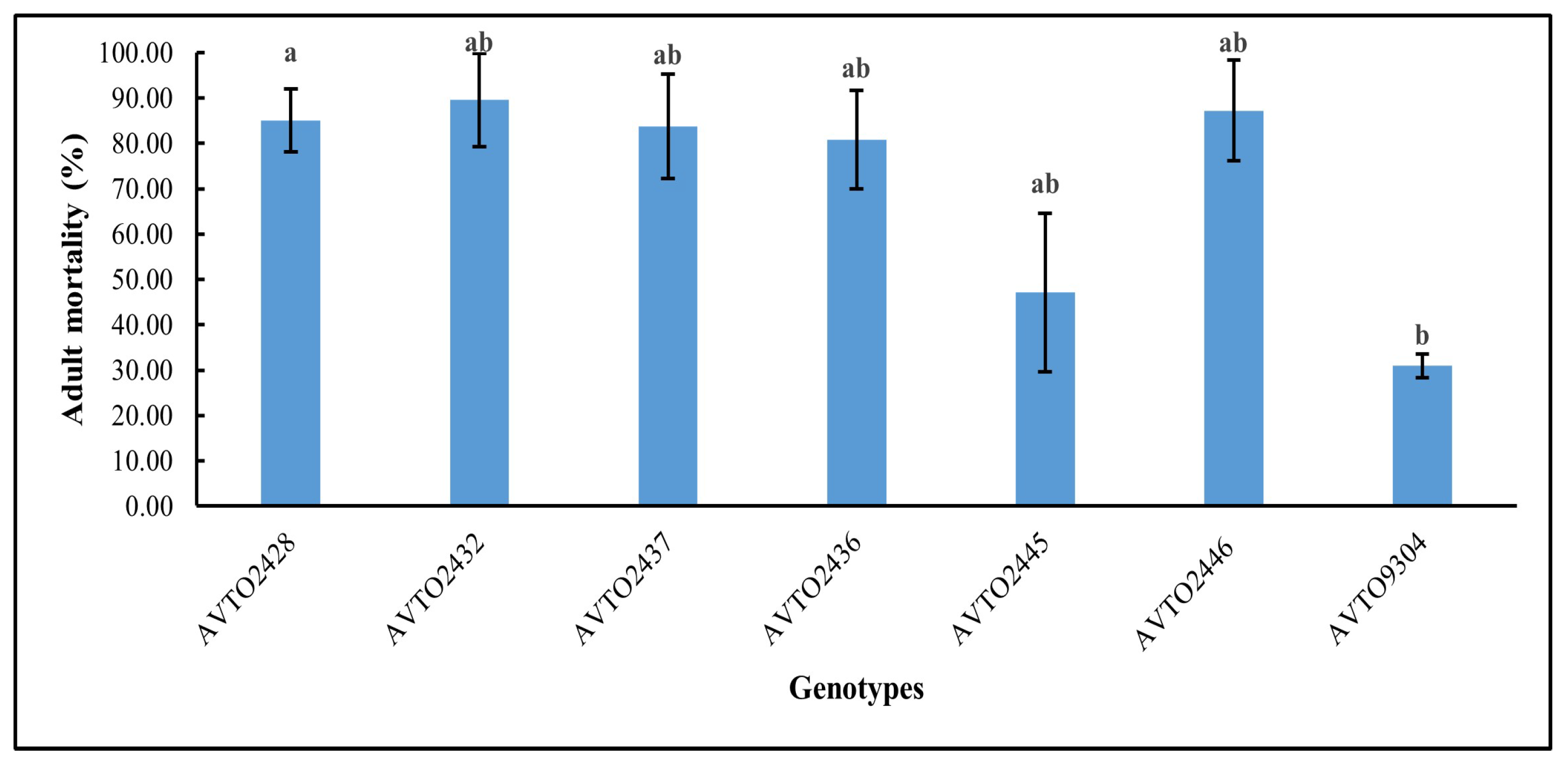
The genotype AVTO2446 attracted the fewest whitefly adults in the choice assay as shown in Figure 4. A significant difference was found between genotypes [F(6,14) = 3.745, p = 0.020], with AVTO2446 (M = 0.74, SD = 0.65) differing significantly from AVTO9304 (M = 2.55, SD = 0.48). However, AVTO2428, AVTO2432, AVTO2437, AVTO2436, and AVTO2445 did not differ significantly from one another, or from AVTO2446 or AVTO9304. Adult mortality (%) was also higher in the multi-disease and insect-resistant lines (Figure 5).
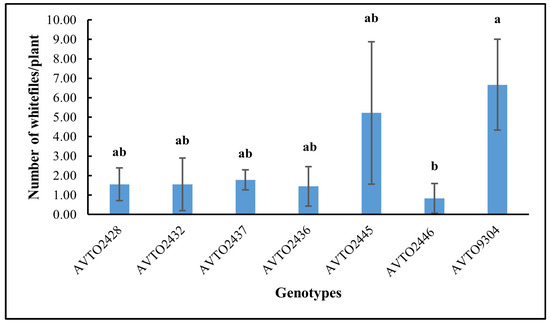
Figure 4.
Average number of adult whiteflies per plant across genotypes. Different letters indicate statistical differences according to Tukey’s HSD test (p < 0.05). Error bars represent standard deviation.
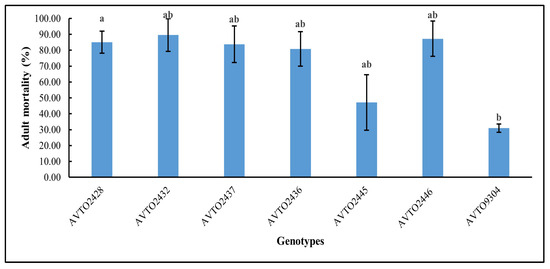
Figure 5.
Percentage of adult whitefly mortality in different plant genotypes. Different letters indicate statistical differences according to Tukey’s HSD test (p < 0.05). Error bars represent standard deviation.
3.3. TYLCTHV Accumulation
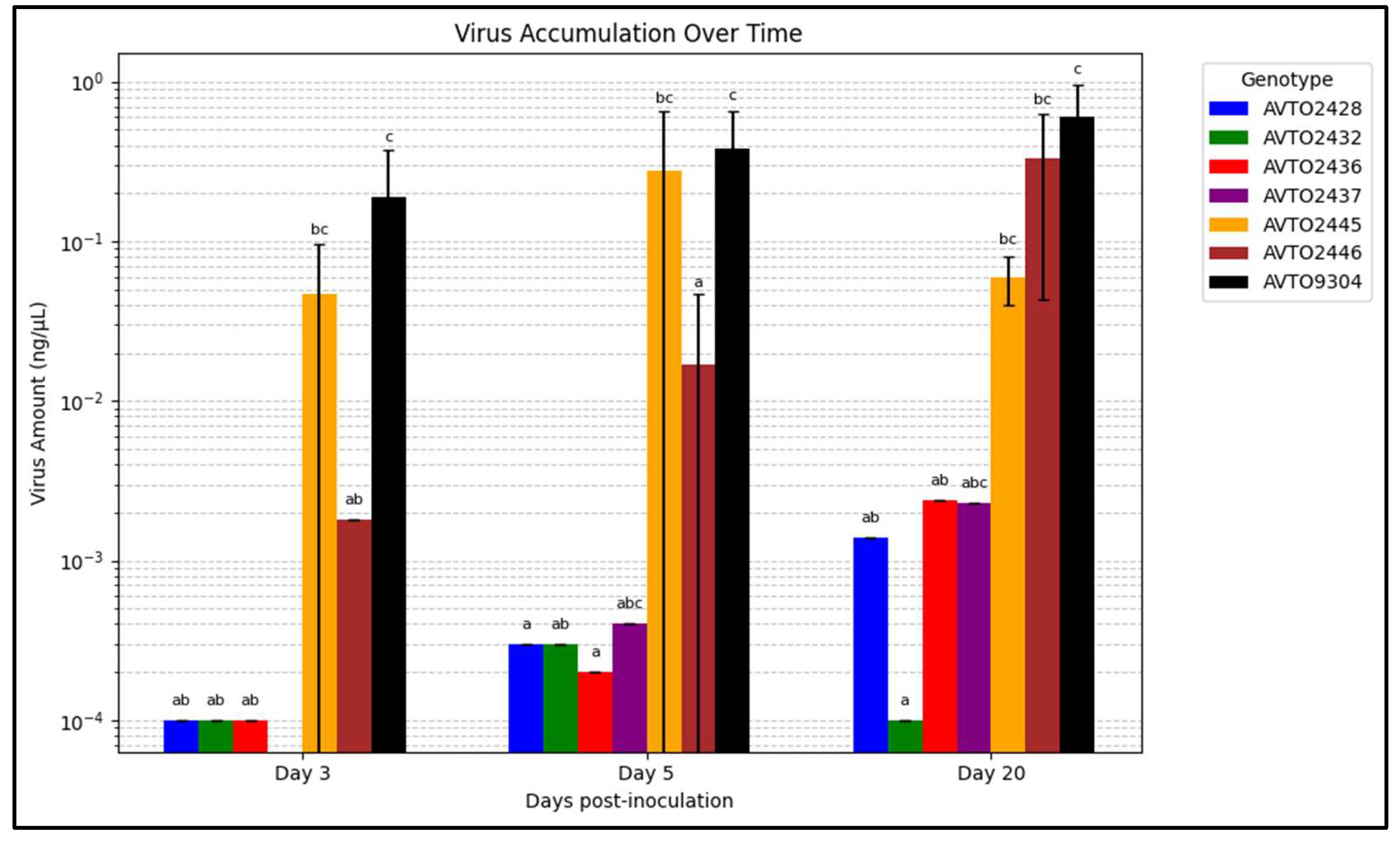
Figure 6 summarizes the viral load on various days post-inoculation. The multi-disease and insect-resistant plants consistently displayed lower viral loads than AVTO2445, AVTO2446, and AVTO9304. At 20 days post-inoculation (dpi), the genotype AVTO2432 had significantly lower virus amounts than AVTO2445, AVTO2446, and AVTO9304.
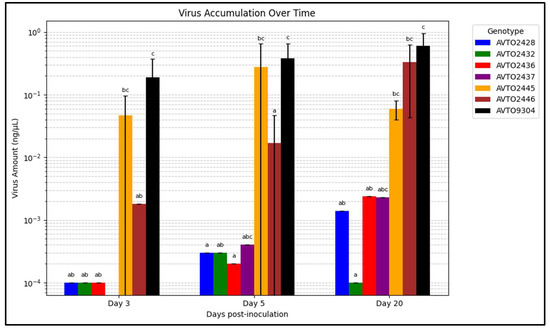
Figure 6.
Graph illustrating the change in virus accumulation over time. Different letters indicate statistical differences according to Dunn’s tests (p < 0.05). Error bars represent standard deviation.
3.4. Disease Scoring
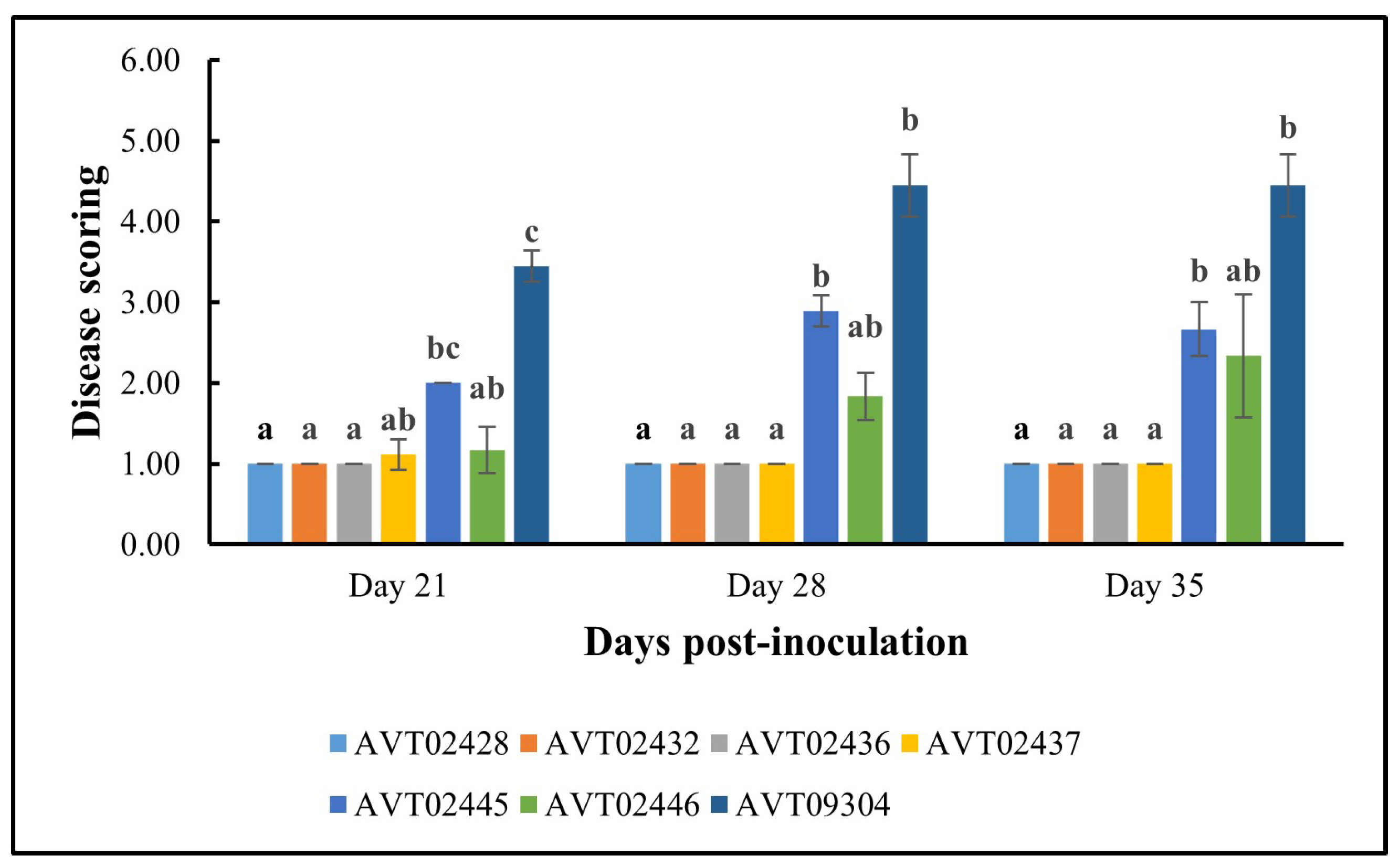
Figure 7 summarizes the disease scoring results. The multi-disease and insect-resistant plants consistently showed lower disease scores and none to very mild symptoms. At 35 dpi, the effectiveness of multi-disease and insect-resistant plants is demonstrated by an average severity rating of 1, indicating that all plants were healthy. In contrast, single-resistant plants and the susceptible check had average severity ratings of 2.50 and 4.44, respectively. At 35 dpi, all four lines differed significantly in disease severity from AVTO2445 and AVTO9304.
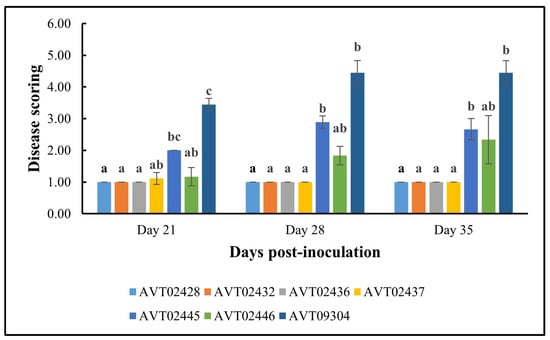
Figure 7.
Results of disease severity scores for different plant genotypes using a 1–6 scale. 1 = Healthy; 6 = Very severe. Different letters indicate statistical differences according to Dunn’s tests (p < 0.05). Error bars represent standard deviation.
4. Discussion
This study demonstrates that combining resistance to B. tabaci based on acylsugar secretion with virus resistance genes (Ty-1/Ty-3) in multi-disease and insect-resistant tomato lines significantly reduced the accumulation of the TYLCTHV compared to plants carrying either insect or virus resistance alone and, as expected, to the susceptible check.
The choice bioassay is expected to reveal lines that confer both antixenosis and antibiosis resistance mechanisms [25]. The initial host plant selection by adults is primarily influenced by preference factors. The preference assays confirmed that the lines with high acylsugar secretion like AVTO2428, AVTO2432, AVTO2437, AVTO2436, and AVTO2446 were less preferred, whereas lines AVTO2445 and AVTO9304 were more preferred. Previous studies have suggested that glandular trichomes on leaves and acylsugar secretion were associated with reduced attractiveness and resistance to B. tabaci [12,15,16,17,26,27,28]. Our results are aligned with these findings, with lines showing higher acylsugar concentration being less preferred by whiteflies. Whitefly feeding, besides vectoring viruses, causes a range of other disorders. The multi-disease and insect-resistant lines might help to reduce the irregular ripening disorder caused by B. tabaci on tomatoes by lowering whitefly infestations [17]. Host resistance to whiteflies presumably also minimizes the spread of other viruses transmitted by whiteflies, such as tomato chlorosis virus [29], thereby lowering the risk of the emergence of recombinant viruses due to mixed infection. Due to the combination of different resistance mechanisms, multi-disease and insect-resistant lines might also exert lower selection pressure on the virus to evolve [30].
Resistance to adult B. tabaci in the tomato lines was associated with high acylsugar concentration in these lines, which reduced the plant’s attractiveness to the insect and, therefore, reduced settling and oviposition. Rodríguez-López et al. (2011) [17] found that the insect-resistant plants showing deterrence due to trichomes and acylsugar concentrations alter the feeding behavior of the insect after it lands on a plant and affect virus acquisition by decreasing the ability to start probing. The tested whitefly resistance did not completely block virus acquisition and transmission in vector-resistant plants but significantly reduced the initial viral concentration. Hence, combining vector resistance with virus resistance to reduce disease development is necessary.
The graph of virus accumulation over time indicated that when the whiteflies were given a free choice between the genotypes, more whiteflies were attracted to the susceptible check AVTO9304. This leads to high initial virus inoculation. Coupled with the lack of any virus resistance mechanism to reduce viral multiplication, higher virus accumulation was seen in the susceptible check. In the virus-resistant genotype AVTO2445, the viral titer was found to be as high as in the susceptible genotypes at the initial stages, due to whitefly-mediated inoculation. However, despite the high initial viral load, further virus multiplication appears to be suppressed, likely due to the presence of Ty-1/Ty-3 resistance genes. This suggests that the genes do not prevent initial infection but play a significant role in limiting viral replication and reducing symptom severity. In the vector-resistant genotype AVTO2446, the initial virus concentration is low due to whitefly resistance, but due to the absence of any mechanism to reduce viral multiplication, even a small initial viral load multiplies at a high rate, resulting in high virus accumulation over time and leading to disease development. In contrast, the multi-disease and insect-resistant lines consistently showed lower virus concentrations. At 20 dpi, the multi-disease and insect-resistant line AVTO2432 performed significantly better than the single-resistant plants and the susceptible check. This could be attributed to the combined resistance targeting the virus and its vector, creating a more comprehensive barrier against TYLCTHV infection and accumulation. These cultivars deter whitefly infestation and exhibit significant efficacy in impeding viral proliferation within the plant tissues.
Lower viral loads corresponded with reduced symptom severity, demonstrating the effectiveness of multi-disease and insect resistance in alleviating disease symptoms. The mild symptoms observed in the dual-resistant plants can be attributed to their enhanced ability to restrict virus replication and movement within the plant tissues and reduced virus acquisition potential.
In conclusion, this study demonstrates that the multi-disease and insect-resistant tomato lines significantly reduce the accumulation of TYLCTHV and disease symptoms. The high acylsugar concentration reduces the preference of whiteflies for settling, resulting in reduced initial viral load. Together with virus resistance, this leads to lower virus accumulation over time and less severe symptoms, underscoring the effectiveness of these lines in managing TYLCTHV. These lines offer a robust and sustainable solution to prevent TYLCD damage in tomato breeding programs and can reduce the reliance on chemical controls to combat disorders caused by whiteflies. The study highlights the potential of multi-disease and insect-resistant tomato lines as a key component of IPM strategies to manage whitefly-transmitted TYLCTHV effectively. These lines are a breakthrough in resistance breeding for TYLCD management. The promising results from this study pave the way for further research and development of multi-disease and insect-resistant lines to combat TYLCD and other whitefly-transmitted viral diseases.
Further research should focus on conducting field trials under diverse environmental conditions and with different virus complexes causing TYLCD to validate the effectiveness of these lines in real-world agricultural settings, exploring the long-term durability of resistance and the potential for resistance breakdown. Studies should be conducted to assess the impact on fruit production, both in controlled conditions comparing controls and inoculated samples, and in field conditions. Further, the effect of pyramiding insect resistance with other Ty resistance genes and resistance genes for other viruses of tomato into these multi-disease and insect-resistant lines needs to be investigated to optimize the implementation of these resistant lines as an IPM component to maximize their benefits in agricultural practices. Further research should also focus on improving the horticultural traits such as fruit size and yield according to the market demands for better adoption by farmers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects16070721/s1, Figure S1: Photo of choice assay setup 1. Photograph depicting the choice assay experimental setup used to evaluate plant preference by adult whiteflies; Figure S2: Photo of choice assay setup 2. Photograph depicting the choice assay experimental setup used to evaluate plant preference by adult whiteflies; File S1: Scored electrophoresis images in Excel; File S2: Raw gel electrophoresis images; File S3: Summary of experimental results; Text S1: Protocol for DNA extraction from tomato leaf samples using a 96-well plate. Detailed step-by-step methodology for extracting DNA from tomato leaf tissues; Text S2: Protocol for acylsugar quantification in plant samples.
Author Contributions
Writing—original draft, S.S.P.; Writing—review & editing, M.S.P., S.O., S.R. and A.E.; Supervision, Y.-C.H., J.Y., H.-Y.C., M.-Y.L. and A.E. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded by the long-term strategic donors to the World Vegetable Center, Taiwan: UK aid from the UK Government, United States Agency for International Development (USAID), Australian Centre for International Agricultural Research (ACIAR), Germany, Thailand, Philippines, Korea, and Japan.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We are grateful to the World Vegetable Center, Shanhua, Taiwan, for providing the resources and facilities essential for conducting this research. We sincerely appreciate all the technical staff and team members contributing to the experimental setup and data collection. Special thanks are extended to our colleagues for their insightful discussions and constructive feedback during the preparation of this manuscript. Finally, we acknowledge the funding sources and administrative support that made this work possible. Their contributions were invaluable in advancing our understanding of biotic stress in tomato crop production.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#home (accessed on 19 May 2024).
- FAO. FAO’s Plant Production and Protection Division; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Brown, J.K.; Frohlich, D.R.; Rosell, R.C. The sweet potato or silverleaf whiteflies: Biotypes of Bemisia tabaci or a species complex? Annu. Rev. Entomol. 1995, 40, 511–534. [Google Scholar] [CrossRef]
- Brown, J.K.; Czosnek, H. Whitefly transmission of plant viruses. In Advances in Botanical Research; Plumb, R.T., Ed.; Plant Virus Vector Interactions; Academic Press: New York, NY, USA, 2002; Volume 36, pp. 65–100. [Google Scholar] [CrossRef]
- Navas-Castillo, J.; Fiallo-Olivé, E.; Sánchez-Campos, S. Emerging virus diseases transmitted by whiteflies. Annu. Rev. Phytopathol. 2011, 49, 219–248. [Google Scholar] [CrossRef] [PubMed]
- Rybicki, E.P. A top ten list for economically important plant viruses. Arch. Virol. 2015, 160, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Mou, D.F.; Hsieh, C.K.; Weng, S.H.; Tsai, W.S.; Tsai, C.W. Vector transmission of tomato yellow leaf curl Thailand virus by the whitefly Bemisia tabaci: Circulative or propagative? Insects 2021, 12, 181. [Google Scholar] [CrossRef]
- Yan, Z.; Wolters, A.M.A.; Navas-Castillo, J.; Bai, Y. The global dimension of Tomato yellow leaf curl disease: Current status and breeding perspectives. Microorganisms 2021, 9, 740. [Google Scholar] [CrossRef]
- Dhaliwal, M.S.; Jindal, S.K.; Sharma, A.; Prasanna, H.C. Tomato yellow leaf curl virus disease of tomato and its management through resistance breeding: A review. J. Hortic. Sci. Biotechnol. 2020, 95, 425–444. [Google Scholar] [CrossRef]
- Vidavski, F.; Czosnek, H.; Gazit, S.; Levy, D.; Lapidot, M. Pyramiding of genes conferring resistance to Tomato yellow leaf curl virus from different wild tomato species. Plant Breed. 2008, 127, 625–631. [Google Scholar] [CrossRef]
- Srinivasan, R.; Riley, D.; Diffie, S.; Sparks, A.; Adkins, S. Whitefly population dynamics and evaluation of whitefly-transmitted tomato yellow leaf curl virus (TYLCV)-resistant tomato genotypes as whitefly and TYLCV Reservoirs. J. Econ. Entomol. 2012, 105, 1447–1456. [Google Scholar] [CrossRef]
- Rakha, M.; Hanson, P.; Ramasamy, S. Identification of resistance to Bemisia tabaci Genn. in closely related wild relatives of cultivated tomato based on trichome type analysis and choice and no-choice assays. Genet. Resour. Crop Evol. 2017, 64, 247–260. [Google Scholar] [CrossRef]
- Rakha, M.; Bouba, N.; Ramasamy, S.; Regnard, J.L.; Hanson, P. Evaluation of wild tomato accessions (Solanum spp.) for resistance to two-spotted spider mite (Tetranychus urticae Koch) based on trichome type and acylsugar content. Genet. Resour. Crop Evol. 2017, 64, 1011–1022. [Google Scholar] [CrossRef]
- Rakha, M.; Zekeya, N.; Sevgan, S.; Musembi, M.; Ramasamy, S.; Hanson, P. Screening recently identified whitefly/spider mite-resistant wild tomato accessions for resistance to Tuta absoluta. Plant Breed. 2017, 136, 562–568. [Google Scholar] [CrossRef]
- Simmons, A.T.; Gurr, G.M. Trichomes of Lycopersicon species and their hybrids: Effects on pests and natural enemies. Agric. For. Entomol. 2005, 7, 265–276. [Google Scholar] [CrossRef]
- Marchant, W.G.; Legarrea, S.; Smeda, J.R.; Mutschler, M.A.; Srinivasan, R. Evaluating acylsugars-mediated resistance in tomato against Bemisia tabaci and transmission of Tomato yellow leaf curl virus. Insects 2020, 11, 842. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, M.J.; Garzo, E.; Bonani, J.P.; Fereres, A.; Fernández-Muñoz, R.; Moriones, E. Whitefly resistance traits derived from the wild tomato Solanum pimpinellifolium affect the preference and feeding behavior of Bemisia tabaci and reduce the spread of Tomato yellow leaf curl virus. Phytopathology 2011, 101, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Romanow, L.R.; Moyer, J.W.; Kennedy, G.G. Alteration of efficiencies of acquisition and inoculation of watermelon mosaic virus 2 by plant resistance to the virus and to an aphid vector. Phytopathology 1986, 76, 1276–1281. [Google Scholar] [CrossRef]
- Yao, Q.; Peng, Z.; Tong, H.; Yang, F.; Xing, G.; Wang, L. Tomato plant flavonoids increase whitefly resistance and reduce spread of tomato yellow leaf curl virus. J. Econ. Entomol. 2019, 112, 2790–2796. [Google Scholar] [CrossRef]
- Atiri, G.I.; Ekpo, E.J.A.; Thottappilly, G. The effect of aphid-resistance in cowpea on infestation and development of Aphis craccivora and the transmission of cowpea aphid-borne mosaic virus. Ann. Appl. Biol. 1984, 104, 339–346. [Google Scholar] [CrossRef]
- Diaz-Montano, J.; Fuchs, M.; Nault, B.A.; Shelton, A.M. Resistance to onion thrips (Thysanoptera: Thripidae) in onion cultivars does not prevent infection by Iris Yellow Spot Virus following vector-mediated transmission. Fla. Entomol. 2012, 95, 156–161. [Google Scholar] [CrossRef]
- Maris, P.C.; Joosten, N.N.; Goldbach, R.W.; Peters, D. Restricted spread of Tomato spotted wilt virus in thrips-resistant pepper. Phytopathology 2003, 93, 1223–1227. [Google Scholar] [CrossRef]
- Fulton, T.M.; Chunwongse, J.; Tanksley, S.D. Microprep protocol for extraction of DNA from tomato and other herbaceous plants. Plant Mol. Biol. Report. 1995, 13, 207–209. [Google Scholar] [CrossRef]
- Savory, E.A. Modification of the PGO Assay for Use in Acylsugar Quantification; Cornell University: Ithaca, NY, USA, 2004; p. 36. [Google Scholar]
- Baldin, E.L.L.; Beneduzzi, R.A. Characterization of antibiosis and antixenosis to the whitefly silverleaf Bemisia tabaci B biotype (Hemiptera: Aleyrodidae) in several squash varieties. J. Pest Sci. 2010, 83, 223–229. [Google Scholar] [CrossRef]
- Dias, D.M.; Resende, J.T.V.; Marodin, J.C.; Matos, R.; Lustosa, I.F.; Resende, N.C.V. Acyl sugars and whitefly (Bemisia tabaci) resistance in segregating populations of tomato genotypes. Genet. Mol. Res. 2016, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Resende, J.T.D.; Maluf, W.R.; Cardoso, M.D.G.; Gonçalves, L.D.; Faria, M.V.; Nascimento, I.R.D. Resistance of tomato genotypes to the silverleaf whitefly mediated by acylsugars. Hortic. Bras. 2009, 27, 345–348. [Google Scholar] [CrossRef]
- Rodríguez-López, M.J.; Garzo, E.; Bonani, J.P.; Fernández-Muñoz, R.; Moriones, E.; Fereres, A. Acylsucrose-producing tomato plants forces Bemisia tabaci to shift its preferred settling and feeding site. PLoS ONE 2012, 7, e33064. [Google Scholar] [CrossRef]
- Fortes, I.M.; Fernández-Muñoz, R.; Moriones, E. Host plant resistance to Bemisia tabaci to control damage caused in tomato plants by the emerging crinivirus tomato chlorosis virus. Front. Plant Sci. 2020, 11, 585510. [Google Scholar] [CrossRef]
- Van den Bosch, F.; Akudibilah, G.; Seal, S.; Jeger, M. Host resistance and the evolutionary response of plant viruses. J. Appl. Ecol. 2006, 43, 506–516. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).