1. Introduction
In 1922 Hambleton [
1] assigned laborers the task of measuring the weight of two beehives over several months at precise hourly intervals, continuously, day and night. The high-precision scales (±10 g) yielded a unique insight into the daily pattern of hive weight change, resulting from the interplay between the honeybee colony and its surroundings. Hambleton [
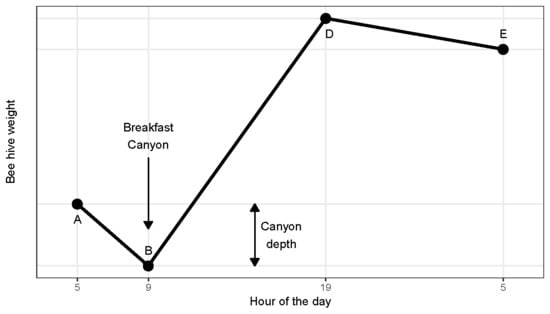
1] identified a pattern which he summarized in a graph, displaying a series of line segments with clearly defined shifts in slope (
Figure 1). After sunrise he found a ‘morning loss’ (from A to B) interpreted as the recruitment of foragers. The morning loss would end abruptly as foragers began returning, thus creating a steady rate of net gain (B to D) due to incoming nectar. He acknowledged that the course of weight change from B to D would vary according to weather and the availability of nectar sources. Between sunset and sunrise (from D to E) he found a constant ‘nocturnal loss’ rate attributed to evaporation and respiration.
The within-day dynamics of beehive weight was not studied further until 70 years later when Buchmann and Thoenes [
2] repeated the procedure of Hambleton [
1], albeit using electronic scales linked to data loggers. Without recognizing the precedence in Hambleton’s work, they identified the same daily pattern during nectar flows as seen in
Figure 1. Later, Meikle et al. [
3,
4,
5] analyzed more extensive data sets of continuously monitored beehive weight but daily variation was reduced to a simple sine wave curve without going into any details of the daily pattern.
The work presented here began as we were intrigued by a regular pattern found in the weight of continuously monitored beehives in Southern Arizona. On many mornings, there would be a distinct dip in weight. This we dubbed ‘Breakfast Canyon’ after a nearby locality, only later realizing that the canyon low point corresponded to the point B of Hambleton (
Figure 1).
Continuous weight curves logged at a frequency of 1 h or less is one of several measures used for automatic monitoring of beehive status [
6]. If the hive resides constantly on a scale, this monitoring method is nonintrusive. The logged readings of total hive weight are directly linked to the accumulation of honey and pollen stores, which is an important measure of bee colony performance. In addition, the daily amplitude of weight change has been used to infer bee colony size [
4,
5]. Recently, the within-day pattern of weight changes was analyzed using segmented linear regression [
7]. It was concluded that the early morning weight loss was mostly due to foragers leaving.
Here we will address three questions: (i) Is it possible to extract consistently the salient features of Hambleton’s Graph from beehive weight curves? Are the extracted parameters useful for (ii) describing honeybee colony behavior and for (iii) diagnosing neonicotinoid stress? Of these questions, the first two explores the quality and usefulness of the extracted parameters, while the last one forms a hypothesis that can be rigorously tested.
We used two earlier-published data sets [
4] for the analysis from which we estimated three daily parameter values for each beehive: (1) nocturnal loss rate (kg/h), (2) depth of Breakfast Canyon (kg) (
Figure 1), and (3) daily weight gain (kg/d). Questions (i) and (ii) were explored graphically while for question (iii) the three parameters were used as response variables, regressed upon the level of neonicotinoid stress brought upon the honeybees in the experiments [
4]. The three parameters were successfully extracted by a common R script and provided detailed information on honeybee colony behavior, in general, as well as under neonicotinoid stress.
3. Results
After discarding data from dates disturbed by rain and hive manipulations, a total of 3750 hive × date instances remained (2014: 2204, 2015: 1546). The segmented regression procedure successfully estimated 3742 4-segmented SLRs. Thus, it failed in only eight cases. However, for some of the estimated SLRs, the nocturnal loss rate was impossibly large. By experimentation, a threshold of 3.4 s.d. from the average was found to detect these cases. The detected 15 hive × date instances had a loss >97 g/h or a gain >84 g/h, both unlikely to occur at night. They were removed, reducing the data set finally to 3727 instances. Typical examples of the 3727 SLR models are shown in
Figure 2.
Depending on the course of the segments (
Figure 3), 1904 of the 3727 SLRs (51%) allowed estimation of canyon depth and thereby nocturnal loss rate as well. The remaining 3727 − 1904 = 1823 SLRs, for which a Breakfast Canyon feature could not be identified, were dominated by segments with negative slopes (
Figure 3); i.e., losses persisted from the night until 4 h after sunrise when the analysis ended.
The nocturnal loss segments had mostly negative slopes indicating, indeed, a weight loss (
Figure 4 top). Weight gains during the night caused by rain had been filtered out before the analysis, so the instances of slight nightly weight gains must have other explanations, maybe light rains or moisture uptake from the air.
The onset of Breakfast Canyon (point A in
Figure 1) mostly occurred at ½ hour before sunrise or later (
Figure 4 bottom). Canyon bottoms (point B in
Figure 1) reached in this interval were also the deepest.
The scatter plots of the three estimated parameters were inspected for visual patterns which were highlighted by background shading (
Figure 5). As these patterns were inspired by the data, they cannot undergo statistical hypothesis testing but only serve to develop plausible biological inferences and hypotheses.
A deep Breakfast Canyon (>0.3 kg) was never followed by a large daily weight gain (>1 kg) (
Figure 5 top, light shading). This could be the result of foragers, which on days with scarce resources would spend more time scouting, thus extending and deepening the canyon before they returned with empty crops. In contrast, shallow canyons (depth < 0.3 kg) might be related to rich, nearby nectar sources, resulting in a high weight gain (
Figure 5 top, dark shading); or to a low foraging effort, e.g., due to a small number of foragers, resulting in a lower weight gain (
Figure 5 top, unshaded points).
A deep Breakfast Canyon (>0.3 kg) only occurred after nights with a distinct weight loss (
Figure 5 middle, dark shading). If the weight loss was caused by drying nectar, foragers might have been cued to leave in high numbers anticipating yet another day of high nectar flow.
The nocturnal loss rate was always high when the weight gain during the previous day had been large (>0.6 kg) (
Figure 4 bottom, dark shading right). This would be expected since most of the gain would be due to nectar, the main source for evaporation during the night. However, a tremendous loss the day before (more than 1 kg), as well led to high losses at night (
Figure 4 bottom, dark shading left). Apparently, these hives suffered a sustained weight loss from the previous day that lasted into the night. For intermediate weight gains during the day (
Figure 4 bottom, light shading), rates of gains as well as losses occurred the following night.
When the three parameters were applied as response variables for the neonicotinoid treatments (
Figure 6), the treatment with the highest concentration pushed the distribution towards lower values for Breakfast Canyon depth, making it shallower. The statistical treatment (see
File S1: Section 1) supported this result (
p = 0.003 for both years). The other two parameters seemed not to react to the treatment.
4. Discussion
The obvious statistic to extract from beehive weight curves is the daily weight gain e.g., [
10,
11]. Easily calculated as the weight difference between subsequent midnights, it is directly related to the hoarding of honey and therefore relevant both to the understanding of honeybee ecology and to monitoring of honeybee colony performance. However, through segmented linear regression and subsequent algorithmic analysis, we successfully extracted more detailed information from the daily weight curves, ultimately obtaining consistent estimates of two features of Hambleton’s Graph (
Figure 1), namely nocturnal loss rate and Breakfast Canyon (BC) depth.
The weight changes of a beehive result as a summation of several concurrent processes: nectar inflow and evaporation, fluctuations in honey and pollen stores, base and heating respiration, worker mortality, precipitation, dew, and physical exchanges of humidity between the air and the beehive construction. Pollen stores tend to be short-lived and are not usually stockpiled during periods of ample pollen resources [
12,
13]. Hence, pollen collection effort is driven by the current demand of the brood [
14] and by its availability; the collected pollen is readily consumed. Nectar, on the other hand, is collected both to cover the current energetic demands of the colony and to process and store as honey. This is the basis for ‘nectar flows’, i.e., periods with a steady increase in honey stores. Overall, colonies usually recruit more foragers for nectar collection than for pollen collection [
14].
Hambleton [
1] expected his graph (
Figure 1) to apply only to periods of nectar flow. In our study, we identified his graph by a distinctive BC which we found in 51% of the cases. Since we consider nectar flows to have been present less than 20% of the time, Hambleton’s Graph seems applicable also outside nectar flows.
Base respiration will, in general, change only slowly as the colony changes in size and distribution among life stages. In the desert climate of the experiments reported here, respiration must have risen every night to maintain hive temperature in face of the cold outdoors. Total respiration rates of 30−50 g/day have been reported from beehives overwintered at 3–5 °C [
15]; we would expect these respiration rates to apply roughly to our hives a well. Water carried in by the bees to cool the hive would not register on the scale, as water is not stored but brought to evaporation immediately. Rain was a rare event but major rainfall events could be detected and were removed from the data set to reduce noise. Thus, disregarding minor showers and physical changes of humidity, major weight changes in these experiments must have been caused by honeybee traffic, together with nectar inflow and its transformation into honey, which involves water evaporation.
The proportion of a bee colony that are foragers varies from 4.1% to 9.6%—larger during nectar flows [
16]. The average weight of a worker honeybee has been reported at 115 mg [
17] and 128 mg [
3]. BC depth varied (
Figure 5 top) with a median value of 85 g and a 95% percentile of 249 g. Assuming an average weight of 122 mg for a bee, we get a median BC depth corresponding to 85/0.122 = 697 bees and a 95% percentile of 249/0.122 = 2041 bees. Assuming further that the 95% percentile applies to nectar flow periods, we get an estimate of the total colony size of 2041/0.096 = 21,260 bees. If the median BC depth applies to non-nectar flow periods, which were dominant during the observation periods, we get a total of 697/0.041 = 17,000 bees. The adult bee population in these experiments was typically in the 2–4 kg range [
4], corresponding to a colony size of 16,400–32,800 bees (122 mg per bee). Thus, the estimated BC depths are consistent with the hypothesis that BC was caused by bees leaving.
The mechanisms connecting daily weight gain, nocturnal loss rate and BC depth (
Figure 5) were interpreted in terms of honeybee biology. Nocturnal weight gains were not unusual. This points to moisture taken up by the hive structure (made of wood) and by open pollen and nectar cells during the desert night when the relative humidity of the air is rising. In Southern France, empty wooden hives will fluctuate as much as 200 g in weight between night and day [
18]; however, in an occupied beehive the fluctuation will likely be less due to the microclimate-regulating habit of the honeybees.
Scout bees will return and recruit foragers through their waggle dance, probably resulting on any given day in a focus on a few high-yielding patches [
19]. A potential forager will instead become a scout, the longer time it waits without meeting a recruiting (dancing) bee [
20]. In the event that a food patch persists, foragers are likely to remember this and will continue to exploit the same patch without further scouting [
21]. These mechanisms will make scouts and foragers out of most of the honeybees leaving during the BC period. If abundant resources are found nearby, the bees will return quickly. This will lead to a shallow BC and a high weight gain on the same day. This mechanism was supported by our data, in which a deep BC never coincided with a high weight gain (
Figure 5 top, light shading).
In the original paper [
4] presenting the data reused here, it was found that the ‘high’ neonicotinoid treatment reduced both colony size and average frame weight. We found a reduced BC depth for the high treatment (
Figure 6 and statistical analysis in
File S1: Section 1), which indicates a lowered foraging capacity of the bee colony. This result supports the original conclusion, that bee colonies were weakened by the high treatment. In conclusion, BC depth might represent a new measure by which to monitor bee colony strength.
We successfully estimated segmented linear regressions in agreement with Hambleton’s Graph (
Figure 1) even outside nectar flows. The shape of this graph, however, may vary among climates. Cooler mornings would delay the onset of Breakfast Canyon. A colony placed near a superabundant nectar resource, such as a commercial field of oilseed rape, might shorten the foraging trip as much as to fill up the Breakfast Canyon with returning scouts and foragers, so fast that the canyon would not show; its detection relies on a delay until the first successful bees return.
In rainy locations, it might be difficult to achieve an undisturbed time series of hive weight. For hives not in some kind of shelter, foraging activity between rain showers would be difficult to filter out from weight fluctuations due to the accumulation of rain on the hive and subsequent drying. For research purposes, the hive and scale should be protected by a rain cover. In addition, hives made of water-inert materials, such as plastic foam, would reduce the noise produced by fluctuations in hive moisture content.
The collection of a variety of sensor data (weight, temperature, humidity, acoustics, digital vision) from honeybee hives has received much attention recently [
6]. However, there is a lack of methods to turn the accumulated body of data into biologically meaningful information. The method described here is a step towards establishing a toolbox to turn beehive sensor data into valuable research and monitoring information.