Variation in a Host–Parasitoid Interaction across Independent Populations
Abstract
:1. Introduction
2. Material and Methods
2.1. The Butterfly and the Parasitoid
2.2. The Study Populations

| Åland (AL) | Uppland (UP) | Öland (OL) * | Saaremaa (SA) | Pikku Tytärsaari (PT) ** | |
|---|---|---|---|---|---|
| Area (km2) | 1,480 | >1,000 | 1,342 | 2,673 | 2.5 |
| Landscape type | fragmented | fragmented | continuous | continuous | small, isolated |
| Butterfly inbreeding a | yes | presumed yes | no | no | yes |
| Wasp inbreeding b | yes | presumed yes | presumed no | no | yes |
2.3. The Reciprocal Parasitism Experiment
2.4. Brood Size, Pupal Weight and Longevity of C. melitaearum
2.5. Statistical Analyses
3. Results
3.1. Early Parasitoid Development and Encapsulation
| Initial brood size | Fraction encapsulated | Fraction eggs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | DF | SS | F | p | SS | F | p | SS | F | p | |
| Model | 31 | 67.95 | 2.30 | <0.001 | 5.61 | 1.32 | 0.132 | 7.08 | 3.90 | <0.001 | |
| Wasp origin | 3 | 13.45 | 4.70 | 0.004 | 0.27 | 0.66 | 0.577 | 0.87 | 4.69 | 0.009 | |
| Host origin | 3 | 3.25 | 1.13 | 0.336 | 0.17 | 0.42 | 0.735 | 1.05 | 3.01 | 0.003 | |
| Host family (origin) | 16 | 37.63 | 2.47 | 0.002 | 3.67 | 1.68 | 0.054 | 8.57 | 2.76 | 0.002 | |
| Host x wasp origin | 9 | 16.49 | 1.92 | 0.052 | 1.27 | 1.03 | 0.417 | 1.84 | 0.005 | ||
| Error | 173 | 164.95 | 23.62 | 12.84 | |||||||
3.2. Parasitoid Development at Host Diapause
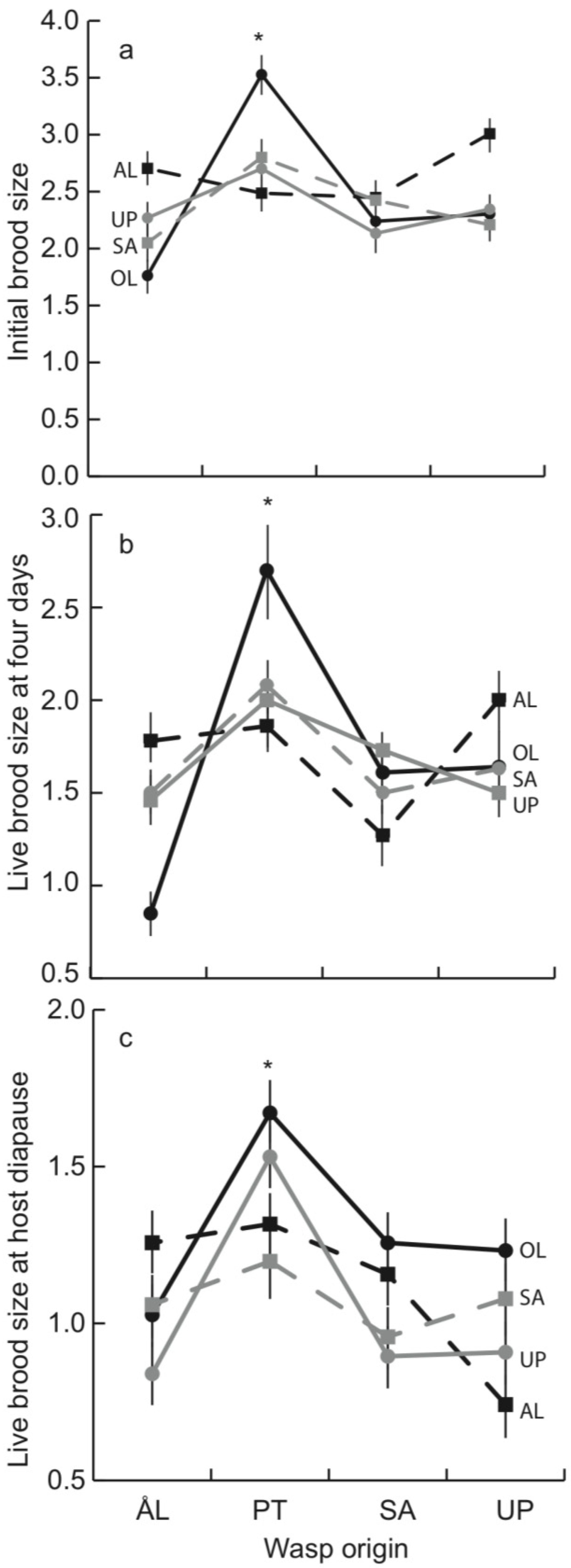
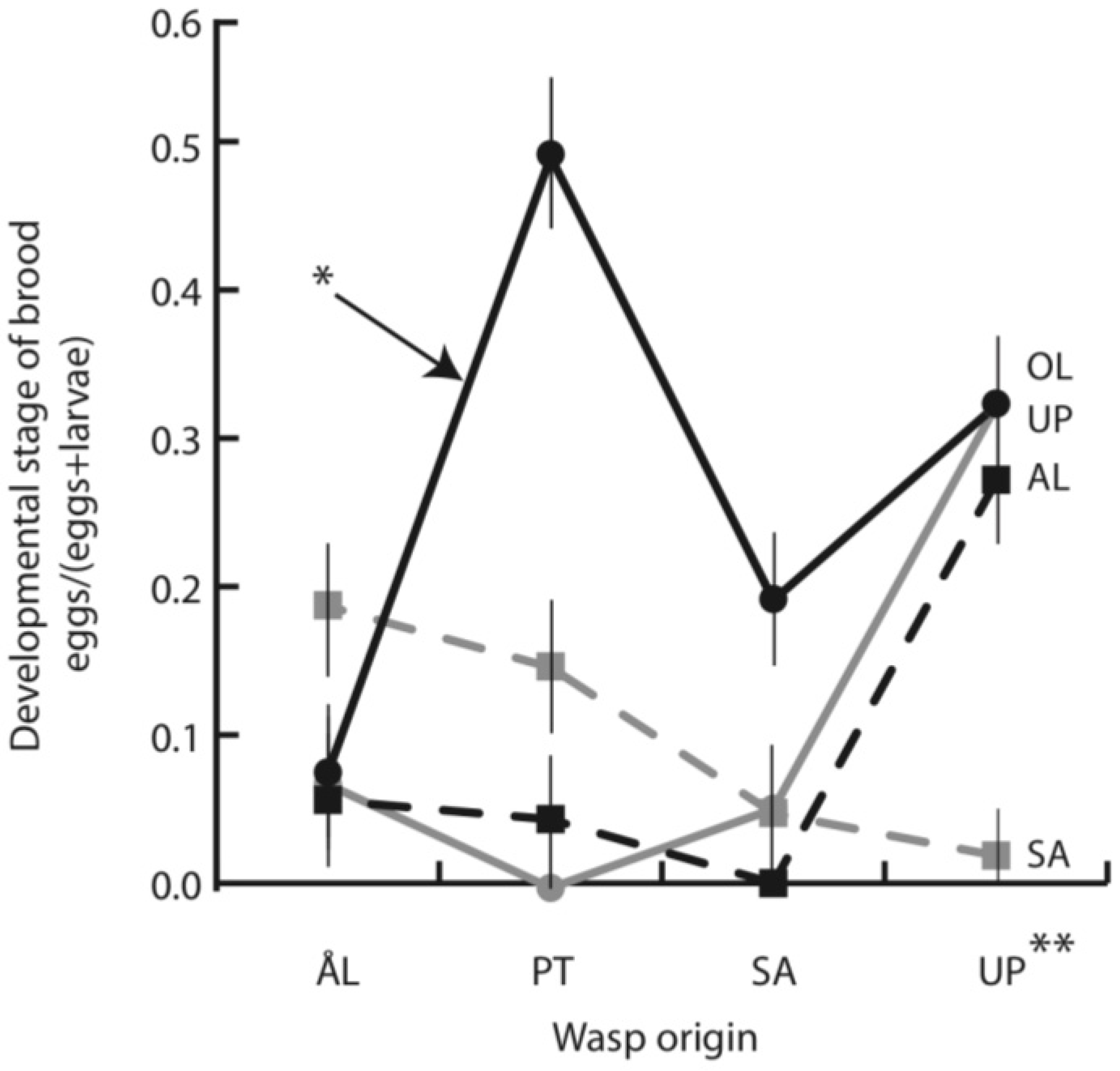
| Live brood size | Fraction encapsulated | Fraction mature in autumn | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | DF | SS | F | p | SS | F | p | SS | F | p |
| Model | 31 | 88.39 | 2.45 | <0.001 | 17.09 | 3.68 | <0.001 | 16.42 | 4.35 | <0.001 |
| Wasp origin | 3 | 12.41 | 3.55 | 0.015 | 0.80 | 1.78 | 0.150 | 4.18 | 11.46 | <0.001 |
| Host origin | 3 | 3.94 | 1.13 | 0.337 | 1.67 | 3.73 | 0.011 | 1.42 | 3.91 | 0.009 |
| Host family (origin) | 16 | 64.87 | 3.48 | <0.001 | 13.00 | 5.43 | <0.001 | 8.90 | 4.57 | <0.001 |
| Host x wasp origin | 9 | 7.39 | 0.70 | 0.704 | 1.40 | 1.04 | 0.405 | 1.65 | 1.51 | 0.143 |
| Error | 382 | 444.91 | 57.6 | 46.42 | ||||||
| Whole brood encapsulation | |||
|---|---|---|---|
| DF | χ2 | p | |
| Model | 31 | 73.83 | <0.001 |
| Wasp origin | 3 | 3.57 | 0.312 |
| Host origin | 3 | 14.77 | 0.002 |
| Host family (origin) | 16 | 48.87 | <0.001 |
| Host x wasp origin | 9 | 6.8 | 0.658 |
| Full-reduced log likelihood = 36.92; AICc = 568.21 | |||
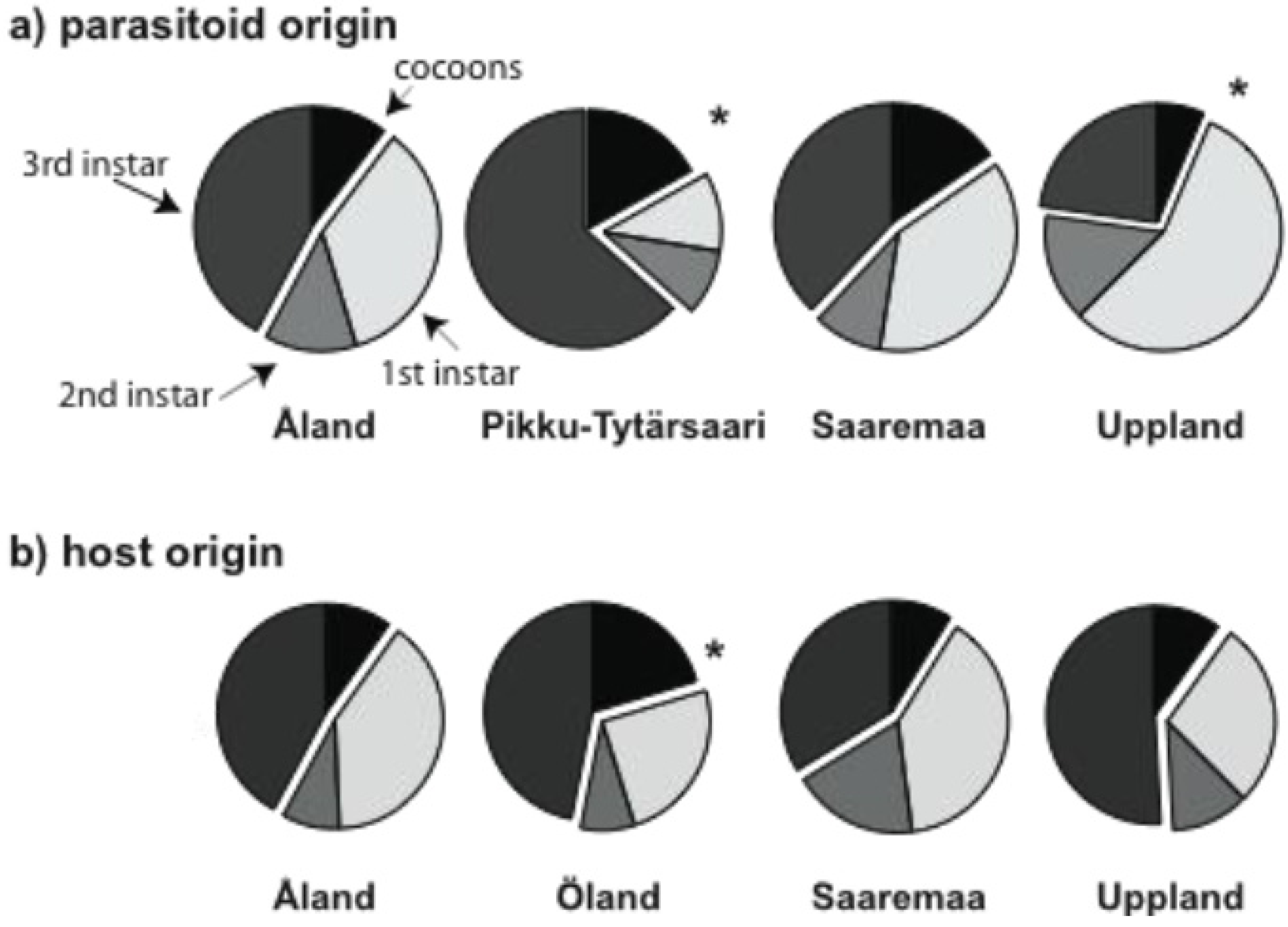
3.3. Brood Size and Adult Longevity in the Spring Generation
| Pupal brood size | Pupal weight | Adult longevity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | DF | SS | F | P | DF | SS | F | P | DF | SS | F | P |
| Model | 7 | 363.62 | 21.98 | <0.001 | 11 | 19.21 | 1.75 | <0.001 | 11 | 713,612.5 | 62.46 | <0.001 |
| Origin | 3 | 300.43 | 42.36 | <0.001 | 3 | 2.49 | 3.63 | 0.013 | 3 | 2,611.66 | 8.38 | <0.001 |
| Sex | 1 | 4.60 | 1.95 | 0.164 | 1 | 3.69 | 16.18 | <0.001 | 1 | 18,138.38 | 174.64 | <0.001 |
| Origin x Sex | 3 | 9.41 | 1.33 | 0.265 | 3 | 0.55 | 0.80 | 0.493 | 3 | 2,785.58 | 8.94 | <0.001 |
| Brood size | - | - | - | - | 1 | 3.82 | 16.73 | <0.001 | - | - | - | - |
| Brood size x Origin | - | - | - | - | 3 | 6.44 | 9.41 | <0.001 | - | - | - | - |
| Pupal weight | - | - | - | - | - | - | - | - | 1 | 3,542.55 | 34.11 | <0.001 |
| Pupal weight x Origin | - | - | - | - | - | - | - | - | 3 | 404.96 | 1.30 | 0.274 |
| Error | 424 | 1,087 | 467 | 104.10 | 413 | 49,285.5 | ||||||
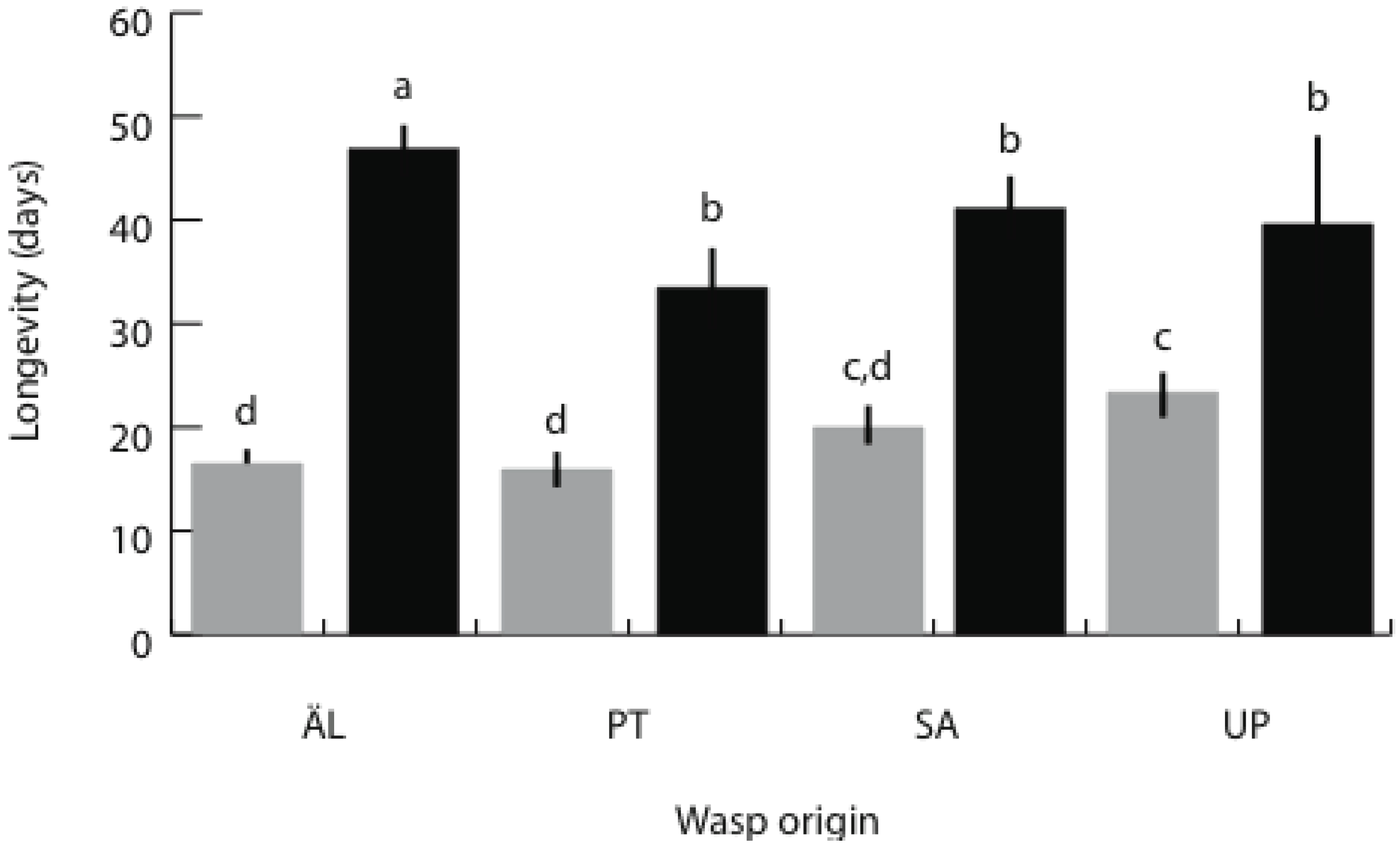
4. Discussion
4.1. Encapsulation Rate
4.2. Parasitoid Brood Size, Pupal Size and Adult Longevity
4.3. Parasitoid Development Rate
4.4. Variation in Host Resistance and Parasitoid Virulence
5. Conclusions
Acknowledgements
References and Notes
- Thompson, J.N. Coevolution: The geographic mosaic of coevolutionary arms races. Curr. Biol. 2005, 15, R992–R994. [Google Scholar] [CrossRef]
- Lively, C.M.; Dybdahl, M.F. Parasite adaptation to locally common host genotypes. Nature 2000, 405, 679–681. [Google Scholar] [CrossRef]
- Gandon, S. Local adaptation and the geometry of host-parasite coevolution. Ecol. Lett. 2002, 5, 246–256. [Google Scholar] [CrossRef]
- Dupas, S.; Dubuffet, A.; Carton, Y.; Poirie, M. Local, geographic and phylogenetic scales of coevolution in Drosophila-parasitoid interactions. In Advances in Parasitology: Parasitoids of Drosophila; Prevost, G., Ed.; Elsevier: London, UK, 2009; Volume 70, pp. 281–295. [Google Scholar]
- Kraaijeveld, A.R.; Godfray, H.C.J. Evolution of host resistance and parasitoid counter-resistance. In Advances in Parasitology; Elsevier: London, UK, 2009; Volume 70, pp. 257–280. [Google Scholar]
- Gitau, C.W.; Dupas, S.; Ngi-Song, A.J.; Mbugi, J.P.; Schulthess, F. Calyx fluid proteins of two Cotesia sesamiae (Cameron) (Hymenoptera: Braconidae) biotypes in kenya: Implications to biological control of the stem borer Busseola fusca (Fuller) (Lepidoptera:Noctuidae). Ann. Soc. Entomol. France 2006, 42, 433–441. [Google Scholar]
- Catherine, G.W.; Schulthess, F.; Stephane, D. An association between host acceptance and virulence status of different populations of Cotesia sesamiae, a braconid larval parasitoid of lepidopteran cereal stemborers in kenya. Biol. Control 2010, 54, 100–106. [Google Scholar] [CrossRef]
- Laurentz, M.; Reudler, J.H.; Mappes, J.; Friman, V.; Ikonen, S.; Lindstedt, C. Diet quality can play a critical role in defence efficacy against parasitoids and pathogens in the glanville fritillary (Melitaea cinxia). J. Chem. Ecol. 2012, 38, 116–125. [Google Scholar] [CrossRef]
- Bukovinszky, T.; Poelman, E.H.; Gols, R.; Prekatsakis, G.; Vet, L.E.M.; Harvey, J.A.; Dicke, M. Consequences of constitutive and induced variation in plant nutritional quality for immune defence of a herbivore against parasitism. Oecologia 2009, 160, 299–308. [Google Scholar] [CrossRef]
- White, J.A.; Kelly, S.E.; Cockburn, S.N.; Perlman, S.J.; Hunter, M.S. Endosymbiont costs and benefits in a parasitoid infected with both Wolbachia and Cardinium. Heredity 2011, 106, 585–591. [Google Scholar] [CrossRef]
- Martinez, J.; Duplouy, A.; Woolfit, M.; Vavre, F.; O'Neill, S.L.; Varaldi, J. Influence of the virus lbfv and of Wolbachia in a host-parasitoid interaction. PLoS One 2012, 7, e35081. [Google Scholar]
- Branca, A.; Le Ru, B.P.; Vavre, F.; Silvain, J.-F.; Dupas, S. Intraspecific specialization of the generalist parasitoid Cotesia sesamiae revealed by polydnavirus polymorphism and associated with different Wolbachia infection. Mol. Ecol. 2011, 20, 959–971. [Google Scholar] [CrossRef]
- Strand, M.R.; Beck, M.H.; Lavine, M.D.; Clark, K.D. Microplitis demolitor bracovirus inhibits phagocytosis by hemocytes from Pseudoplusia includens. Arch. Insect Biochem. 2006, 61, 134–145. [Google Scholar] [CrossRef]
- Oliver, K.M.; Moran, N.A.; Hunter, M.S. Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proc. Natl. Acad. Sci. USA 2005, 102, 12795–12800. [Google Scholar] [CrossRef]
- Veldtman, R.; McGeoch, M.A.; Scholtz, C.H. Can life-history and defence traits predict the population dynamics and natural enemy responses of insect herbivores? Ecol. Entomol. 2007, 32, 662–673. [Google Scholar] [CrossRef]
- Potting, R.P.J.; Vermeulen, N.E.; Conlong, D.E. Active defence of herbivorous hosts against parasitism: Adult parasitoid mortality risk involved in attacking a concealed stemboring host. Entomol. Exp. Appl. 1999, 91, 143–148. [Google Scholar]
- Tanaka, S. The impact of host aggressiveness on sex allocation by the gregarious parasitoid wasp Cotesia glomerata (L.). Biol. Lett. 2009, 5, 197–199. [Google Scholar] [CrossRef]
- Altizer, S.M. Migratory behaviour and host-parasite co-evolution in natural populations of monarch butterflies infected with a protozoan parasite. Evol. Ecol. Res. 2001, 3, 611–632. [Google Scholar]
- Nieminen, M.; Suomi, J.; van Nouhuys, S.; Sauri, P.; Riekkola, M.L. Effect of iridoid glycoside content on oviposition host plant choice and parasitism in a specialist herbivore. J. Chem. Ecol. 2003, 29, 823–844. [Google Scholar] [CrossRef]
- van Nouhuys, S.; Via, S. Natural selection and genetic differentiation of behavior between parasitoids from wild and cultivated habitats. Heredity 1999, 83, 127–137. [Google Scholar] [CrossRef]
- Pannebakker, B.A.; Garrido, N.R.T.; Zwaan, B.J.; van Alphen, J.J.M. Geographic variation in host-selection behaviour in the Drosophila parasitoid Leptopilina clavipes. Entomol. Exp. Appl. 2008, 127, 48–54. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Hassell, M.P.; Holt, R.D. The population dynamic consequences of phenological asynchrony between parasitoids and their hosts. J. Anim. Ecol. 1994, 63, 1–10. [Google Scholar] [CrossRef]
- Waage, J.K.; Godfray, H.C.J. Reproductive strategies and populaiton ecology of insect parasitoids. In Behavioural Ecology; Sibly, R.M., Smith, R.H., Eds.; Blackwell Scientific: Oxford, UK, 1985; pp. 449–470. [Google Scholar]
- Kapranas, A.; Tena, A.; Luck, R.F. Dynamic virulence in a parasitoid wasp: The influence of clutch size and sequential oviposition on egg encapsulation. Anim. Behav. 2012, 83, 833–838. [Google Scholar] [CrossRef]
- van Nouhuys, S. Effects of habitat fragmentation at different trophic levels in insect communities. Ann. Zool. Fennici 2005, 42, 433–447. [Google Scholar]
- Thompson, J.N.; Burdon, J.J. Gene-for-gene coevolution between plants and parasites. Nature 1992, 360, 121–126. [Google Scholar] [CrossRef]
- Brown, J.K.M.; Tellier, A. Plant-parasite coevolution: Bridging the gap between genetics and ecology. Annu. Rev. Phytopathol. 2011, 49, 345–367. [Google Scholar] [CrossRef]
- Dupas, S.; Carton, Y.; Poirie, M. Genetic dimension of the coevolution of virulence-resistance in Drosophila-parasitoid wasp relationships. Heredity 2003, 90, 84–89. [Google Scholar] [CrossRef]
- Lozier, J.D.; Roderick, G.K.; Mills, N.J. Evolutionarily significant units in natural enemies: Identifying regional populations of Aphidius transcaspicus (Hymenoptera: Braconidae) for use in biological control of mealy plum aphid. Biol. Control 2008, 46, 532–541. [Google Scholar] [CrossRef]
- Henter, H.J. Inbreeding depression and haplodiploidy: Experimental measures in a parasitoid and comparisons across diploid and haplodiploid insect taxa. Evolution 2003, 57, 1793–1803. [Google Scholar]
- Henter, H.J. The potential for coevolution in a host-parasitoid system. II. Genetic variation within a population of wasps in the ability to parasitize an aphid host. Evolution 1995, 49, 439–445. [Google Scholar] [CrossRef]
- Henter, H.J.; Via, S. The potential for coevolution in a host—Parasitoid system. I. Genetic variation within an aphid population in susceptibility to a parasitic wasp. Evolution 1995, 49, 427–438. [Google Scholar] [CrossRef]
- Hufbauer, R.A. Pea aphid-parasitoid interactions: Have parasiotids adapted to differential resistance? Ecology 2001, 82, 717–725. [Google Scholar]
- Hufbauer, R.A.; Via, S. Evolution of an aphid-parasitoid interaction: Variation in resistance to parasitism among aphid populations specialized on different plants. Evolution 1999, 53, 1435–1445. [Google Scholar] [CrossRef]
- Degnan, P.H.; Moran, N.A. Evolutionary genetics of a defensive facultative symbiont of insects: Exchange of toxin-encoding bacteriophage. Mol. Ecol. 2008, 17, 916–929. [Google Scholar] [CrossRef]
- Ferrari, J.; Darby, A.C.; Daniell, T.J.; Godfray, H.C.J.; Douglas, A.E. Linking the bacterial community in pea aphids with host-plant use and natural enemy resistance. Ecol. Entomol. 2004, 29, 60–65. [Google Scholar] [CrossRef]
- Kraaijeveld, A.R.; van Alphen, J.J.M.; Godfray, H.C.J. The coevolution of host resistance and parasitoid virulence. Parasitology 1998, 116, S29–S45. [Google Scholar] [CrossRef]
- Russo, J.; Brehelin, M.; Carton, Y. Haemocyte changes in resistant and susceptible strains of D. melanogaster caused by virulent and avirulent strains of the parasitic wasp Leptopilina boulardi. J. Insect Physiol. 2001, 47, 167–172. [Google Scholar] [CrossRef]
- Russo, J.; Dupas, S.; Frey, F.; Carton, Y.; Brehelin, M. Insect immunity: Early events in the encapsulation process of parasitoid (Leptopilina boulardi) eggs in resistant and susceptible strains of Drosophila. Parasitology 1996, 112, 135–142. [Google Scholar] [CrossRef]
- Kraaijeveld, A.R.; van Alphen, J.J.M. Geographical variation in resistance of the parasitoid Asobara tabida, against encapsulation by Drosophila melanogaser larvae- the mechanism explored. Physiol. Entomol. 1994, 19, 9–14. [Google Scholar] [CrossRef]
- Dupas, S.; Gitau, C.W.; Branca, A.; Le Ru, B.P.; Silvain, J.F. Evolution of a polydnavirus gene in relation to parasitoid-host species immune resistance. J. Hered. 2008, 99, 491–499. [Google Scholar] [CrossRef]
- Gueguen, G.; Rajwani, R.; Paddibhatla, I.; Morales, J.; Govind, S. Vlps of Leptopilina boulardi share biogenesis and overall stellate morphology with vlps of the heterotoma clade. Virus Res. 2011, 160, 159–165. [Google Scholar] [CrossRef]
- Fytrou, A.; Schofield, P.G.; Kraaijeveld, A.R.; Hubbard, S.F. Wolbachia infection suppresses both host defence and parasitoid counter-defence. Proc. R. Soc. B Biol. Sci. 2006, 273, 791–796. [Google Scholar] [CrossRef]
- Ndemah, R.; Schulthess, F.; Abang, A.; Ghogomu, R.T.; Ntonifor, N.; Dupas, S.; LeRu, B. Suitability of cereal stemborers in Cameroon to Kenyan populations of the braconid larval parasitoid Cotesia sesamiae. J. Appl. Entomol. 2012, 136, 60–69. [Google Scholar] [CrossRef]
- Calatayud, P.A.; Gitau, C.; Calatayud, S.; Dupas, S.; Le Ru, B.; Silvain, J.F. Variability in the reproductive biology and in resistance against Cotesia sesamiae among two Busseola fusca populations. J. Appl. Entomol. 2011, 135, 423–429. [Google Scholar] [CrossRef]
- Kraaijeveld, A.R.; Hutcheson, K.A.; Limentani, E.C.; Godfray, H.C.J. Costs of counterdefenses to host resistance in a parasitoid of Drosophila. Evolution 2001, 44, 1815–1821. [Google Scholar]
- Dubuffet, A.; Dupas, S.; Frey, F.; Drezen, J.M.; Poirie, M.; Carton, Y. Genetic interactions between the parasitoid wasp Leptopilina boulardi and its Drosophila hosts. Heredity 2007, 98, 21–27. [Google Scholar] [CrossRef]
- Vorburger, C.; Sandrock, C.; Gouskov, A.; Castaneda, L.E.; Ferrari, J. Genotypic variation and the role of defensive endosymbionts in an all-parthenogenetic host-parasidoid interaction. Evolution 2009, 63, 1439–1450. [Google Scholar] [CrossRef] [Green Version]
- Dion, E.; Zele, F.; Simon, J.C.; Outreman, Y. Rapid evolution of parasitoids when faced with the symbiont-mediated resistance of their hosts. J. Evol. Biol. 2011, 24, 741–750. [Google Scholar] [CrossRef]
- Dupas, S.; Boscaro, R. Geographic variation and evolution of immunosuppressive genes in a Drosophila parasitoid. Ecography 1999, 22, 284–291. [Google Scholar] [CrossRef]
- Mattila, A.L.K.; Duplouy, A.; Kirjokangas, M.; Lehtonen, R.; Rastas, P.; Hanski, I. High genetic load in an old isolated butterfly population. Proc. Natl. Acad. Sci. USA 2012, in press. [Google Scholar]
- Kuussaari, M.; Singer, M.; Hanski, I. Local specialization and landscape-level influence on host use in an herbivorous insect. Ecology 2000, 81, 2177–2187. [Google Scholar] [CrossRef]
- Kankare, M.; van Nouhuys, S.; Hanski, I. Genetic divergence among host-specific cryptic species in Cotesia melitaearum aggregate (Hymenoptera: Braconidae), parasitoids of checkerspot butterflies. Ann. Entomol. Soc. Am. 2005, 98, 382–394. [Google Scholar] [CrossRef]
- Lei, G.C.; Hanski, I. Metapopulation structure of Cotesia melitaearum, a specialist parasitoid of the butterfly Melitaea cinxia. Oikos 1997, 78, 91–100. [Google Scholar] [CrossRef]
- van Nouhuys, S.; Lei, G.C. Parasitoid and host metapopulation dynamics: The influences of temperature mediated phenological asynchrony. J. Anim. Ecol. 2004, 73, 526–535. [Google Scholar] [CrossRef]
- Saastamoinen, M.; Hirai, N.; van Nouhuys, S. Direct and trans-generational responses to food deprivation during development in the glanville fritillary butterfly. Oecologia 2012, in press. [Google Scholar]
- Saastamoinen, M.; Rantala, M.P. University of Helsinki: Helsinki, Finland, 2012; Unpublished work.
- Hanski, I. Eco-evolutionary spatial dynamics in the glanville fritillary butterfly. Proc. Natl. Acad. Sci. USA 2011, 108, 14397–14404. [Google Scholar] [CrossRef]
- van Nouhuys, S.; Hanski, I. Colonization rates and distances of a host butterfly and two specific parasitoids in a fragmented landscape. J. Anim. Ecol. 2002, 71, 639–650. [Google Scholar] [CrossRef]
- Nieminen, M.; Singer, M.C.; Fortelius, W.; Schöps, K.; Hanski, I. Experimental confirmation that inbreeding depression increases extinction risk in butterfly populations. Am. Nat. 2001, 157, 237–244. [Google Scholar] [CrossRef]
- Saccheri, I.; Kuussaari, M.; Kankare, M.; Vikman, P.; Fortelius, W.; Hanski, I. Inbreeding and extinction in a butterfly metapopulation. Nature 1998, 392, 491–494. [Google Scholar] [CrossRef]
- Kankare, M.; van Nouhuys, S.; Gaggiotti, O.; Hanski, I. Metapopulation genetic structure of two coexisting parasitoids of the glanville fritillary butterfly. Oecologia 2005, 143, 77–84. [Google Scholar] [CrossRef]
- Wahlberg, N.; Saccheri, I. The effects of pleistocene glaciations on the phylogeography of Melitaea cinxia (Lepidoptera: Nymphalidae). Eur. J. Entomol. 2007, 104, 675–684. [Google Scholar]
- Somervuo, P.; Lehtonen, R.; Hanski, I. University of Helsinki: Helsinki, Finland, 2012; Unpublished work.
- van Nouhuys, S.; Punju, E. Coexistence of competing parasitoids: Which is the fugitive and where does it hide? Oikos 2010, 119, 61–70. [Google Scholar] [CrossRef]
- Mochiah, M.B.; Ngi-Song, A.J.; Overholt, W.A.; Stouthamer, R. Variation in encapsulation sensitivity of Cotesia sesamiae biotypes to Busseola fusca. Entomol. Exp. Appl. 2002, 105, 111–118. [Google Scholar]
- Gounou, S.; Chabi-Olaye, A.; Poehling, H.M.; Schulthess, F. Reproductive compatibility of several east and west african Cotesia sesamiae (Hymenoptera: Braconidae) populations and their crosses and backcrosses using Sesamia calamistis (Lepidoptera: Noctuidae) as the host. Biocontrol Sci. Technol. 2008, 18, 255–266. [Google Scholar] [CrossRef]
- Kankare, M.; Stefanescu, C.; van Nouhuys, S.; Shaw, M.R. Host specialization by Cotesia (Hymenoptera: Braconidae) species parasitizing rich assemblages of checkerspot (Melitaeine) butterflies in Spain. Biol. J. Linn. Soc. 2005, 86, 45–65. [Google Scholar] [CrossRef]
- Godfray, H.C.J. Parasitoids: Behavioural and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994; p. 473. [Google Scholar]
- Strand, M.R. The insect cellular immune response. Insect Sci. 2008, 15, 1–14. [Google Scholar] [CrossRef]
- Pakarinen, S. Host-parasitoid relationship in different Cotesia melitaearum and Melitaea cinxia populations around the baltic sea. M.Sc. Thesis, University of Helsinki, Helsinki, Finland, 2011. [Google Scholar]
- Lei, G.C.; Vikberg, V.; Nieminen, M.; Kuussaari, M. The parasitoid complex attacking the finnish populations of glanville fritillary Melitaea cinxia (Lep: Nymphalidae), an endangered butterfly. J. Nat. Hist. 1997, 31, 635–648. [Google Scholar] [CrossRef]
- Pexton, J.J.; Mayhew, P.J. Competitive interactions between parasitoid larvae and the evolution of gregarious development. Oecologia 2004, 141, 179–190. [Google Scholar]
- van Nouhuys, S.; Singer, M.C.; Nieminen, M. Spatial and temporal patterns of caterpillar performance and the suitability of two host plant species. Ecol. Entomol. 2003, 28, 193–202. [Google Scholar] [CrossRef]
- Sasaki, A. Host-parasite coevolution in a multilocus gene-for-gene system. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2000, 267, 2183–2188. [Google Scholar] [CrossRef]
- Greischar, M.A.; Koskella, B. A synthesis of experimental work on parasite local adaptation. Ecol. Lett. 2007, 10, 418–434. [Google Scholar] [CrossRef]
- Sandrock, C.; Gouskov, A.; Vorburger, C. Ample genetic variation but no evidence for genotype specificity in an all-parthenogenetic host-parasitoid interaction. J. Evol. Biol. 2010, 23, 578–585. [Google Scholar] [Green Version]
- Dupas, S.; Turnbull, M.W.; Webb, B.A. Diversifying selection in a parasitoid's symbiotic virus among genes involved in inhibiting host immunity. Immunogenetics 2003, 55, 351–361. [Google Scholar] [CrossRef]
- Kraaijeveld, A.R.; Godfray, H.C.J. Geographic patterns in the evolution of resistance and virulence in Drosophila and its parasitoids. Am. Nat. 1999, 153, S61–S74. [Google Scholar] [CrossRef]
- Ngi-Song, A.J.; Overholt, W.A.; Stouthamer, R. Suitability of Busseola fusca and Sesamia calamistis (Lepidoptera: Noctuidae) for the development of two populations of Cotesia sesamiae (Hymenoptera: Braconidae) in Kenya. Biol. Control 1998, 12, 208–214. [Google Scholar]
- Chinwada, P.; Overholt, W.A.; Omwega, C.O.; Mueke, J.M. Geographic differences in host acceptance and suitability of two Cotesia sesamiae populations in Zimbabwe. Biol. Control 2003, 28, 354–359. [Google Scholar] [CrossRef]
- Keller, L.F.; Waller, D.M. Inbreeding effects in wild populations. Trends Ecol. Evol. 2002, 17, 230–241. [Google Scholar] [CrossRef]
- Heimpel, G.E.; de Boer, J.G. Sex determination in the hymenoptera. Annu. Rev. Entomol. 2008, 53, 209–230. [Google Scholar] [CrossRef]
- Hall, A.R.; Scanlan, P.D.; Morgan, A.D.; Buckling, A. Host-parasite coevolutionary arms races give way to fluctuating selection. Ecol. Lett. 2011, 14, 635–642. [Google Scholar] [CrossRef]
- Laine, A.L. Role of coevolution in generating biological diversity: Spatially divergent selection trajectories. J. Exp. Bot. 2009, 60, 2957–2970. [Google Scholar] [CrossRef]
- Shaw, M.R.; Stefanescu, C.; van Nouhuys, S. Parasitoids of European butterflies. In Ecology of Butterflies in Europe; Settele, J., Shreeve, T.G., Konvicka, M., van Dyck, H., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 130–156. [Google Scholar]
- Wertheim, B.; Kraaijeveld, A.R.; Hopkins, M.G.; Walther Boer, M.; Godfray, H.C.J. Functional genomics of the evolution of increased resistance to parasitism in Drosophila. Mol. Ecol. 2011, 20, 932–949. [Google Scholar] [CrossRef]
- Hansen, A.K.; Vorburger, C.; Moran, N.A. Genomic basis of endosymbiont-conferred protection against an insect parasitoid. Genome Res. 2012, 22, 106–114. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Van Nouhuys, S.; Niemikapee, S.; Hanski, I. Variation in a Host–Parasitoid Interaction across Independent Populations. Insects 2012, 3, 1236-1256. https://doi.org/10.3390/insects3041236
Van Nouhuys S, Niemikapee S, Hanski I. Variation in a Host–Parasitoid Interaction across Independent Populations. Insects. 2012; 3(4):1236-1256. https://doi.org/10.3390/insects3041236
Chicago/Turabian StyleVan Nouhuys, Saskya, Suvi Niemikapee, and Ilkka Hanski. 2012. "Variation in a Host–Parasitoid Interaction across Independent Populations" Insects 3, no. 4: 1236-1256. https://doi.org/10.3390/insects3041236
APA StyleVan Nouhuys, S., Niemikapee, S., & Hanski, I. (2012). Variation in a Host–Parasitoid Interaction across Independent Populations. Insects, 3(4), 1236-1256. https://doi.org/10.3390/insects3041236




