Abstract
Blow flies of the subfamily Luciliinae (Diptera: Calliphoridae) are one of the main forensically important subfamilies globally. In addition to being used to estimate the minimum post-mortem interval (PMImin), assuming colonization occurred after death, blow fly specimens found infesting a human corpse are used to determine if the corpse was relocated or if the individual ingested narcotics prior to death. The presence of these blow flies in a given area is strongly influenced by abiotic and biotic factors, such as temperature, elevation, and habitat. Having this information, along with geographical distributions and the characteristics of preferred habitats, is necessary to better understand the biology of this group. This study aimed to characterize the spatial distribution of Luciliinae throughout 18 sampling sites within six ecozones (disturbed mixed deciduous forest, mixed deciduous forest, mixed orchard, paddy field, lowland village, and city/town) in central Chiang Mai Province, northern Thailand over one year (May 2009–May 2010). The purpose of the study was to elucidate the relationship of blow fly species composition with environmental abiotic factors (e.g., temperature, relative humidity, light intensity), and to predict the distribution of the common species within this subfamily using GIS. Adult collections were performed biweekly, baited with one-day-old beef offal. A total of 2331 Luciliinae flies trapped, comprising eight species, of which the four predominant species were Hemipyrellia ligurriens (Wiedemann) (n = 1428; 61.3%), Lucilia porphyrina (Walker) (n = 381; 16.3%), Hemipyrellia pulchra (Wiedemann) (n = 293; 12.6%), and Lucilia papuensis Macquart (n = 129; 5.5%). Population density across species varied seasonally, peaking in August 2009 coinciding with the rainy season. Predicting population composition was based on a model developed with ArcGIS 9.2, which utilized environmental variables (temperature, relative humidity, and light intensity) in conjunction with abundance data. Models indicated H. ligurriens had the most widespread geographic distribution, while H. pulchra was predicted to occur largely in mixed orchards and lowland villages. Lucilia porphyrina and L. papuensis were less widespread, restricted mainly to mixed deciduous forest. This model, along with knowledge of forensic information, may be useful under certain investigations where the corpse may have been relocated.
1. Introduction
Blow flies (Diptera: Calliphoridae) draw much attention from the forensic community due to their close association with decomposing remains and subsequent value as evidence [1,2,3,4]. They are the first group of insects to arrive at a corpse, often within minutes of death, thus being used in the crime scene investigation especially in estimating a minimum postmortem interval (PMImin) [3,5,6]. In many countries, blow flies of the subfamily Chrysomyinae (e.g., genus Chrysomya) account for the predominant taxa found infesting human corpses; however, those of the subfamily Luciliinae (e.g., genus Lucilia, Hemipyrellia, Hypopygiopsis) are still of consequence because of their distribution and close association with such resources [7,8,9,10]. Examples of cases where remains were infested with Luciliinae flies included L. papuensis in Australia [11], H. ligurriens in Malaysia [12], China [8], and Australia [11], Hemipyrellia tagaliana (Bigot) in Malaysia [13], and Hypopygiopsis violacea Macquart in Malaysia [14]. In Thailand, 10 species of Luciliinae flies have been recorded, including the genera Lucilia, Hemipyrellia, and Hypopygiopsis [15]. Among these, H. ligurriens, Lucilia cuprina (Wiedemann), and L. porphyrina, were found in association with human corpses [4,16]. Although the Luciliinae flies found in Thailand occur mainly in Asia, Australia, and Oceania [17,18,19,20,21], very little information about their spatial and temporal distributions and forensic cases have been reported. This limitation may be due to Luciliinae flies accounting for a small proportion of the populations that infest dead bodies. A recent study in Thailand reported that Luciliinae flies accounted for only 0.63% of a total of 147,248 calliphorids collected throughout a year [22]. Furthermore, Luciliinae accounted for 5.9% of blow flies collected from human corpses in Malaysia [12] and 6.5% of blow flies from cases in Thailand [4].
Knowledge of the distribution, biology, and behavior of forensically important flies is helpful in forensic investigations by providing information about time, location, and condition of the death [2,3,23,24]. In Thailand, the majority of studies on Luciliinae have focused on species identification [25,26,27] and developmental rate of the immature stages [28,29]. Although surveys of forensically important flies have been conducted on a local scale in Thailand, a comprehensive landscape assessment of the distribution of Luciliinae that are forensically important is limited [30,31,32]. Previous work on spatial analysis of forensically important blow flies has focused on Chrysomya megacephala (Fabricius) [33], Chrysomya rufifacies (Macquart) [34], Chrysomya pinguis (Walker), Chrysomya chani Kurahashi, Chrysomya villeneuvi Patton and Ceylonomyia nigripes (Aubertin) [35]. However, studies on Luciliinae flies are lacking. Thus, this study aimed to investigate the occurrence of Luciliinae blow flies across six diverse land use categories in central Chiang Mai Province, northern Thailand. The influence of climatic factors (temperature, relative humidity, and light intensity) on their geographic distributions was investigated. Furthermore, the predicted distributions of the predominant species sampled were modeled using ArcGIS 9.2 (ESRI, Redlands, CA, USA). To our knowledge, this is the first study to spatially and temporally characterize the Luciliinae fly population over a variety of land use types in Thailand.
2. Materials and Methods
2.1. Study Areas
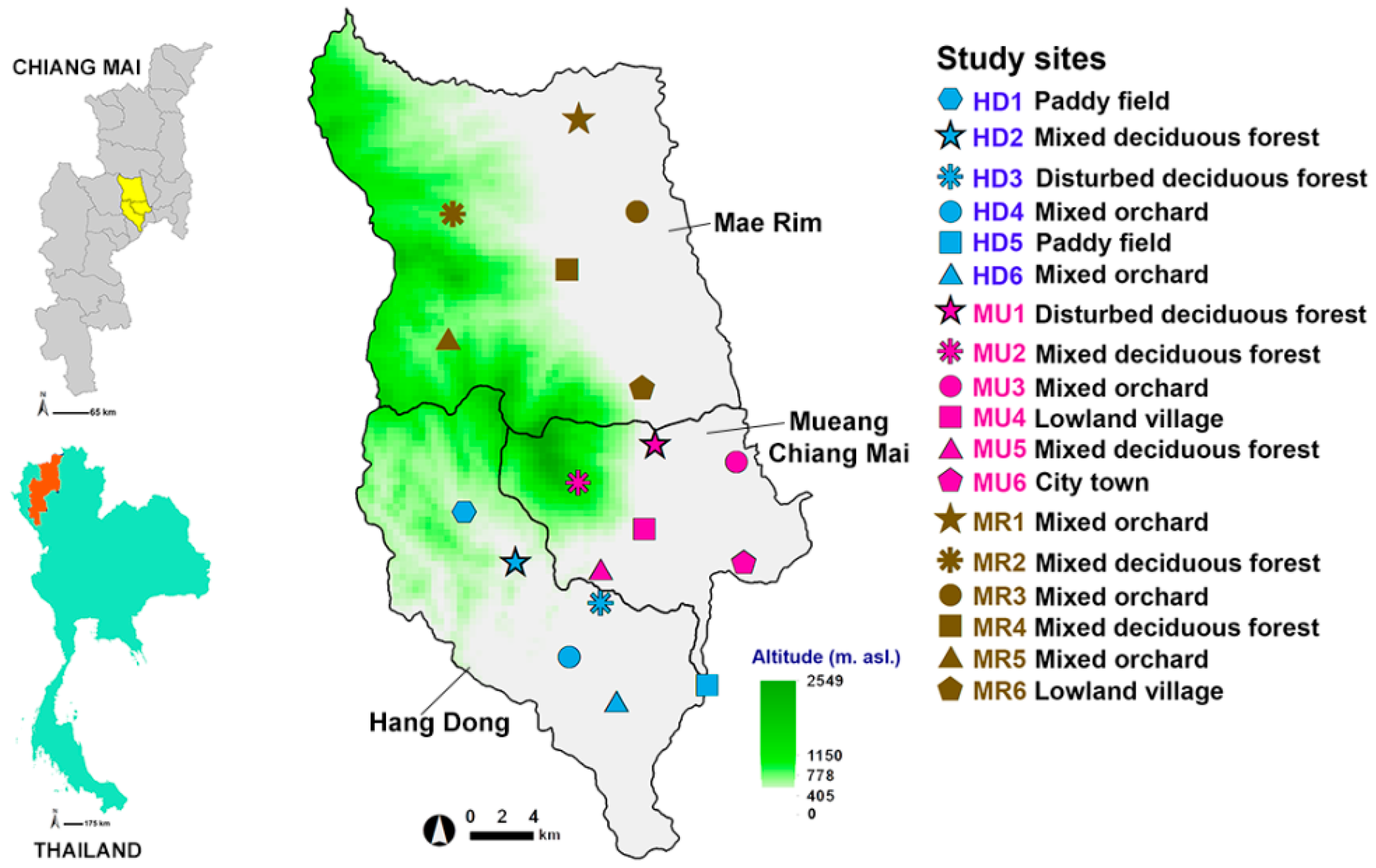
In order to develop a prediction model, flies of forensic importance were sampled in May 2009 to May 2010 by selecting 18 study sites located within three districts of Chiang Mai Province, northern Thailand. These calibrated study sites were distributed across the following districts: one urban (Mueang Chiang Mai (MU)) and two suburban sites (Hang Dong (HD) and Mae Rim (MR)) (Figure 1). Following a systematic random sampling method [36], the study area within the three districts was partitioned into a sampling frame of 5 × 5 km for suburban areas (Mae Rim and Hang Dong districts) and 3 × 3 km for the urban area (Mueang Chiang Mai district). The study area was plotted using the contour maps of Chiang Mai Province (MapMagic™ scale 1:150,000 with a UTM projection type, Everest Spheroid and the Indian 1975 Datum). More detail of the sample site selection procedure and land use classification is provided in File S1. Six land uses were categorized: mixed deciduous forest, disturbed mixed deciduous forest, paddy field, mixed orchard, lowland village, and city/town. The 18 study sites covering six land uses were used to model the likely spatial distribution of Luciliinae flies.
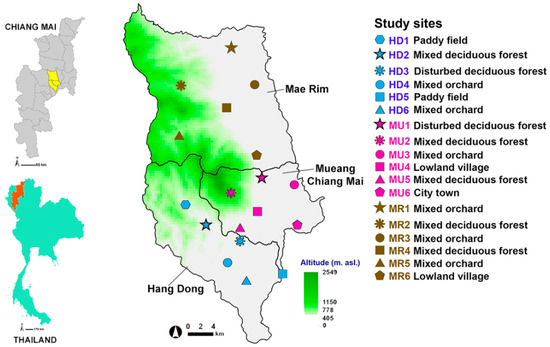
Figure 1.
Map of Thailand showing three sample districts (Mueang Chiang Mai, MU; Mae Rim, MR; and Hang Dong, HD) and 18 sample locations. Green shading indicates a mountainous zone.
2.2. Adult Flies Collection
Adult flies were trapped every two weeks from May 2009 to May 2010, using an in-house prototype portable funnel trap kit [33]. The trapping method was described previously [33]. Briefly, the trap consisted of a polyvinyl chloride (PVC) frame box (30 × 30 × 50 cm), a fly entrance module, and a black fly net (30 × 30 × 80 cm). Two replicate sets of traps were placed in a single row (50 m apart) on the ground [33], each baited with 250 g of one-day-old beef offal [37], which was kept in a translucent plastic container. The bait was placed underneath the fly entrance module after it had been tied to a fly net with elastic bands and installed with the PVC frame box. Flies lured by the smell from the offal were collected by their passive movement up toward the light through the fly entrance module to the fly collection net after landing on the bait. Traps were placed in the shade to prevent thermal stress for trapped flies [38]. The collection was done for a 1-h period between 09:30 a.m. and 12:00 p.m. Physical data of each study site were collected including light intensity (lux) (LUX/FC light meter TM-204 Tenmars, Tenmars Electronics Co., Ltd., Taipei, Taiwan), temperature (°C), relative humidity, RH (%) (Digital Hygro-Thermo Meter (DHT-1), Daeyoon Scale Industrial Co., Ltd., Seoul, South Korea) and the co-ordinates (Garmin™ eTrex Handheld GPS, Garmin China Co., Ltd., Chaoyang, China).
All specimens were carried to the laboratory of the Department of Parasitology, Faculty of Medicine, Chiang Mai University. They were sacrificed by placement in a freezer set at 0 °C for 2 h. Flies were then identified under a dissecting microscope (model SZ2-ILST, Olympus Corporation, Tokyo, Japan) using taxonomic keys, sexed, and counted [20,21].
2.3. Statistical Analysis
Three seasons were considered: a rainy season occurring from June through October, winter from November through February, and summer from March through May (File S2). Species sampled with fewer than 100 specimens captured year-round were excluded from the analysis. We analyzed the relationship of environmental variables (temperature, relative humidity, and light intensity) with trap catch using negative binomial regression analysis. We used one-way analysis of variance (ANOVA) followed by the post hoc Bonferroni test (homogeneity of variance: p > 0.05) or Dunnett T3 test (homogeneity of variance: p < 0.05) to determine the relationship between land use types and mean total number of flies using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA) (α = 0.05).
To predict the spatial distribution of Luciliinae flies by season and aggregated for the full year, we conducted additional geospatial analysis using a kriging/co-kriging approach in ArcGIS 9.2. Kriging is a kind of linear least squares estimation method [39] that is associated with spatial optimal linear prediction in which the unknown random-process mean is estimated with the best linear unbiased estimator [40]. To create a continuous surface of the phenomenon, predictions are made for each location in the study area based on the semivariogram and the spatial arrangement of measured values that are nearby. Co-kriging is an extension of kriging for prediction of one variable using other variables. The co-variables must have a relationship and this relationship must be defined [39]. The use of co-kriging requires the spatial covariance model of each variable and the cross-covariance model of the variables is defined [39].
In this study, the parameters that were used in co-kriging included climatic factors that had a statistically significant relationship with fly numbers and the total trap catch during the study period. The data were log-transformed to normalize the distribution and minimize standard error of the geographical analysis [40]. Moreover, the data of land use types were defined as dummy variables and were incorporated into the ordinary kriging/co-kriging analysis. The mathematical models for evaluating the semivariogram/covariance function were accepted when the model provided the lowest root-mean-square prediction error [33].
3. Results
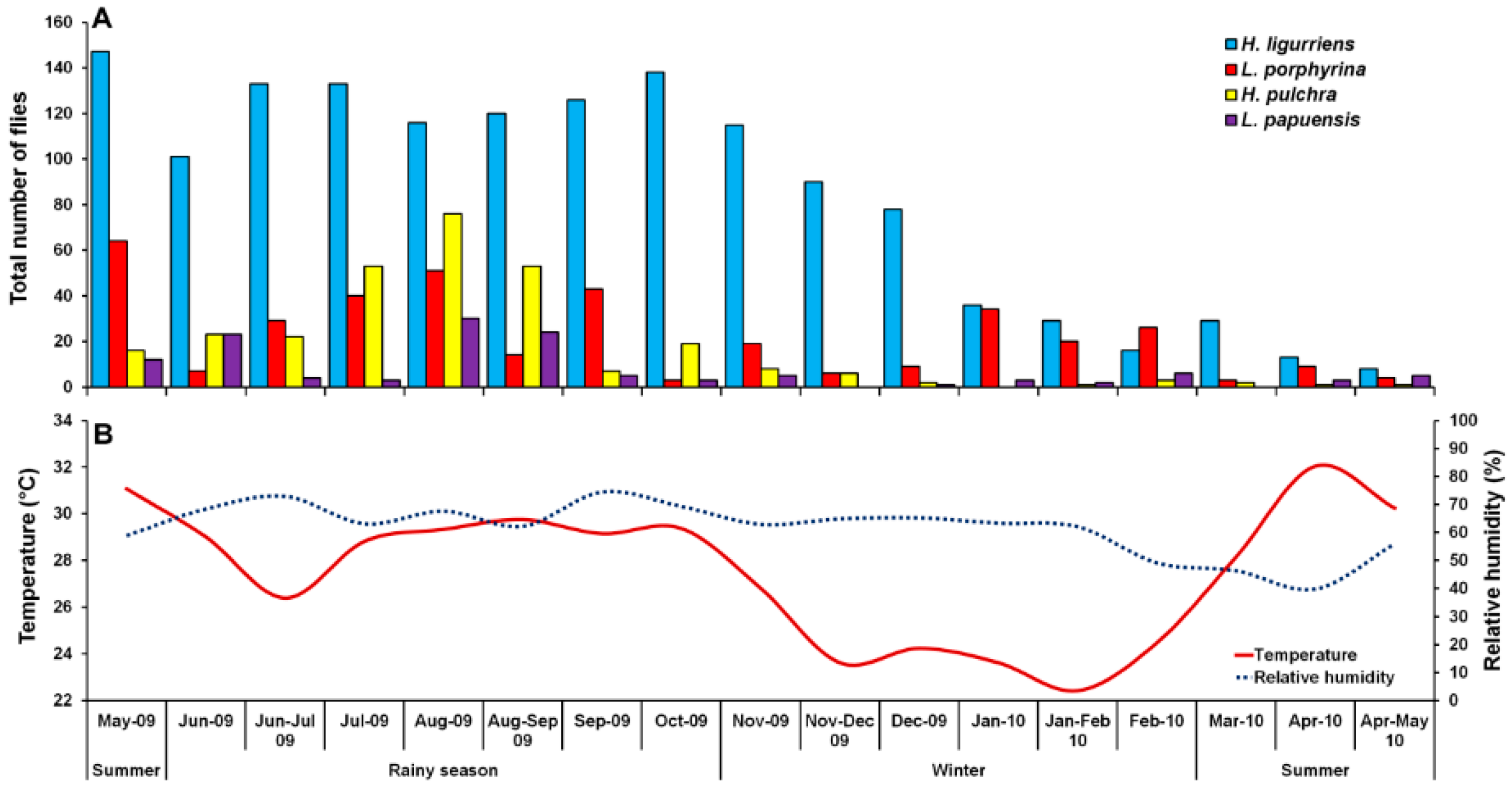
A total of 2331 Luciliinae flies were sampled, comprising eight species, of which the predominant species was H. ligurriens, representing 61.3% of the individuals (n = 1428). Other associated species were L. porphyrina (n = 381; 16.3%), H. pulchra (n = 293; 12.6%), and L. papuensis (n = 129; 5.5%). Small numbers of L. cuprina (n = 67; 2.9%), Hypopygiopsis tumrasvini Kurahashi (n = 23; 1.0%), Lucilia sinensis Aubertin (n = 6; 0.3%) and Hypopygiopsis infumata (Bigot) (n = 4; 0.2%) (Table 1). Therefore, this paper focused mainly on the four most sampled species, namely H. ligurriens, L. porphyrina, H. pulchra, and L. papuensis. The sampled fly numbers varied seasonally, peaking in August 2009, coinciding with the rainy season, while a minor peak was in summer (May 2009). The sample numbers decreased gradually during the late rainy season (September 2009) and remained low throughout the winter and summer (Figure 2).

Table 1.
Climatic factors (temperature, relative humidity, and light intensity) recorded and total numbers of Luciliinae flies collected at each land use type.
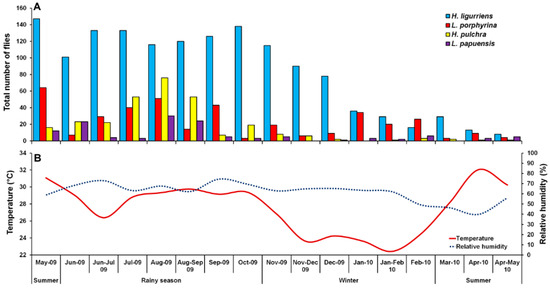
Figure 2.
Monthly fluctuations in trap catches of H. ligurriens, L. porphyrina, H. pulchra, and L. papuensis determined using a portable funnel trap baited with one-day-old beef offal in Chiang Mai Province, northern Thailand, May 2009 to May 2010 (A), and variation of temperature and relative humidity recorded during the fly survey (B).
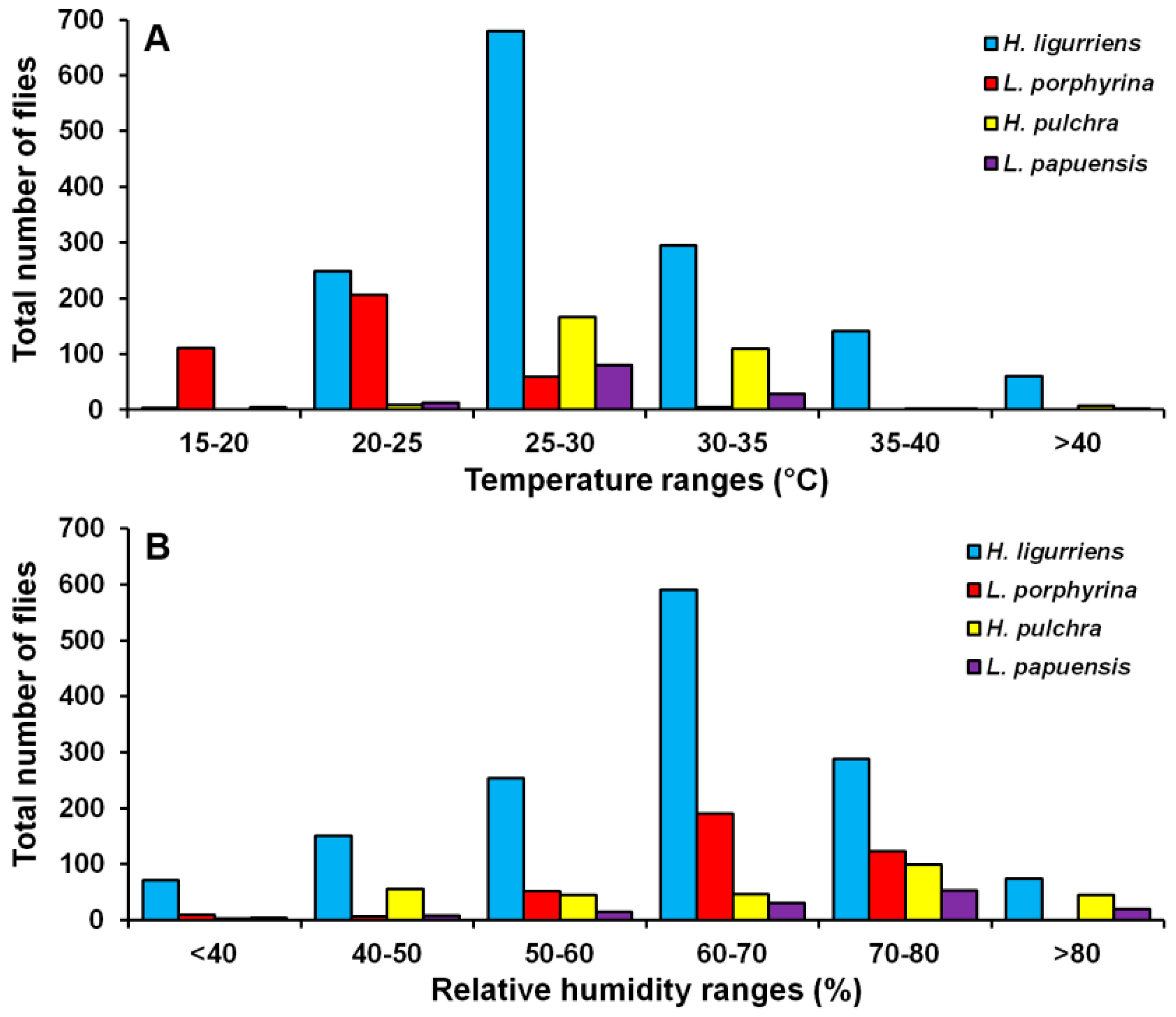
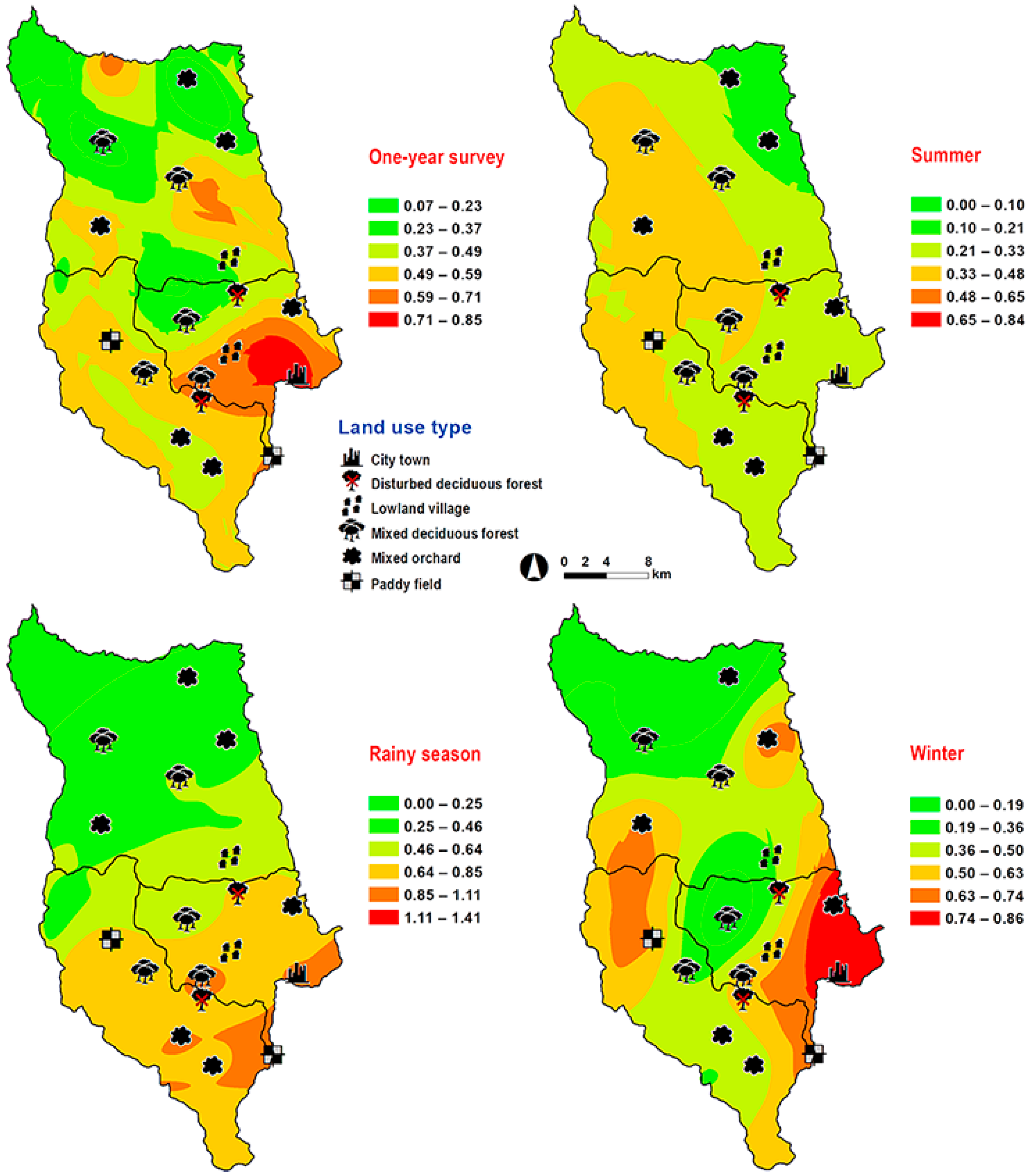
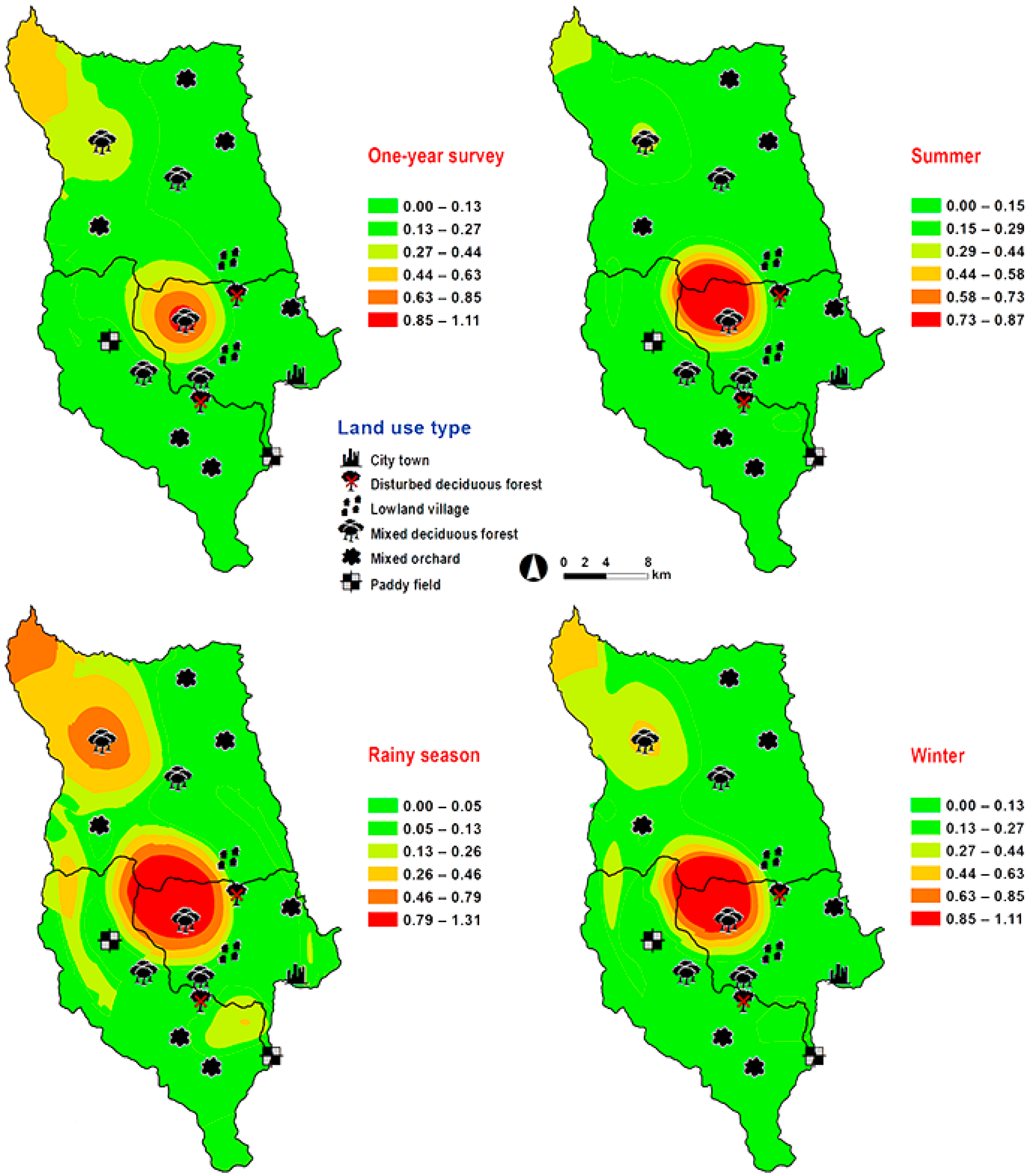
Hemipyrellia ligurriens had widespread geographic distribution (Table 1). Although this species was sampled throughout the year, a bimodal peak was observed—the major peak occurring in late summer (May 2009), while a minor peak occurred in the rainy season (June–October 2009). As expected, sample numbers gradually decreased at the onset of winter (November 2009) and remained minimal throughout winter and summer (January–May 2010) (Figure 2). H. ligurriens trap catch was positively correlated with temperature and relative humidity (p < 0.05), with increased temperature (B = 0.103, p = 0.0001) and relative humidity (B = 0.035, p = 0.0001) associated with increases in trap catch (Table 2). No correlation with light intensity was found (p = 0.954). The greatest numbers were collected at 25–30 °C and 60–70% RH (Figure 3). Analyses using co-kriging showed the spatial distribution of fly density across a variety of land uses in Mueang Chiang Mai district (Figure 4).

Table 2.
The association of climatic factors with Luciliinae trap catches.
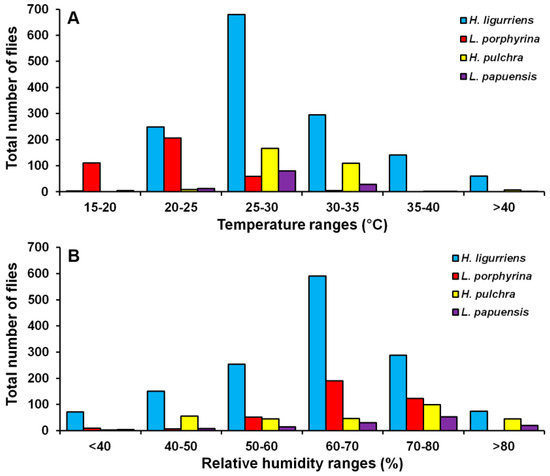
Figure 3.
Total number of flies captured at different temperature (A) and relative humidity ranges (B).
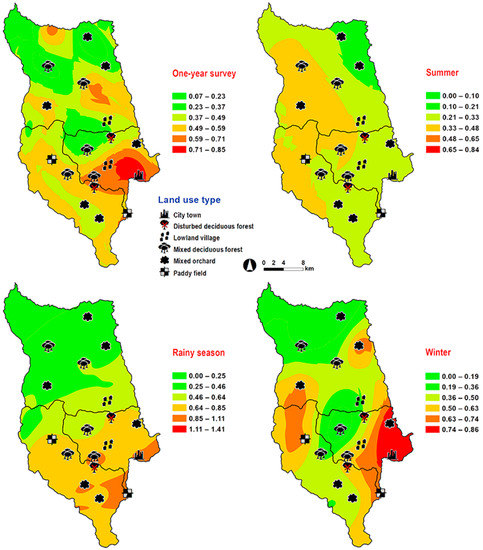
Figure 4.
Predictive distribution maps of Hemipyrellia ligurriens. The color scheme reflects different fly density categories. The red areas indicate the highest fly population, while the green areas indicate the lowest population density. The scale corresponds to natural logarithm of (fly density + 1).
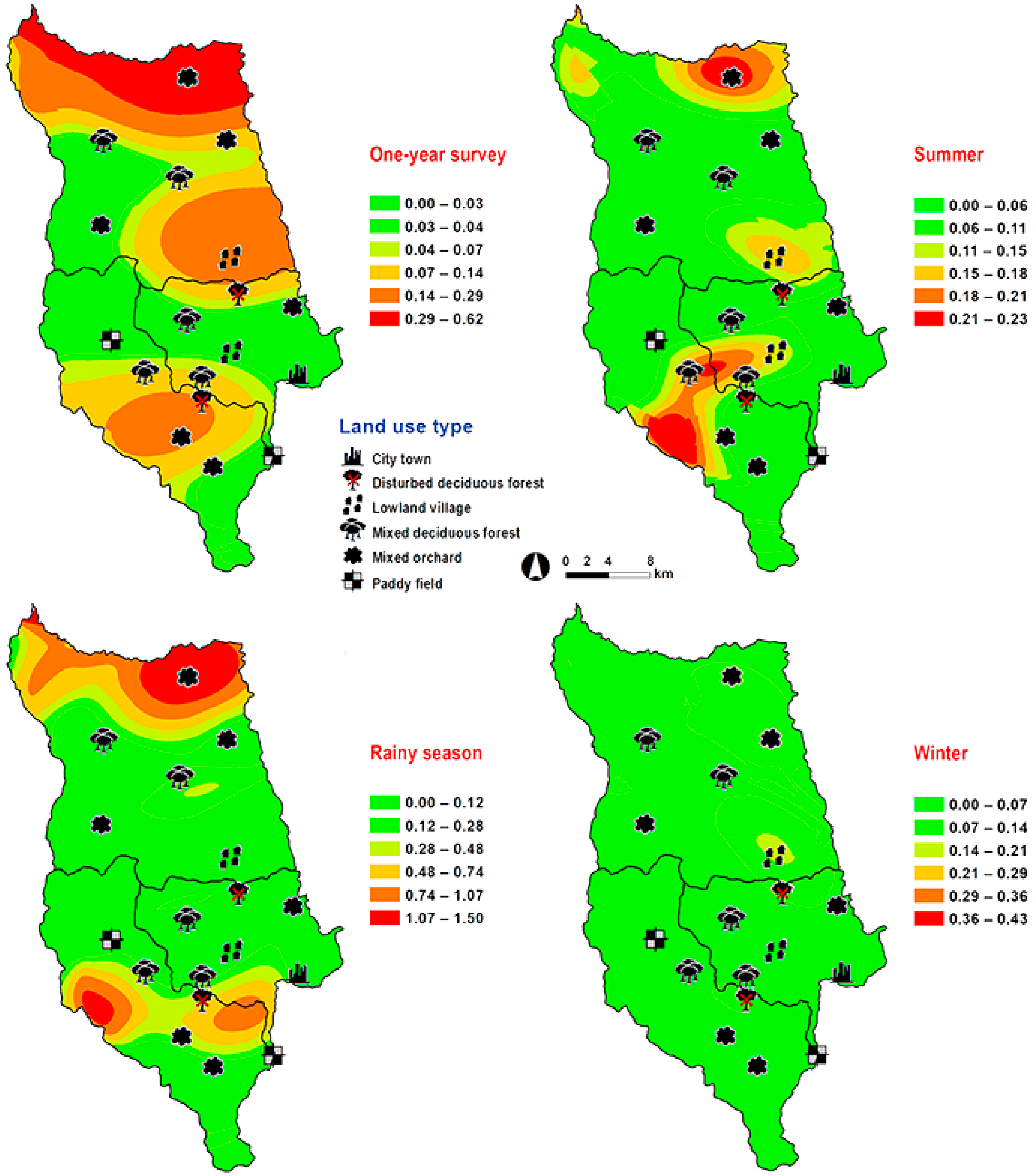
Lucilia porphyrina was prevalent in mixed deciduous forest (Table 1). Although it was trapped throughout the year, no particular seasonal fluctuation pattern was found. Collections were highest in late summer (May 2009), and in the rainy season (June–September 2009), while a small peak was in the winter (January–February 2010) (Figure 2). L. porphyrina trap catch was positively correlated with temperature and light intensity (p < 0.05), with increased temperature (B = −0.263, p = 0.0001) and light intensity (B = −0.000034, p = 0.0001) significantly associated with the decreased trap catch. No correlation with relative humidity was found (p = 0.741) (Table 2). The greatest numbers of this species was collected at 20–25 °C and 60–70% RH (Figure 3). Analyzed using co-kriging, the predicted geographical distributions were found in mixed deciduous forest at 950 m a.s.l. of Mueang Chiang Mai district (MU2) and mixed deciduous forest at 407 m a.s.l. of Mae Rim district (MR2) throughout the year (Figure 5).
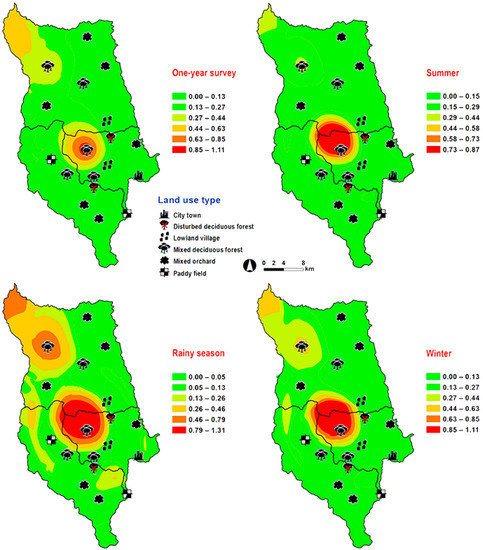
Figure 5.
Predictive distribution maps of Lucilia porphyrina. The color scheme represented different fly density categories. The red areas represent the highest fly population, while the green represent the lowest population density. The scale corresponds to natural logarithm of (fly density + 1).
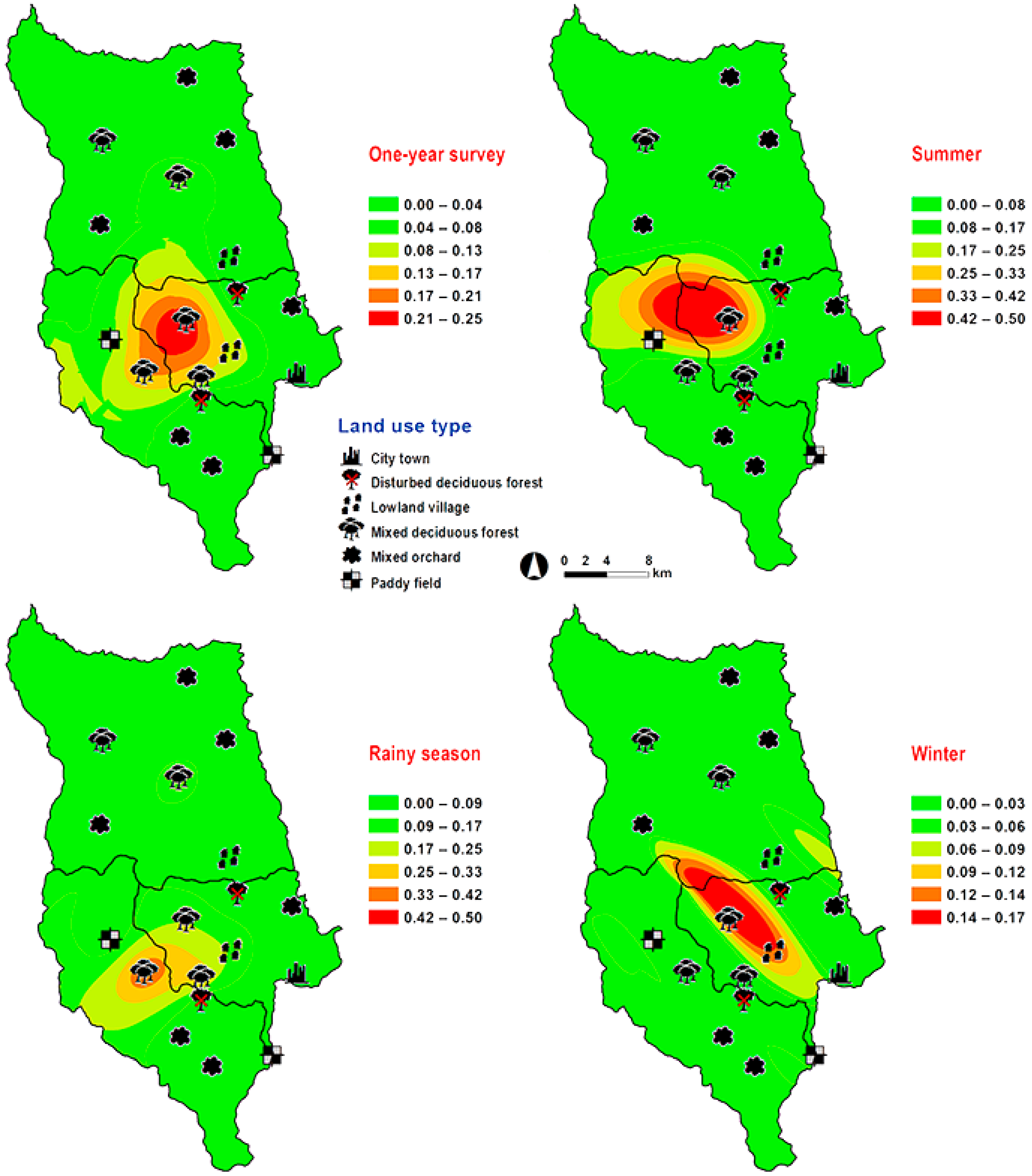
Hemipyrellia pulchra was collected primarily in mixed orchard and lowland village; this species was not found in paddy field or city/town (Table 1). Peak population was observed in the rainy season (August 2009), and decreased gradually in late rainy season (September 2009) throughout winter (November 2009–February 2010) and summer (March–May 2010). Regression analysis showed a positive significant association between trap catch of H. pulchra and climatic factors (temperature and relative humidity) (p < 0.05). Increased temperature (B = 0.325, p = 0.0001) and relative humidity (B = 0.077, p = 0.0001) were significantly associated with increased trap catch. No correlation with light intensity was found (p = 0.0578) (Table 2). The greatest number of this species was captured at 25–30 °C and 60–70% RH (Figure 3). Co-kriged prediction maps were produced, showing abundance in mixed orchard and lowland village. In summer, the spatial pattern was similar to the aggregated one-year survey. This species was predicted to occur largely in mixed orchard and disturbed mixed deciduous forest in the rainy season, while the fewest numbers were predicted in winter, with little apparent spatial variation (Figure 6).
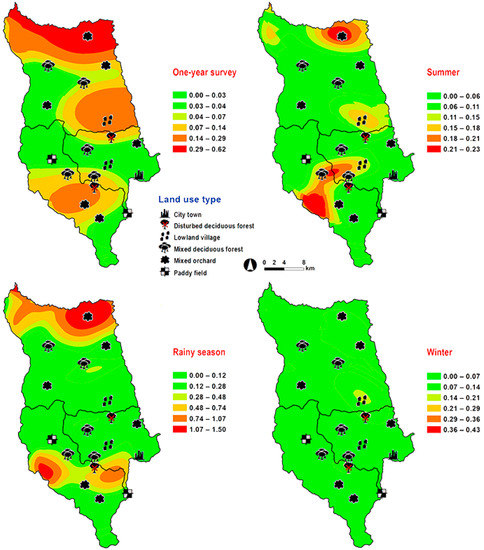
Figure 6.
Predictive distribution maps of Hemipyrellia pulchra. The color scheme represented different fly density categories. The red areas represent the highest fly population, while the green represent the lowest population density. The scale corresponds to natural logarithm of (fly density + 1).
Lucilia papuensis was significantly prevalent (ANOVA, p < 0.05) in mixed deciduous forest and lowland village, respectively (Table 1). A bimodal peak population was evident, with a major peak in the rainy season (August–September 2009) and a minor peak at the onset of the rainy season (June 2009). Fly numbers decreased dramatically during the late rainy season (September–October 2009) throughout winter and summer. No flies of this species were sampled in November‒December 2009 and March 2010 (Figure 2). The trap catch of this species was significantly associated with the climatic factors (p < 0.05). Increased temperature (B = 0.126, p = 0.022) and relative humidity (B = 0.033, p = 0.048) were significantly associated with increased trap catch. However, the increase of light intensity was associated with a significantly reduced trap catch of flies (B = −0.00003, p = 0.003) (Table 2). The greatest numbers of flies were collected at 25–30 °C and 70–80% RH (Figure 3). Co-kriged prediction maps were produced. A one-year survey and seasonal prediction maps exhibited a similar pattern, as this species was predicted to occur largely in mixed deciduous forest along the mountainous areas and in lowland villages (Figure 7).
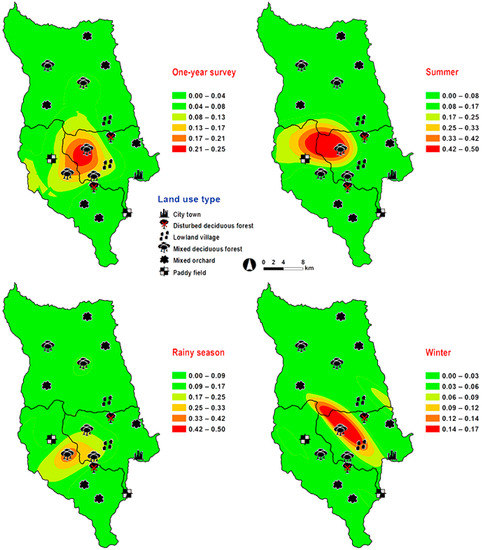
Figure 7.
Predictive distribution maps of Lucilia papuensis. The color scheme represented different fly density categories. The red areas represent the highest fly population, while the green represent the lowest population density. The scale corresponds to natural logarithm of (fly density + 1).
4. Discussion
An adequate knowledge of the ecology and geographical abundance of blow flies is important for the forensic sciences [2,3,41]. However, in Thailand, such information on geographic distribution of forensically important flies was limited prior to this study, which is the first to report the distribution of four forensically important Luciliinae flies; H. ligurriens, H. pulchra, L. porphyrina, and L. papuensis, over a full year. Maps of predicted fly distribution were generated using the Geostatistical Analyst Extension of ArcGIS 9.2.
Abundance relative to ecotype differed among the four species. The study sites, which represented different broad ecological locations, were chosen based on a systematic random sampling method. Some of the 18 study sites under the six land use categories, covering the city to forested areas, may not be suitable for some fly species [17,20,21,42]. We found H. ligurriens had widespread geographic distribution, while H. pulchra was collected mainly in mixed orchard and lowland village land uses. This result may reflect that H. ligurriens can live and develop in many substances such as animal dung, carcasses, human feces, and decomposed organic matter [17,19], while the adult of H. pulchra prefer flowers and fruits in the orchards [19]. A study in Phitsanulok Province, northern Thailand, also reported a wide variety of habitat types, where H. ligurriens can be found, ranging from residential areas to the mountainous zones, but H. pulchra was collected only in the agricultural areas and in the forests [43]. A previous study reported various altitude distribution ranges of H. ligurriens, indicating greater adaptation compared with H. pulchra [32].
Lucilia porphyrina and L. papuensis were less widespread, and were restricted mainly to the mixed deciduous forest (at 950 m a.s.l.). Likewise, others have reported that L. porphyrina and L. papuensis are likely to have their distribution restricted to forested highland areas in Thailand [21,32,43]. A study in Japan indicated that the females of L. porphyrina preferred to lay their eggs in the mountains and/or highlands [44]. Additionally, their larvae have been reported to feed on animals’ carcasses [19,21], which could be found in the forests. However, L. porphyrina could be found in human residences in India and in the mainland of Japan (for overwintering) [19,44]. A small number of L. papuensis was collected in the present study, but it was found in all six of the land uses, with the greatest number in mixed deciduous forest. Many studies also supported its preference for the forest areas [17,19,42,43]. This may be caused by the adults gathering around decaying animals (e.g., earthworms, land snails, and snakes) and other vertebrate carcasses [19,21] that could be found in the forest areas.
The peak trap catch of these species was found in the rainy season (August 2009), and the nadir occurred during the summer (March–May 2010). This finding is in contrast with many studies where more blow flies were collected during the warmer months [22,45,46]. The conditions in the rainy season (mean temperature 29.1 ± 0.3 °C, mean RH 69.7 ± 1.0%) may be suitable for the development of Luciliinae flies. A previous study with H. ligurriens suggested the optimal condition for the development of this species is a temperature range of 16 to 28 °C, not 31 °C or above [47]; therefore, the temperature of the summer months in this study (mean temperature 30.6 ± 0.5 °C) (see Figure 2) may exceed the optimal range. However, the present study was carried out during a single year. To obtain reliable seasonal patterns, the season needs to be replicated (i.e., the study should be replicated during more than one year). In this study, fly trapping was done before noon (9:30 a.m. and 12:00 p.m.). Blow fly activity was reported to be higher around noontime and in the afternoon than in the early morning [22,48,49], so our collection time may have affected the resulting collections.
The yearly sampling across 18 study sites allows us to spatially predict the population distribution of Luciliinae flies. Our study is not the first to model the prediction of forensically important blow flies. Previous investigations have shown the spatial models of C. megacephala, C. rufifacies, C. pinguis, C. chani, C. villeneuvi and Cey. nigripes [33,34,35]. According to this study, H. ligurriens was predicted to occur across a variety of land uses in Mueang Chiang Mai district especially nearby residential areas (e.g., city/town and lowland village), which is similar to C. megacephala and C. rufifacies [33,34]. On the other hand, L. porphyrina and L. papuensis showed preference in the mixed deciduous forest, which is consistent with C. pinguis, C. chani, C. villeneuvi and Cey. nigripes [35]. The greatest number of H. pulchra was predicted mainly in mixed orchard and lowland village, with low altitudes ranging between 300 and 400 m a.s.l., which is in contrast with a previous study that found this species only at 901–1050 m a.s.l. [32]. However, their studies were carried out in the forested area of altitudes ranging from 400–1050 m a.s.l. and only one specimen of H. pulchra was collected by a sweep net.
According to our results, the prediction maps indicate H. ligurriens occurred in a variety of habitats and is closely associated with human activity. Previous studies reported H. ligurriens infested a human corpse in a forested area of northern Thailand [4]. This species has also been associated with human remains in an outdoor environment of Australia [11]. The widespread distribution of H. ligurriens (ranging from residential areas, forests, and highland) was also observed in Bangladesh and Malaysia [7,42]. In contrast, L. porphyrina was predicted mainly in the mixed deciduous forest at 905 m a.s.l. This species may be used as insect evidence when corpses are found in the forest of mountainous areas. Recently, L. porphyrina was found on a human corpse discovered in an open mountainous area (1200 m a.s.l.) during the winter time of northern Thailand [16]. Additionally, this species infested rabbit carcasses in the highlands of Malaysia (1517.3 m a.s.l.), suggesting that it is a forensically important species in the highlands.
5. Conclusions
We provide predictive maps of the spatial distribution for forensically important blow flies within the subfamily Luciliinae as related to land use types and climatic factors. Hemipyrellia ligurriens and H. pulchra have spread through a wide range of land use types, implying that their application for explaining the location of corpse movement is limited, especially when the immature specimens of these species were found. On the other hand, L. porphyrina and L. papuensis evidently show a preference to the mixed deciduous forest. This database may be incorporated as a useful tool in forensic entomology. To be used for PMImin estimation, biological information such as developmental rate or insect succession of these species are mandatory.
Supplementary Materials
The following are available online at http://www.mdpi.com/2075-4450/9/4/181/s1, File S1: Stratified random sampling, File S2: General description of Thailand climate.
Author Contributions
Conceptualization, K.S. and K.L.S.; Methodology, K.S., K.N.I., R.N.-k., K.M. and T.K.-k.; Formal Analysis, T.K.-k.; Investigation, K.M., R.N.-k. and T.K.-k.; Resources, K.N.I. and H.K.; Data Curation, T.K.-k. and R.N.-k.; Writing—Original Draft Preparation, T.K.-k. and K.L.S.; Writing—Review & Editing, K.L.S., J.K.T. and K.N.I.; Visualization, T.K.-k.; Supervision, K.S. and K.L.S.; Project Administration, K.S. and K.L.S.; Funding Acquisition, T.C., K.L.S., K.S. and P.S.
Funding
Funding to support project activities came from the Thailand Research Fund (RSA5580010 to K.L.S., K.S.; IRN58W0003 to T.C., K.L.S., T.K.-k.), the Royal Golden Jubilee Ph.D. Program (PHD/0246/2550 to K.L.S. and T.K.-k.), Chiang Mai University (CMU) through Excellence Center of Insect Vector, and Diamond Research Grant of the Faculty of Medicine, Chiang Mai University (PAR-2560-04663).
Acknowledgments
We are grateful to the Department of Geography, Faculty of Social Science, CMU, and Geo-Informatics and Space Technology Development Agency, Northern Region, Thailand for providing geographic data.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Tomberlin, J.K.; Crippen, T.L.; Tarone, A.M.; Chaudhury, M.F.B.; Singh, B.; Cammack, J.A.; Meisel, R.P. A review of bacterial interactions with blow flies (Diptera: Calliphoridae) of Medical, Veterinary, and Forensic importance. Ann. Entomol. Soc. Am. 2017, 110, 19–36. [Google Scholar] [CrossRef]
- Catts, E.P.; Goff, M.L. Forensic entomology in criminal investigations. Annu. Rev. Entomol. 1992, 37, 253–272. [Google Scholar] [CrossRef] [PubMed]
- Tomberlin, J.K.; Mohr, R.; Benbow, M.E.; Tarone, A.M.; VanLaerhoven, S. A roadmap for bridging basic and applied research in forensic entomology. Annu. Rev. Entomol. 2011, 56, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Sukontason, K.; Narongchai, P.; Kanchai, C.; Vichairat, K.; Sribanditmongkol, P.; Bhoopat, T.; Kurahashi, H.; Chockjamsai, M.; Piangjai, S.; Bunchu, N.; et al. Forensic entomology cases in Thailand: A review of cases from 2000 to 2006. Parasitol. Res. 2007, 101, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Amendt, J.; Richards, C.S.; Campobasso, C.P.; Zehner, R.; Hall, M.J. Forensic entomology: Applications and limitations. Forensic Sci. Med. Pathol. 2011, 7, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Benecke, M. Six Forensic entomology cases: Description and commentary. J. Forensic Sci. 1998, 43, 797–805. [Google Scholar] [CrossRef]
- Silahuddin, S.A.; Latif, B.; Kurahashi, H.; Heo, C.C. The importance of habitat in the ecology of decomposition on rabbit carcasses in Malaysia: Implications in forensic entomology. J. Med. Entomol. 2015, 52, 9–23. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, M.Y.; Jiang, X.Y.; Wang, J.F.; Li, L.L.; Yin, X.J.; Wang, M.; Lai, Y.; Tao, L.Y. Insect succession on remains of human and animals in Shenzhen, China. Forensic Sci. Int. 2017, 271, 75–86. [Google Scholar] [CrossRef]
- Bernhardt, V.; Balint, M.; Verhoff, M.A.; Amendt, J. Species diversity and tissue specific dispersal of necrophagous Diptera on human bodies. Forensic Sci. Med. Pathol. 2018, 14, 76–84. [Google Scholar] [CrossRef]
- Vanin, S.; Tasinato, P.; Ducolin, G.; Terranova, C.; Zancaner, S.; Montisci, M.; Ferrara, S.D.; Turchetto, M. Use of Lucilia species for forensic investigations in Southern Europe. Forensic Sci. Int. 2008, 177, 37–41. [Google Scholar] [CrossRef]
- Farrell, J.F.; Whittington, A.E.; Zalucki, M.P. A review of necrophagous insects colonising human and animal cadavers in South-East Queensland, Australia. Forensic Sci. Int. 2015, 257, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, R.; Nazni, W.A.; Tan, T.C.; Lee, H.L.; Azirun, M.S. Review of forensically important entomological specimens collected from human cadavers in Malaysia (2005–2010). J. Forensic Leg. Med. 2013, 20, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Kumara, T.K.; Disney, R.H.; Abu Hassan, A.; Flores, M.; Hwa, T.S.; Mohamed, Z.; CheSalmah, M.R.; Bhupinder, S. Occurrence of oriental flies associated with indoor and outdoor human remains in the tropical climate of North Malaysia. J. Vector Ecol. 2012, 37, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Firdaus, M.S.; Marwi, M.A.; Syamsa, R.A.; Zuha, R.M.; Ikhwan, Z.; Omar, B. Morphological descriptions of second and third instar larvae of Hypopygiopsis violacea Macquart (Diptera: Calliphoridae), a forensically important fly in Malaysia. Trop. Biomed. 2010, 27, 134–137. [Google Scholar] [PubMed]
- Bunchu, N. Blow fly (Diptera: Calliphoridae) in Thailand: Distribution, morphological identification and medical importance appraisals. Int. J. Parasitol. Res. 2012, 4, 57–64. [Google Scholar] [CrossRef]
- Monum, T.; Sukontason, K.L.; Sribanditmongkol, P.; Sukontason, K.; Samerjai, C.; Limsopatham, K.; Suwannayod, S.; Klong-klaew, T.; Wannasan, A. Forensically important blow flies Chrysomya pinguis, C. villeneuvi, and Lucilia porphyrina (Diptera: Calliphoridae) in a case of human remains in Thailand. Korean. J. Parasitol. 2017, 55, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, H.; Chowanadisai, L. Blow flies (Insecta: Diptera: Calliphoridae) from Indochina. Spec. Div. 2001, 6, 185–242. [Google Scholar] [CrossRef]
- James, T.M. New species are records of Australasian Calliphorinae, with special reference to the fauna of New Guinea. Pac. Insects 1971, 13, 1–12. [Google Scholar]
- Nandi, B.C. Blow flies (Diptera: Calliphoridae) of West Bengal, India with a note on their biodiversity. Rec. Zool. Surv. India 2002, 100, 117–129. [Google Scholar]
- Tumrasvin, W.; Kurahashi, H.; Kano, R. Studies on medically important flies in Thailand VII. Report on 42 species of Calliphorid flies, including the taxonomic keys (Diptera: Calliphoridae). Bull. Tokyo Med. Dent. Univ. 1979, 26, 243–272. [Google Scholar] [PubMed]
- Tumrasvin, W.; Kurahashi, H.; Kano, R. Studies on medically important flies in Thailand II. Record of four species of Lucilia Robineau-Desvoidy (Diptera: Calliphoridae). Bull. Tokyo Med. Dent. Univ. 1977, 24, 1–8. [Google Scholar] [PubMed]
- Klong-klaew, T.; Sontigun, N.; Sanit, S.; Samerjai, C.; Sukontason, K.; Kurahashi, H.; Koehler, P.G.; Pereira, R.M.; Limsopatham, K.; Suwannayod, S.; et al. Field evaluation of a semi-automatic funnel trap targeted the medically important non-biting flies. Acta Trop. 2017, 176, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Amendt, J.; Campobasso, C.P.; Gaudry, E.; Reiter, C.; LeBlanc, H.N.; Hall, M.J. Best practice in forensic entomology—standards and guidelines. Int. J. Legal Med. 2007, 121, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Amendt, J.; Krettek, R.; Zehner, R. Forensic entomology. Naturwissenschaften 2004, 91, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Klong-klaew, T.; Sukontason, K.; Sribanditmongkol, P.; Moophayak, K.; Sanit, S.; Sukontason, K.L. Observations on morphology of immature Lucilia porphyrina (Diptera: Calliphoridae), a fly species of forensic importance. Parasitol. Res. 2012, 111, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Sanit, S.; Sukontason, K.; Kurahashi, H.; Tomberlin, J.K.; Wannasan, A.; Kraisittipanit, R.; Sukontason, K.L. Morphology of immature stages of blow fly, Lucilia sinensis Aubertin (Diptera: Calliphoridae), a potential species of forensic importance. Acta Trop. 2017, 176, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Sontigun, N.; Sanit, S.; Wannasan, A.; Sukontason, K.; Amendt, J.; Yasanga, T.; Sukontason, K.L. Ultrastructure of male genitalia of blow flies (Diptera: Calliphoridae) of forensic importance. Acta Trop. 2018, 179, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Bunchu, N.; Thaipakdee, C.; Vitta, A.; Sanit, S.; Sukontason, K.; Sukontason, K.L. Morphology and developmental rate of the blow fly, Hemipyrellia ligurriens (Diptera: Calliphoridae): Forensic entomology applications. J. Parasitol. Res. 2012, 2012, 371243. [Google Scholar] [CrossRef]
- Sanit, S.; Sukontason, K.; Klong-klaew, T.; Tomberlin, J.K.; Limsopatham, K.; Samerjai, C.; Sontigun, N.; Sukontason, K.L. Ontogenensis and developmental rate of the blow fly, Hypopygiopsis tumrasvini Kurahashi (Diptera: Calliphoridae). Trop. Biomed. 2014, 31, 760–768. [Google Scholar]
- Sukontason, K.; Sukontason, K.L.; Piangjai, S.; Tippanun, J.; Lertthamnongtham, S.; Vogtsberger, R.C.; Olson, J.K. Survey of forensically-relevant fly species in Chiang Mai, northern Thailand. J. Vector Ecol. 2003, 28, 135–138. [Google Scholar]
- Sukontason, K.L.; Samerjai, C.; Sanit, S.; Klong-klaew, T.; Limsopatham, K.; Sontigun, N.; Suwannayod, S.; Kurahashi, H.; Bunchu, N.; Chaiwong, T.; et al. Survey of forensically important fly species in northern Thailand. Southeast Asian. J. Trop. Med. Public Health. 2018, 49, 580–589. [Google Scholar]
- Moophayak, K.; Klong-klaew, T.; Sukontason, K.; Kurahashi, H.; Tomberlin, J.K.; Sukontason, K.L. Species composition of carrion blow flies in northern Thailand: Altitude appraisal. Rev. Inst. Med. Trop. São Paulo 2014, 56, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Ngoen-klan, R.; Moophayak, K.; Klong-klaew, T.; Irvine, K.N.; Sukontason, K.L.; Prangkio, C.; Somboon, P.; Sukontason, K. Do climatic and physical factors affect populations of the blow fly Chrysomya megacephala and house fly Musca domestica? Parasitol. Res. 2011, 109, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Klong-klaew, T.; Sukontason, K.; Ngoen-klan, R.; Moophayak, K.; Irvine, K.N.; Kurahashi, H.; Prangkio, C.; Sanit, S.; Sukontason, K.L. Impact of abiotic factor changes in blowfly, Achoetandrus rufifacies (Diptera: Calliphoridae), in northern Thailand. Parasitol. Res. 2014, 113, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Klong-klaew, T.; Ngoen-klan, R.; Moophayak, K.; Sukontason, K.; Irvine, K.N.; Tomberlin, J.K.; Chareonviriyaphap, T.; Kurahashi, H.; Sukontason, K.L. Predicting geographic distribution of forensically significant blow flies of subfamily Chrysomyinae (Diptera: Calliphoridae) in northern Thailand. Insects 2018, 9, 106. [Google Scholar] [CrossRef]
- Rogers, D.J.; Williams, B.G. Monitoring trypanosomiasis in space and time. Parasitology 1993, 106, S77–S92. [Google Scholar] [CrossRef]
- Bunchu, N.; Sukontason, K.L.; Olson, J.K.; Kurahashi, H.; Sukontason, K. Behavioral responses of Chrysomya megacephala to natural products. Parasitol. Res. 2008, 102, 419–429. [Google Scholar] [CrossRef]
- Hwang, C.; Turner, B.D. Spatial and temporal variability of necrophagous Diptera from urban to rural areas. Med. Vet. Entomol. 2005, 19, 379–391. [Google Scholar] [CrossRef]
- Monfared, S.; Khorshidian, K.; Monfared, A.; Mohammadi, Z.; Khodaparast, R. Prediction of bees (Apoidea, Hymenoptera) frequency and distribution using best linear spatial prediction techniques (kriging and cokriging). Entomofauna 2013, 34, 81–104. [Google Scholar]
- Jacob, B.G.; Burkett-Cadena, N.D.; Luvall, J.C.; Parcak, S.H.; McClure, C.J.W.; Estep, L.K.; Hill, G.E.; Cupp, E.W.; Novak, R.J.; Unnasch, T.R. Developing GIS-based eastern equine encephalitis vector-host models in Tuskegee, Alabama. Int. J. Health Geogr. 2010, 9, 12. [Google Scholar] [CrossRef]
- Zabala, J.; Díaz, B.; Saloña-Bordas, M.I. Seasonal blowfly distribution and abundance in fragmented landscapes. Is it useful in forensic inference about where a corpse has been decaying? PLoS ONE. 2014, 9, e99668. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, H.; Banu, Q. Notes on the Bangladesh calliphorid flies of medical importance (Insecta: Diptera). Jpn. J. Sanit. Zool. 1989, 40, 97–111. [Google Scholar] [CrossRef]
- Bunchu, N.; Sukontason, K.; Sanit, S.; Chidburee, P.; Kurahashi, H.; Sukontason, K.L. Occurrence of blow fly species (Diptera: Calliphoridae) in Phitsanulok Province, northern Thailand. Trop. Biomed. 2012, 29, 532–543. [Google Scholar] [PubMed]
- Suenaga, O.; Kurahashi, H. Life cycle of an oriental blow fly, Lucilia porphyrina (Walker) in Nagasaki, Western Japan. Trop. Med. 1995, 37, 99–107. [Google Scholar]
- Lertthamnongtham, S.; Sukontason, K.L.; Sukontason, K.; Piangjai, S.; Choochote, W.; Vogtsberger, R.C.; Olson, J.K. Seasonal fluctuations in populations of the two most forensically important fly species in northern Thailand. Ann. Trop. Med. Parasitol. 2003, 97, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Weidner, L.M.; Jennings, D.E.; Tomberlin, J.K.; Hamilton, G.C. Seasonal and geographic variation in biodiversity of forensically important blow flies (Diptera: Calliphoridae) in New Jersey, USA. J. Med. Entomol. 2015, 52, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Lyu, Z.; Li, X.B.; Li, K.; Yao, L.; Wan, L.H. Technical note: Development of Hemipyrellia ligurriens (Wiedemann) (Diptera: Calliphoridae) at constant temperatures: Applications in estimating postmortem interval. Forensic Sci. Int. 2015, 253, 48–54. [Google Scholar] [CrossRef]
- Sontigun, N.; Sukontason, K.L.; Klong-klaew, T.; Sanit, S.; Samerjai, C.; Somboon, P.; Thanapornpoonpong, S.N.; Amendt, J.; Sukontason, K. Bionomics of the oriental latrine fly Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae): Temporal fluctuation and reproductive potential. Parasit. Vectors 2018, 11, 415. [Google Scholar] [CrossRef]
- Klong-klaew, T.; Sontigun, N.; Sanit, S.; Samerjai, C.; Sukontason, K.; Koehler, P.G.; Pereira, R.M.; Chareonviriyaphap, T.; Kurahashi, H.; Sukontason, K.L. Daily and seasonal prevalence of the blow fly Chrysomya rufifacies (Diptera: Calliphoridae) as revealed by semi-automatic trap collections in suburban Chiang Mai Province, northern Thailand. Fla. Entomol. 2018, 101, 1–6. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).