Abiotic and Biotic Factors Regulating Inter-Kingdom Engagement between Insects and Microbe Activity on Vertebrate Remains
Abstract
:1. Introduction
1.1. A Need for Integrative Research of Entomology and Microbiology
1.2. What Is Known about Microbes Associated with Decomposing Vertebrate Remains?
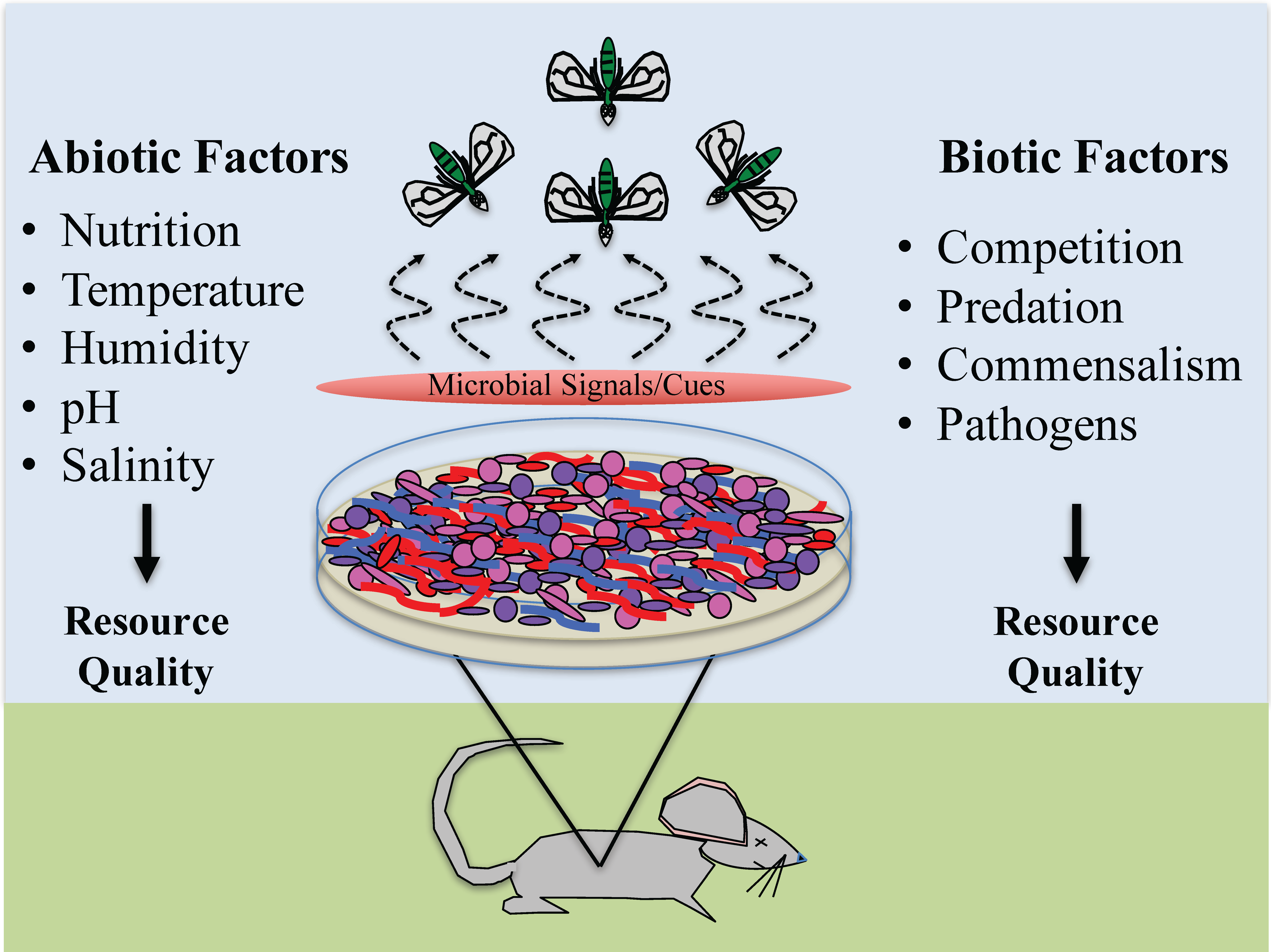
2. Abiotic Factors
2.1. Temperature
2.2. Water Activity
2.3. Resource Quality
2.4. Narcotics
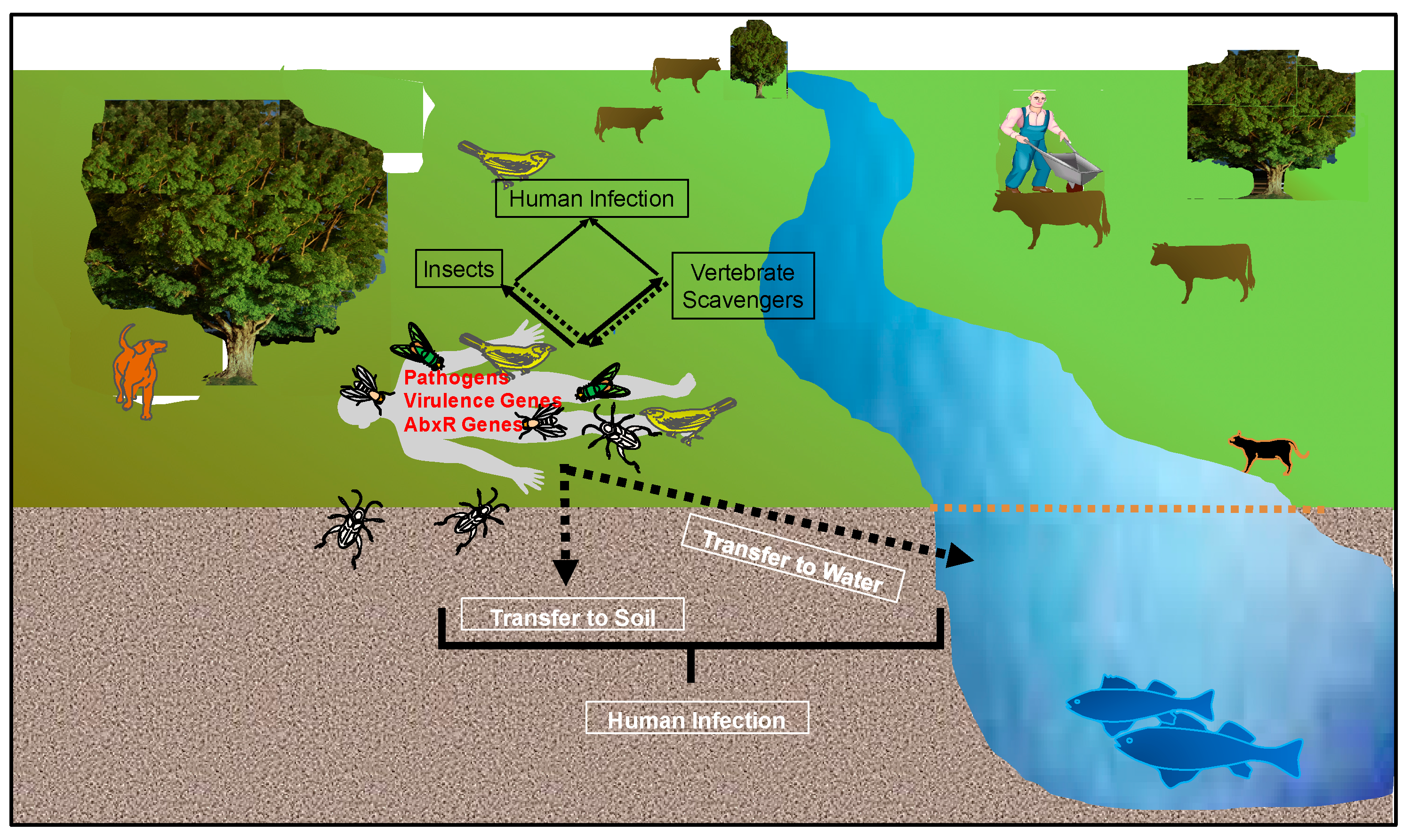
3. Biotic Factors
3.1. Other Microbes
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Sanford, M.R.; Whitworth, T.L.; Phatak, D.R. Human wound colonization by Lucilia eximia and Chrysomya rufifacies (Diptera: Calliphoridae): Myiasis, perimortem, or postmortem colonization? J. Med. Entomol. 2014, 51, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Benecke, M.; Lessig, R. Child neglect and forensic entomology. Forensic Sci. Int. 2001, 120, 155–159. [Google Scholar] [CrossRef]
- Forensic Entomology: International Dimensions and Frontiers; Tomberlin, J.K.; Benbow, M.E. (Eds.) CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Goff, M.L. A Fly for the Prosecution: How Insect Evidence Helps Solve Crimes; Harvard University Press: Cambridge, MA, USA, 2000; p. 225. [Google Scholar]
- Sanford, M.R. Forensic entomology of decomposing humans and their decomposing pets. Forensic Sci. Int. 2015, 247, e11–e17. [Google Scholar] [CrossRef] [PubMed]
- Tomberlin, J.K.; Sanford, M.R. Forensic Entomology and Wildlife. In Wildlife Forensics; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 81–107. [Google Scholar]
- Forensic Entomology: The Utility of Arthropods in Legal Investigations, 2nd ed.; Byrd, J.; Castner, J. (Eds.) CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Lord, W.D.; Goff, M.L.; Adkins, T.R.; Haskell, N.H. The black soldier fly Hermetia illucens (Diptera: Stratiomyidae) as a potential measure of human postmortem interval: Observations and case histories. J. Forensic Sci. 1994, 39, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Catts, E.P.; Goff, M.L. Forensic entomology in criminal investigations. Annu. Rev. Entomol. 1992, 37, 253–272. [Google Scholar] [CrossRef] [PubMed]
- Tomberlin, J.K.; Wallace, J.R.; Byrd, J.H. Forensic entomology: Myths busted! Forensic Mag. 2006, 3, 10–14. [Google Scholar]
- Amendt, J.; Campobasso, C.P.; Gaudry, E.; Reiter, C.; LeBlanc, H.N.; Hall, M.J.R. Best practice in forensic entomology—Standards and guidelines. Int. J. Leg. Med. 2007, 121, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.D.; Anderson, P.C.; Clark, D.P. A case of human myiasis caused by Phormia regina (Diptera, Calliphoridae) in Missouri, USA. J. Med. Entomol. 1986, 23, 578–579. [Google Scholar] [CrossRef] [PubMed]
- Archer, M.S.; Elgar, M.A.; Briggs, C.A.; Ranson, D.L. Fly pupae and puparia as potential contaminants of forensic entomology samples from sites of body discovery. Int. J. Leg. Med. 2006, 120, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Owings, C.; Spiegelman, C.; Tarone, A.; Tomberlin, J. Developmental variation among Cochliomyia macellaria Fabricius (Diptera: Calliphoridae) populations from three ecoregions of Texas, USA. Int. J. Leg. Med. 2014, 128, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, M.B.; Sandhu, S.; Kimsey, R. Variation in development time for geographically distinct populations of the common green bottle fly, Lucilia sericata (Meigen). J. Forensic Sci. 2010, 55, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Tarone, A.M.; Picard, C.J.; Spiegelman, C.; Foran, D.R. Population and temperature effects on Lucilia sericata (Diptera: Calliphoridae) body size and minimum development time. J. Med. Entomol. 2011, 48, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Haskell, N.H.; Williams, R.E. Entomology & Death: A Procedural Guide, 2nd ed.; East Park Printing: Clemson, SC, USA, 2008. [Google Scholar]
- Archer, M.S. The effect of time after body discovery on the accuracy of retrospective weather station ambient temperature corrections in forensic entomology. J. Forensic Sci. 2004, 49, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Archer, M.S. Annual variation in arrival and departure times of carrion insects at carcasses: Implications for succession studies in forensic entomology. Aust. J. Zool. 2003, 51, 569–576. [Google Scholar] [CrossRef]
- Archer, M.S. Rainfall and temperature effects on the decomposition rate of exposed neonatal remains. Sci. Justice 2004, 44, 35–41. [Google Scholar] [CrossRef]
- Amendt, J.; Zehner, R.; Reckel, F. The nocturnal oviposition behavior of blowflies (Diptera: Calliphoridae) in Central Europe and its forensic implications. Forensic Sci. Int. 2008, 175, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Baldridge, R.S.; Wallace, S.G.; Kirkpatrick, R. Investigation of nocturnal oviposition by necrophilous flies in central Texas. J. Forensic Sci. 2006, 51, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Stamper, T.; Davis, P.; Debry, R.W. The nocturnal ovipositing behaviour of carrion flies in Cincinnati, Ohio. J. Forensic Sci. 2009, 54, 1450–1452. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Fonseca, A.; Liu, W.; Fields, A.T.; Pimsler, M.L.; Spindola, A.F.; Tarone, A.M.; Crippen, T.L.; Tomberlin, J.K.; Wood, T.K. Proteus mirabilis interkingdom swarming signals attract blow flies. Int. Soc. Microb. Ecol. J. 2012, 6, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
- Tomberlin, J.K.; Crippen, T.L.; Tarone, A.M.; Singh, B.; Adams, K.; Rezenom, Y.H.; Benbowd, M.E.; Floresa, M.; Longneckere, M.; Pechald, J.L. Interkingdom responses of flies to bacteria mediated by fly physiology and bacterial quorum sensing. Anim. Behav. 2012, 84, 1449–1456. [Google Scholar] [CrossRef]
- Liu, W.L.; Longnecker, M.; Tarone, A.M.; Tomberlin, J.K. Response of Lucilia sericata (Diptera: Caliphoridae) to compounds from microbial decomposition of larval resources. Anim. Behav. 2016, 115, 217–225. [Google Scholar] [CrossRef]
- Chaudhury, M.F.; Skoda, S.R.; Sagel, A.; Welch, J.B. Volatiles emitted from eight wound-isolated bacteria differentially attract gravid screwworms (Diptera: Calliphoridae) to oviposit. J. Med. Entomol. 2010, 47, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Carrion Ecology, Evolution, and Their Applications; Benbow, M.E.; Tomberlin, J.K.; Tarone, A.M. (Eds.) CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Brundage, A.L.; Benbow, M.E.; Tomberlin, J.K. Priority effects on the life-history traits of two carrion blow fly (Diptera, Calliphoridae) species. Ecol. Entomol. 2014, 39, 539–547. [Google Scholar] [CrossRef]
- Mohr, R.M.; Tomberlin, J.K. Environmental factors affecting early carcass attendance by four species of blow flies (Diptera: Calliphoridae) in Texas. J. Med. Entomol. 2014, 51, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Mohr, R.M.; Tomberlin, J.K. Development and validation of a new technique for estimating a minimum postmortem interval using adult blow fly (Diptera: Calliphoridae) carcass attendance. Int. J. Leg. Med. 2015, 129, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Arnaldos, M.I.; Romera, E.; Presa, J.J.; Luna, A.; Garcia, M.D. Studies on seasonal arthropod succession on carrion in the southeastern Iberian Peninsula. Int. J. Leg. Med. 2004, 118, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Avila, F.W.; Goff, M.L. Arthropod succession patterns onto burnt carrion in two contrasting habitats in the Hawaiian Islands. J. Forensic Sci. 1998, 43, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Bharti, M.; Singh, D. Insect faunal succession on decaying rabbit carcasses in Punjab, India. J. Forensic Sci. 2003, 48, 1–11. [Google Scholar] [CrossRef]
- Grassberger, M.; Frank, C. Initial study of arthropod succession on pig carrion in a central European urban habitat. J. Med. Entomol. 2004, 41, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Tomberlin, J.K.; Byrd, J.H.; Wallace, J.R.; Benbow, M.E. Assessment of decomposition studies indicates need for standardized and repeatable methods in forensic entomology. J. Forensic Res. 2012, 3. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Mohr, R.; Benbow, M.E.; Tarone, A.M.; VanLaerhoven, S.L. A roadmap for bridging basic and applied research in forensic entomology. Annu. Rev. Entomol. 2011, 56, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, S. Estimating the pre-appearance interval from temperature in Necrodes littoralis L. (Coleoptera: Silphidae). Forensic Sci. Int. 2011, 212, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Janzen, D.H. Why fruits rot, seeds mold, and meat spoils. Am. Nat. 1977, 111, 691–713. [Google Scholar] [CrossRef]
- Burkepile, D.E.; Parker, J.D.; Woodson, C.B.; Mills, H.J.; Kubanek, J.; Sobecky, P.A.; Hay, M.E. Chemically mediated competition between microbes and animals: Microbes as consumers in food webs. Ecology 2006, 87, 2821–2831. [Google Scholar] [CrossRef]
- Foltan, P.; Puza, V. To complete their life cycle, pathogenic nematode–bacteria complexes deter scavengers from feeding on their host cadaver. Behav. Proc. 2009, 80, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.M.; Gennard, D.E.; Dixon, R.A. An assessment of the antibacterial activity in larval excretion/secretion of four species of insects recorded in association with corpses, using Lucilia sericata Meigen as the marker species. Bull. Entomol. Res. 2010, 100, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, G.R.; Khalil, S.K.W. Isolation and identification of two antibacterial agents produced by a strain of Proteus mirabilis isolated from larvae of the screwworm (Cochliomyia hominivorax) (Diptera: Calliphoridae). J. Med. Entomol. 1986, 23, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Fenchel, T.; Field, J.G.; Gray, J.S.; Meyer-Reil, L.A.; Thingstad, F. The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 1983, 10, 257–263. [Google Scholar] [CrossRef]
- Pechal, J.L.; Crippen, T.L.; Benbow, M.E.; Tarone, A.M.; Dowd, S.; Tomberlin, J.K. The potential use of bacterial community succession in forensics as described by high throughput metagenomic sequencing. Int. J. Leg. Med. 2013, 128, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.L.; Wegener Parfrey, L.; Gonzalez, A.; Lauber, C.L.; Knights, D.; Ackermann, G.; Humphrey, G.C.; Gebert, M.J.; Van Treuren, W.; Berg-Lyons, D.; et al. A microbial clock provides an accurate estimate of the postmortem interval in a mouse model system. eLife 2013, 2, e01104. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.L.; Xu, Z.Z.; Weiss, S.; Lax, S.; Van Treuren, W.; Hyde, E.R.; Song, S.J.; Amir, A.; Larsen, P.; Sangwan, N.; et al. Microbial community assembly and metabolic function during mammalian corpse decomposition. Science 2016, 351, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Hyde, E.R.; Haarmann, D.P.; Lynne, A.M.; Bucheli, S.R.; Petrosino, J.F. The living dead: Bacterial community structure of a cadaver at the onset and end of the bloat stage of decomposition. PLoS ONE 2013, 8, e77733. [Google Scholar] [CrossRef] [PubMed]
- Hyde, E.R.; Haarmann, D.P.; Petrosino, J.F.; Lynne, A.M.; Bucheli, S.R. Initial insights into bacterial succession during human decomposition. Int. J. Leg. Med. 2015, 129, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Gounot, A.M. Bacterial life at low temperature: Physiological aspects and biotechnological implications. J. Appl. Bacteriol. 1991, 71, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Shakhnovich, E.I. Thermal adaptation of viruses and bacteria. Biophys. J. 2010, 98, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Los, D.A.; Murata, N. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta 2004, 1666, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Tansey, M.R.; Brock, T.D. The upper temperature limit for eukaryotic organisms. Proc. Natl. Acad. Sci. USA 1972, 69, 2426–2428. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.H.; Marahiel, M.A. Bacterial cold shock responses. Sci. Prog. 2003, 86, 9–75. [Google Scholar] [CrossRef] [PubMed]
- Ranawat, P.; Rawat, S. Stress response physiology of thermophiles. Arch. Microbiol. 2017, 199, 391–414. [Google Scholar] [CrossRef] [PubMed]
- Maleki, F.; Khosravi, A.; Nasser, A.; Taghinejad, H.; Azizian, M. Bacterial heat shock protein activity. J. Clin. Diagn. Res. 2016, 10, BE01–BE03. [Google Scholar] [CrossRef] [PubMed]
- Gasser, B.; Saloheimo, M.; Rinas, U.; Dragosits, M.; Rodriguez-Carmona, E.; Baumann, K.; Giuliani, M.; Parrilli, E.; Branduardi, P.; Lang, C.; et al. Protein folding and conformational stress in microbial cells producing recombinant proteins: A host comparative overview. Microb. Cell Fact. 2008, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Baatout, S.; De Boever, P.; Max, M. Temperature-induced changes in bacterial physiology as determined by flow cytometry. Ann. Microbiol. 2005, 55, 73–80. [Google Scholar]
- Al-Fageeh, M.B.; Smales, C.M. Control and regulation of the cellular responses to cold shock: The responses in yeast and mammalian systems. Biochem. J. 2006, 397, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Hiscox, J.; Clarkson, G.; Savoury, M.; Powell, G.; Savva, I.; Lloyd, M.; Shipcotta, J.; Choimesa, A.; Cumbriua, X.A.; Boddya, L.; et al. Effects of pre-colonisation and temperature on interspecific fungal interactions in wood. Fungal Ecol. 2016, 21, 32–42. [Google Scholar] [CrossRef]
- Wessner, D.; Dupont, C.; Charles, T.; Neufeld, J. Cultivating Microorganisms. In Microbiology, 2nd ed.; Wessner, D., Dupont, C., Charles, T., Neufeld, J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Mansilla, M.C.; Cybulski, L.E.; Albanesi, D.; de Mendoza, D. Control of membrane lipid fluidity by molecular thermosensors. J. Bacteriol. 2004, 186, 6681–6688. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Sakumoto, N.; Kaneko, Y.; Harashima, S. Mga2p is a putative sensor for low temperature and oxygen to induce OLE1 transcription in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2002, 291, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Hightower, L.E. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell 1991, 66, 191–197. [Google Scholar] [CrossRef]
- Schade, B.; Jansen, G.; Whiteway, M.; Entian, K.D.; Thomas, D.Y. Cold adaptation in budding yeast. Mol. Biol. Cell. 2004, 15, 5492–5502. [Google Scholar] [CrossRef] [PubMed]
- Rivers, D.B.; Thompson, C.; Brogan, R. Physiological trade-offs of forming maggot masses by necrophagous flies on vertebrate carrion. Bull. Entomol. Res. 2011, 101, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Slone, D.H.; Gruner, S.V. Thermoregulation in larval aggregations of carrion-feeding blow flies (Diptera: Calliphoridae). J. Med. Entomol. 2007, 44, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.O.; Yellowlees, D.; Tibbett, M. Cadaver decomposition in terrestrial ecosystems. Naturwissenschaften 2007, 94, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.O.; Yellowlees, D.; Tibbett, M. Temperature affects microbial decomposition of cadavers (Rattus rattus) in contrasting soils. Appl. Soil Ecol. 2008, 40, 129–137. [Google Scholar] [CrossRef]
- Forbes, S.L.; Perrault, K.A.; Stefanuto, P.H.; Nizio, K.D.; Focant, J.F. Comparison of the decomposition VOC profile during winter and summer in a moist, mid-latitude (Cfb) climate. PLoS ONE 2014, 9, e113681. [Google Scholar] [CrossRef] [PubMed]
- Pechal, J.L.; Crippen, T.L.; Tarone, A.M.; Lewis, A.J.; Tomberlin, J.K.; Benbow, M.E. Microbial community functional change during vertebrate carrion decomposition. PLoS ONE 2013, 8, e79035. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.O.; Tibbett, M. Microbial decomposition of skeletal muscle tissue (Ovis aries) in a sandy loam soil at different temperatures. Appl. Soil Ecol. 2006, 38, 1139–1145. [Google Scholar] [CrossRef]
- Carter, D.O.; Metcalf, J.L.; Bibat, A.; Knight, R. Seasonal variation of postmortem microbial communities. Forensic Sci. Med. Pathol. 2015, 11, 202–207. [Google Scholar] [CrossRef] [PubMed]
- ICMSF. Microbial Ecology of Foods; Elsevier: Amsterdam, The Netherlands, 1980. [Google Scholar]
- Baird, R.; Bloomfield, S.F. Microbial Quality Assurance in Pharmaceuticals, Cosmetics, and Toiletries; Taylor & Francis: Abingdon, UK, 1996. [Google Scholar]
- Pitt, J.I.; Hocking, A.D. The Ecology of Fungal Food Spoilage. In Fungi and Food Spoilage; Springer Science+Business Media, LLC: New York, NY, USA, 2009. [Google Scholar]
- Lennon, J.T.; Aanderud, Z.T.; Lehmkuhl, B.K.; Schoolmaster, D.R., Jr. Mapping the niche space of soil microorganisms using taxonomy and traits. Ecology 2012, 93, 1867–1879. [Google Scholar] [CrossRef] [PubMed]
- Barton-Browne, L.B. Water regulation in insects. Ann. Rev. Entomol. 1964, 9, 63–82. [Google Scholar] [CrossRef]
- Rowley, M.; Hanson, F. Humidity detection and hygropreference behavior in larvae of the tobacco hornworm, Manduca sexta. J. Insect Sci. 2007, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tichy, H.; Kallina, W. The evaporative function of cockroach hygroreceptors. PLoS ONE 2013, 8, e53998. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.S.; Hardie, J. Hygroreception in olfactometer studies. Physiol. Entomol. 2009, 34, 211–216. [Google Scholar] [CrossRef]
- Schroeder, H.; Klotzbach, H.; Puschel, K. Insects’ colonization of human corpses in warm and cold season. Leg. Med. 2003, 5 (Suppl. 1:S3), 72–74. [Google Scholar] [CrossRef]
- Benbow, M.E.; Lewis, A.J.; Tomberlin, J.K.; Pechal, J.L. Seasonal necrophagous insect community assembly during vertebrate carrion decomposition. J. Med. Entomol. 2013, 50, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Doll, K.; Dastjerdi, R.; Karlovsky, P.; Dehne, H.W.; Altincicek, B. Effect of fungal colonization of wheat grains with Fusarium spp. on food choice, weight gain and mortality of meal beetle larvae (Tenebrio molitor). PLoS ONE 2014, 9, e100112. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.S.; Crippen, T.L.; Hofstetter, R.W.; Tomberlin, J.K. Microbial volatile emissions as insect semiochemicals. J. Chem. Ecol. 2013, 39, 840–859. [Google Scholar] [CrossRef] [PubMed]
- Carrero-Colon, M.; Nakatsu, C.H.; Konopka, A. Effect of nutrient periodicity on microbial community dynamics. Appl. Environ. Microbiol. 2006, 72, 3175–3183. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.H.; Dighton, J.; Frankland, J.C.; Roberts, J.D. Fungal communities on decaying wheat straw of different resource qualities. Soil Biol. Biochem. 1994, 26, 1053–1058. [Google Scholar] [CrossRef]
- Griffith, G.S.; Bardgett, R.D. Influence of resource unit distribution and quality on the activity of soil fungi in a particulate medium. N. Phytol. 2000, 148, 143–151. [Google Scholar] [CrossRef]
- Paczkowski, S.; Maibaum, F.; Paczkowska, M.; Schutz, S. Decaying mouse volatiles perceived by Calliphora vicina Rob.-Desv. J. Forensic Sci. 2012, 57, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Frederickx, C.; Dekeirsschieter, J.; Verheggen, F.J.; Haubruge, E. Responses of Lucilia sericata Meigen (Diptera: Calliphoridae) to cadaveric volatile organic compounds. J. Forensic Sci. 2012, 57, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Crippen, T.L.; Singh, B. Forensic and Decomposition Microbiology. In Forensic Entomology: International Dimensions and Frontiers; Tomberlin, J.K., Benbow, M.E., Eds.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Bell, T.H.; Callender, K.L.; Whyte, L.G.; Greer, C.W. Microbial competition in polar soils: A review of an understudied but potentially important control on productivity. Biology 2013, 2, 533–554. [Google Scholar] [CrossRef] [PubMed]
- Burcham, Z.M.; Hood, J.A.; Pechal, J.L.; Krausz, K.L.; Bose, J.L.; Schmidt, C.J.; Benbow, M.E.; Jordan, H.R. Fluorescently labeled bacteria provide insight on post-mortem microbial transmigration. Forensic Sci. Int. 2016, 264, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Boelke, S.; Fischer, A.; Haag, L.M.; Loddenkemper, C.; Kuhl, A.A.; Göbel, U.B.; Bereswill, S. Comprehensive postmortem analyses of intestinal microbiota changes and bacterial translocation in human flora associated mice. PLoS ONE 2012, 7, e40758. [Google Scholar] [CrossRef] [PubMed]
- Melvin, J.R., Jr.; Cronholm, L.S.; Simson, L.R., Jr.; Isaacs, A.M. Bacterial transmigration as an indicator of time of death. J. Forensic Sci. 1984, 29, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Alfredsson, H.; Clymans, W.; Stadmark, J.; Conley, D.; Rousk, J. Bacterial and fungal colonization and decomposition of submerged plant litter: Consequences for biogenic silica dissolution. FEMS Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef] [PubMed]
- Parmenter, R.R.; MacMahon, J.A. Carrion decomposition and nutrient cycling in a semiarid shrub-steppe ecosystem. Ecol. Monogr. Ecol. Soc. Am. 2009, 79, 637–661. [Google Scholar] [CrossRef]
- Clark, K.; Evans, L.; Wall, R. Growth rates of the blowfly, Lucilia sericata, on different body tissues. Forensic Sci. Int. 2006, 156, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, H.; Denef, K.; Six, J.; Frey, S.D.; Merckx, R.; Paustian, K. Influence of microbial populations and residue quality on aggregate stability. Appl. Soil Ecol. 2001, 16, 195–208. [Google Scholar] [CrossRef]
- Rousk, J.; Baath, E. Fungal and bacterial growth in soil with plant materials of different C/N ratios. FEMS Microbiol. Ecol. 2007, 62, 258–267. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.K.; Williams, M.A.; Bottomley, P.J.; Myrold, D.D. Dynamics of microbial communities during decomposition of carbon-13 labeled ryegrass fractions in soil. Soil Sci. Soc. Am. J. 2005, 69, 1238–1247. [Google Scholar] [CrossRef]
- Keiblinger, K.M.; Hall, E.K.; Wanek, W.; Szukics, U.; Hammerle, I.; Ellersdorfer, G.; Böck, S.; Strauss, J.; Sterflinger, K.; Richter, A.; et al. The effect of resource quantity and resource stoichiometry on microbial carbon-use-efficiency. FEMS Microbiol. Ecol. 2010, 73, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Strickland, M.S.; Osburn, E.; Lauber, C.; Fierer, N.; Bradford, M.A. Litter quality is in the eye of the beholder: Initial decomposition rates as a function of inoculum characteristics. Funct. Ecol. 2009, 23, 627–636. [Google Scholar] [CrossRef]
- Klappenbach, J.A.; Dunbar, J.M.; Schmidt, T.M. rRNA operon copy number reflects ecological strategies of bacteria. Appl. Environ. Microbiol. 2000, 66, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, T.; Ghosh, A.; Pazhani, G.P.; Shinoda, S. Current perspectives on viable but non-culturable (VBNC) pathogenic bacteria. Front. Public Health 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Lemos, P.C.; Serafim, L.S.; Santos, H.; Reis, M.A. Production of polyhydroxyalkanoates by a mixed culture in a sequencing batch reactor: The use of propionate as carbon source. Commun. Agric. Appl. Biol. Sci. 2003, 68, 109–114. [Google Scholar] [PubMed]
- Reis, M.A.; Serafim, L.S.; Lemos, P.C.; Ramos, A.M.; Aguiar, F.R.; Van Loosdrecht, M.C. Production of polyhydroxyalkanoates by mixed microbial cultures. Bioprocess Biosyst. Eng. 2003, 25, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Hung, T.H.; Shiao, S.F. Molecular identification of forensically important blow fly species (Diptera: Calliphoridae) in Taiwan. J. Med. Entomol. 2004, 41, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Joseph, I.; Mathew, D.G.; Sathyan, P.; Vargheese, G. The use of insects in forensic investigations: An overview on the scope of forensic entomology. J. Forensic Dent. Sci. 2011, 3, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Dayananda, R.; Kiran, J. Entomotoxicology. Int. J. Med. Toxicol. Forensic Sci. 2013, 3, 71–74. [Google Scholar]
- Pyndt Jorgensen, B.; Krych, L.; Pedersen, T.B.; Plath, N.; Redrobe, J.P.; Hansen, A.K.; Nielsen, D.S.; Pedersen, C.S.; Larsen, C.; Sørensen, D.B. Investigating the long-term effect of subchronic phencyclidine-treatment on novel object recognition and the association between the gut microbiota and behavior in the animal model of schizophrenia. Physiol. Behav. 2015, 141, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.; Salonen, A.; Virta, L.J.; Kekkonen, R.A.; Forslund, K.; Bork, P.; de Vos, W.M. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nature Commun. 2016, 7, 10410. [Google Scholar] [CrossRef] [PubMed]
- Murthy, C.R.V.; Mohanty, M. Entomotoxicology: A review. J. Indian Acad. Forensic Med. 2010, 32, 82–84. [Google Scholar]
- Tomberlin, J.K.; Crippen, T.L.; Wu, G.; Griffin, A.S.; Wood, T.K.; Kilner, R.M. Indole: An evolutionarily conserved influencer of behavior across kingdoms. BioEssays 2016, 39, 1600203. [Google Scholar] [CrossRef] [PubMed]
- Morris, B.E.; Henneberger, R.; Huber, H.; Moissl-Eichinger, C. Microbial syntrophy: Interaction for the common good. FEMS Microbiol. Rev. 2013, 37, 384–406. [Google Scholar] [CrossRef] [PubMed]
- Ghoul, M.; Mitri, S. The Ecology and Evolution of Microbial Competition. Trends Microbiol. 2016, 24, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B. Persistence of bacteria in the developmental stages of the housefly: III. Quantitative distribution in prepupae and pupae. Am. J. Trop. Med. Hyg. 1959, 8, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B. Model for destruction of bacteria in the midgut of blow fly maggots. J. Med. Entomol. 1968, 5, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Heil, M. Host manipulation by parasites: Cases, patterns, and remaining doubts. Front. Ecol. Evol. 2016, 4. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Le, Q.T.; Garnier, J.; Janeau, J.L.; Rochelle-Newall, E. Seasonal variability of faecal indicator bacteria numbers and die-off rates in the Red River basin, North Viet Nam. Sci. Rep. 2016, 6, 21644. [Google Scholar] [CrossRef] [PubMed]
- Seyedmousavi, S.; Guillot, J.; Tolooe, A.; Verweij, P.E.; de Hoog, G.S. Neglected fungal zoonoses: Hidden threats to man and animals. Clin. Microbiol. Infect. 2015, 21, 416–425. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.M.; Auvermann, B.W.; Dzikamunhenga, R.S.; Glanville, J.M.; Higgins, J.P.T.; Kirychuk, S.P.; Sargeant, J.M.; Totton, S.C.; Wood, H.; Von Essen, S.G. Updated systematic review: Associations between proximity to animal feeding operations and health of individuals in nearby communities. Syst. Rev. 2017, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Rozen, Y.; Belkin, S. Survival of enteric bacteria in seawater. FEMS Microbiol. Rev. 2001, 25, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Walther, B.A.; Ewald, P.W. Pathogen survival in the external environment and the evolution of virulence. Biol. Rev. Camb. Philos. Soc. 2004, 79, 849–869. [Google Scholar] [CrossRef]
- Winfield, M.D.; Groisman, E.A. Role of nonhost environments in the lifestyles of Salmonella and Escherichia coli. Appl. Environ. Microbiol. 2003, 69, 3687–3694. [Google Scholar] [CrossRef] [PubMed]
- National Research Council; Division on Earth and Life Studies; Board on Life Sciences; Water Science and Technology Board; Committee on Indicators for Waterborne Pathogens. Indicators for Waterborne Pathogens; The National Academies Press: Washington, DC, USA, 2004. [Google Scholar]
- Faust, M.A.; Aotaky, A.E.; Hargadon, M.T. Effect of physical parameters on the in situ survival of Escherichia coli MC-6 in an estuarine environment. Appl. Microbiol. 1975, 30, 800–806. [Google Scholar] [PubMed]
- Gerba, C.P.; McLeod, J.S. Effect of sediments on the survival of Escherichia coli in marine waters. Appl. Environ. Microbiol. 1976, 32, 114–120. [Google Scholar] [PubMed]
- Temple, K.L.; Camper, A.K.; McFeters, G.A. Survival of two enterobacteria in feces buried in soil under field conditions. Appl. Environ. Microbiol. 1980, 40, 794–797. [Google Scholar] [PubMed]
- Lim, C.H.; Flint, K.P. The effects of nutrients on the survival of Escherichia coli in lake water. J. Appl. Bacteriol. 1989, 66, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, J.; Brackett, R.E.; Beuchat, L.R. Survival of salmonellae on and in tomato plants from the time of inoculation at flowering and early stages of fruit development through fruit ripening. Appl. Environ. Microbiol. 2001, 67, 4760–4764. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, J.; Brackett, R.E.; Beuchat, L.R. Survival of Salmonella on tomatoes stored at high relative humidity, in soil, and on tomatoes in contact with soil. J. Food Prot. 2002, 65, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, C.W. Increased recovery rate of salmonellae from stream bottom sediments versus surface waters. Appl. Microbiol. 1971, 21, 379–380. [Google Scholar] [PubMed]
- Baudart, J.; Lemarchand, K.; Brisabois, A.; Lebaron, P. Diversity of Salmonella strains isolated from the aquatic environment as determined by serotyping and amplification of the ribosomal DNA spacer regions. Appl. Environ. Microbiol. 2000, 66, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Kouznetsov, M.Y.; Roodsari, R.; Pachepsky, Y.A.; Shelton, D.R.; Sadeghi, A.M.; Shirmohammadi, A.; Starr, J.L. Modeling manure-borne bromide and fecal coliform transport with runoff and infiltration at a hillslope. J. Environ. Manag. 2007, 84, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Armisen, T.; Servais, P. Partitioning and fate of particle-associated E. coli in river waters. Water Environ. Res. 2009, 81, 21–28. [Google Scholar] [PubMed]
- Gleason, F.H.; Crawford, J.W.; Neuhauser, S.; Henderson, L.E.; Lilje, O. Resource seeking strategies of zoosporic true fungi in heterogeneous soil habitats at the microscale level. Soil Biol. Biochem. 2012, 45, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.C. Clay Mineralogy in relation to survival of soil bacteria. Annu. Rev. Phytopathol. 1975, 13, 357–373. [Google Scholar] [CrossRef]
- Boyer, D.G. Fecal coliform dispersal by rain splash on slopes. Agric. For. Meteorol. 2008, 148, 1395–1400. [Google Scholar] [CrossRef]
- Abu-Ashour, J.; Lee, H. Transport of bacteria on sloping soil surfaces by runoff. Environ. Toxicol. 2000, 15, 149–153. [Google Scholar] [CrossRef]
- Bitton, G.; Lahav, N.; Henis, Y. Movement and retention of Klebsiella aerogenes in soil columns. Plant Soil 1974, 40, 373–380. [Google Scholar] [CrossRef]
- Rochelle-Newall, E.; Nguyen, T.M.; Le, T.P.; Sengtaheuanghoung, O.; Ribolzi, O. A short review of fecal indicator bacteria in tropical aquatic ecosystems: Knowledge gaps and future directions. Front. Microbiol. 2015, 6, 308. [Google Scholar] [CrossRef] [PubMed]
- Smither-Kopperl, M.L.; Charudattan, R.; Berger, R.D. Dispersal of spores of Fusarium culmorum in aquatic systems. Phytopathology 1998, 88, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Ingham, C.J.; Kalisman, O.; Finkelshtein, A.; Ben-Jacob, E. Mutually facilitated dispersal between the nonmotile fungus Aspergillus fumigatus and the swarming bacterium Paenibacillus vortex. Proc. Natl. Acad. Sci. USA 2011, 108, 19731–19736. [Google Scholar] [CrossRef] [PubMed]
- Mircea, G.; Ioana, D.; Emoke, P.; Mihaela, N.; Marina, S. Wild birds as potential vectors for pathogen dissemination on migration routes in the Danube Delta Wetlands. Int. J. Curr. Micobiol. Appl. Sci. 2014, 3, 890–897. [Google Scholar]
- Baldacchino, F.; Muenworn, V.; Desquesnes, M.; Desoli, F.; Charoenviriyaphap, T.; Duvallet, G. Transmission of pathogens by Stomoxys flies (Diptera, Muscidae): A review. Parasite 2013, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M. Insect vectors involving in mechanical transmission of human pathogens for serious diseases. Int. J. Bioinform. Biomed. Eng. 2015, 1, 300–306. [Google Scholar]
- Soto-Arias, J.P.; Groves, R.L.; Barak, J.D. Transmission and retention of Salmonella enterica by phytophagous hemipteran insects. Appl. Environ. Microbiol. 2014, 80, 5447–5456. [Google Scholar] [CrossRef] [PubMed]
- Fotedar, R.; Banerjee, U. Nosocomial fungal infections--study of the possible role of cockroaches (Blattella germanica) as vectors. Acta Trop. 1992, 50, 339–343. [Google Scholar] [CrossRef]
- Mian, L.S.; Maag, H.; Tacal, J.V. Isolation of Salmonella from muscoid flies at commercial animal establishments in San Bernardino County, California. J. Vector Ecol. 2002, 27, 82–85. [Google Scholar] [PubMed]
- Knuckles, J.L. Survival of enteric pathogens in the pupae of Phormia regina (Meigen). J. Med. Entomol. 1972, 9, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Olafson, P.U.; Lohmeyer, K.H.; Edrington, T.S.; Loneragan, G.H. Survival and fate of Salmonella enterica serovar Montevideo in adult horn flies (Diptera: Muscidae). J. Med. Entomol. 2014, 51, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, C.; Albano, H.; Silva, J.; Teixeira, P. Role of flies as vectors of foodborne pathogens in rural areas. Int. Soc. Res. Not. Microbiol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Armed Forces Pest Management Board (Board AFPM). Filth Flies: Significance, Surveillance, and Control in Contingency Operations; Board AFPM, Information Services Division: Washington, DC, USA, 2011. [Google Scholar]
- Lemon, K.M. Dispersal of the ergot fungus Claviceps purpurea by the lauxaniid fly Minettia lupulina. J. N. Y. Entomol. Soc. 1992, 100, 182–184. [Google Scholar]
- Cammack, J.A.; Pimsler, M.L.; Crippen, T.L.; Tomberlin, J.K. Chemical Ecology of Vertebrate Carrion. In Carrion Ecology, Evolution, and Their Applications; Benbow, M.E., Tomberlin, J.K., Tarone, A.M., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 187–212. [Google Scholar]
- Burgess, N.; Cowan, G.O. A Colour Atlas of Medical Entomology; Springer Science & Business Media: Berlin, Germany, 2012; p. 144. [Google Scholar]


| Temperature Condition | Mechanism | Effect | Citation |
|---|---|---|---|
| Cold shock | Membrane rigidity | Decreased energy, decreased fluidity | [52] |
| Cold shock | Ice crystals form (in presence of water) | Cell lysis | [54] |
| Cold shock | Enzyme activity slows or ceases | Cell Growth slows or ceases | [54] |
| Optimal temperature | Increased membrane fluidity | Cell Growth increases | [55] |
| Optimal temperature | Increased metabollic activity and enzyme rate | Cell Growtn increases | [55] |
| Heat Shock | Protein denaturation | Cell Growth ceases | [56] |
| Heat or cold shock | Regulation of protein folding | Regulation of protein secretion | [57] |
| Extreme cold or heat shock | Increased membrane permeability and potential | Effects on active transport and ATP synthesis; Inability to form functional, stable organelles (eukaryotes) | [52,53,58] |
| Extreme cold or heat shock | Increased pH | Effects on DNA transcription, protein synthesis, and enzymatic activity | [58,59] |
| Extreme cold or heat shock | Increased reactive oxygen species | Cell death | [58] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jordan, H.R.; Tomberlin, J.K. Abiotic and Biotic Factors Regulating Inter-Kingdom Engagement between Insects and Microbe Activity on Vertebrate Remains. Insects 2017, 8, 54. https://doi.org/10.3390/insects8020054
Jordan HR, Tomberlin JK. Abiotic and Biotic Factors Regulating Inter-Kingdom Engagement between Insects and Microbe Activity on Vertebrate Remains. Insects. 2017; 8(2):54. https://doi.org/10.3390/insects8020054
Chicago/Turabian StyleJordan, Heather R., and Jeffery K. Tomberlin. 2017. "Abiotic and Biotic Factors Regulating Inter-Kingdom Engagement between Insects and Microbe Activity on Vertebrate Remains" Insects 8, no. 2: 54. https://doi.org/10.3390/insects8020054





