Effect of 8 Weeks Aerobic Training and Saffron Supplementation on Inflammation and Metabolism in Middle-Aged Obese Women with Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Administration of Saffron and Placebo Capsules
2.2. Controlling Participant Diet
2.3. Training Program
2.4. Measurement of Variables
2.5. Statistical Analysis
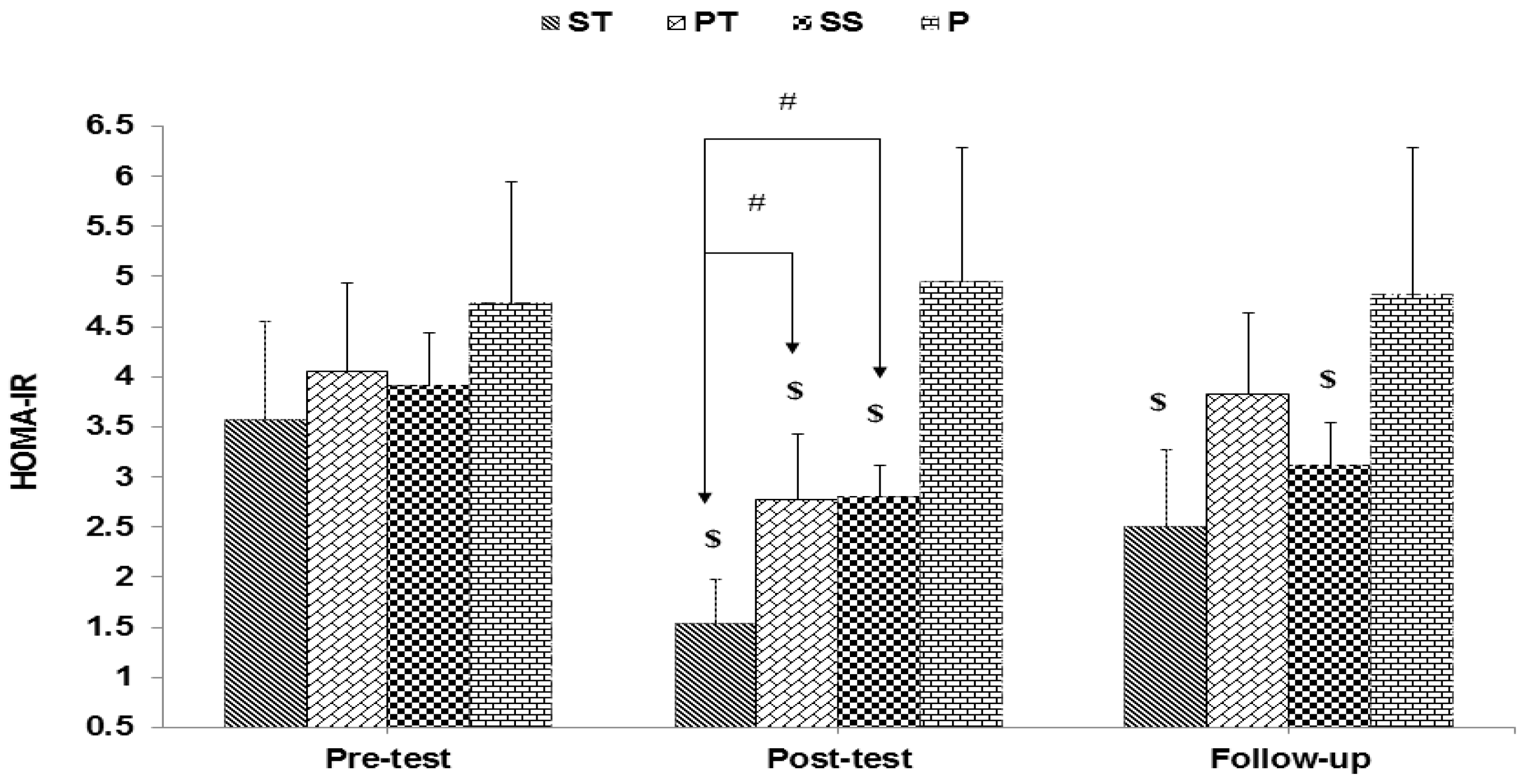
3. Results
4. Discussion
5. Conclusions
6. Limitations and Strength
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Messina, G.; Palmieri, F.; Monda, V.; Messina, A.; Dalia, C.; De Luca, V. Exercise causes muscle GLUT4 translocation in an insulin. Biol. Med. 2015, 1, 1–4. [Google Scholar]
- Khoramipour, K.; Sandbakk, Ø.; Keshteli, A.H.; Gaeini, A.A.; Wishart, D.S.; Chamari, K. Metabolomics in exercise and sports: A systematic review. Sports Med. 2021, 52, 547–583. [Google Scholar] [CrossRef] [PubMed]
- Bragazzi, N.L.; Khoramipour, K.; Chaouachi, A.; Chamari, K. Toward sportomics: Shifting from sport genomics to sport postgenomics and metabolomics specialties. Promises, challenges, and future perspectives. Int. J. Sports Physiol. Perform. 2020, 15, 1201–1202. [Google Scholar] [CrossRef]
- Vasseur, F.; Helbecque, N.; Dina, C.; Lobbens, S.; Delannoy, V.; Gaget, S.; Boutin, P.; Vaxillaire, M.; Leprêtre, F.; Dupont, S.; et al. Single-nucleotide polymorphism haplotypes in the both proximal promoter and exon 3 of the APM1 gene modulate adipocyte-secreted adiponectin hormone levels and contribute to the genetic risk for type 2 diabetes in French Caucasians. Hum. Mol. Genet. 2002, 11, 2607–2614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, N.; Ruan, Y.; Gao, X.; Sun, J. Systematic review and meta-analysis of randomized, controlled trials on the effect of exercise on serum leptin and adiponectin in overweight and obese individuals. Horm. Metab. Res. 2017, 49, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Khoramipour, K.C.K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, physiological functions, role in diseases, and effects of nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef]
- Adeghate, E. An update on the biology and physiology of resistin. Cell. Mol. Life Sci. CMLS 2004, 61, 2485–2496. [Google Scholar] [CrossRef]
- Shaikh-Kader, A.; Houreld, N.N.; Rajendran, N.K.; Abrahamse, H. Levels of Cyclooxygenase 2, Interleukin-6, and Tumour Necrosis Factor-α in Fibroblast Cell Culture Models after Photobiomodulation at 660 nm. Oxidative Med. Cell. Longev. 2021, 2021, 6667812. [Google Scholar] [CrossRef]
- Piché, M.-E.; Tchernof, A.; Després, J.-P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Dovio, A.; Angeli, A. Cytokines and type 2 diabetes mellitus. JAMA 2001, 286, 2233. [Google Scholar]
- Smitka, K.; Marešová, D. Adipose tissue as an endocrine organ: An update on pro-inflammatory and anti-inflammatory microenvironment. Prague Med. Rep. 2015, 116, 87–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, S.I.; Tamura, Y.; Kakehi, S.; Sanada, H.; Kawamori, R.; Watada, H. Exercise-induced increase in IL-6 level enhances GLUT4 expression and insulin sensitivity in mouse skeletal muscle. Biochem. Biophys. Res. Commun. 2016, 473, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Korta, P.; Pocheć, E.; Mazur-Biały, A. Irisin as a multifunctional protein: Implications for health and certain diseases. Medicina 2019, 55, 485. [Google Scholar] [CrossRef] [Green Version]
- Küçükkaraca, H.; Söğüt, M.Ü. Investigation of the Effect of Exercise on Irisin Hormone in Experimentally Induced Diabetic Rats. Int. J. Health Serv. Res. Policy 2017, 2, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Kercher, V.M.; Kercher, K.; Bennion, T.; Yates, B.A.; Feito, Y.; Alexander, C.; Amaral, P.C.; Soares, W.; Li, Y.M.; Han, J.; et al. Fitness trends from around the globe. ACSM’s Health Fit. J. 2021, 25, 20–31. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Rahmaty, S.; Dehghan, P.; Khoramipour, K.; Saboory, M. The effect of listening to brain waves’ relaxing and exciting music during intense endurance training on blood cortisol levels of adult men. Am. J. Sports Sci. Med. 2015, 3, 77–81. [Google Scholar]
- Hua, L.; Lei, M.; Xue, S.; Li, X.; Li, S.; Xie, Q. Effect of fish oil supplementation combined with high-intensity interval training in newly diagnosed non-obese type 2 diabetes: A randomized controlled trial. J. Clin. Biochem. Nutr. 2020, 66, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Mobasseri, M.; Ostadrahimi, A.; Tajaddini, A.; Asghari, S.; Barati, M.; Akbarzadeh, M.; Nikpayam, O.; Houshyar, J.; Roshanravan, N.; Alamdari, N.M. Effects of saffron supplementation on glycemia and inflammation in patients with type 2 diabetes mellitus: A randomized double-blind, placebo-controlled clinical trial study. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 527–534. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Nassiri-Asl, M. Avicenna’s (Ibn Sina) the canon of medicine and saffron (Crocus sativus): A review. Phytother. Res. 2013, 27, 475–483. [Google Scholar] [CrossRef]
- Dehghan, F.; Hajiaghaalipour, F.; Yusof, A.; Muniandy, S.; Hosseini, S.A.; Heydari, S.; Salim, L.Z.A.; Azarbayjani, M.A. Saffron with resistance exercise improves diabetic parameters through the GLUT4/AMPK pathway in-vitro and in-vivo. Sci. Rep. 2016, 6, 25139. [Google Scholar] [CrossRef] [Green Version]
- Khatir, S.A.; Bayatian, A.; Barzegari, A.; Roshanravan, N.; Safaiyan, A.; Pavon-Djavid, G.; Ostadrahimi, A. Saffron (Crocus sativus L.) supplements modulate circulating MicroRNA (miR-21) in atherosclerosis patients; a randomized, double-blind, placebo-controlled trial. Iran. Red Crescent Med. J. 2018, 20, e80260. [Google Scholar]
- Hooshmand Moghadam, B.; Rashidlamir, A.; Attarzadeh Hosseini, S.R.; Gaeini, A.A.; Kaviani, M. The effects of saffron (Crocus sativus L.) in conjunction with concurrent training on body composition, glycaemic status, and inflammatory markers in obese men with type 2 diabetes mellitus: A randomized double-blind clinical trial. Br. J. Clin. Pharmacol. 2022, 88, 3256–3271. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, A.; Akbarnejad, A.; Siahkouhian, M.; Yari, M. Effect of Saffron supplementation and exercise training on blood pressure, pulmonary function and spirometery indicators in obese and overweight women affected by type 2 diabetes. J. Gorgan Univ. Med. Sci. 2019, 21, 59–69. [Google Scholar]
- Mohammadabadi, F.; Vafaiyan, Z.; Hosseini, S.M.; Aryaie, M.; Eshghinia, S. Assessment of insulin resistance with two methods: HOMA-IR and TyG index in Iranian obese women. Iran. J. Diabetes Obes. 2014, 6, 23–27. [Google Scholar]
- Chowdhury, A.I.; Habib, M.A.; Ghosh, S. Effect of Saffron (Crocus sativus L.) on Common Non-Communicable Disease: Review from Current Clinical Findings. J. Ayurvedic Herb. Med. 2021, 7, 93–108. [Google Scholar] [CrossRef]
- Iaccarino, G.; Franco, D.; Sorriento, D.; Strisciuglio, T.; Barbato, E.; Morisco, C. Modulation of insulin sensitivity by exercise training: Implications for cardiovascular prevention. J. Cardiovasc. Transl. Res. 2021, 14, 256–270. [Google Scholar] [CrossRef]
- Shaibi, G.Q.; Roberts, C.K.; Goran, M.I. Exercise and insulin resistance in youth. Exerc. Sport Sci. Rev. 2008, 36, 5–11. [Google Scholar] [CrossRef]
- Choi, K.M.; Kim, T.N.; Yoo, H.J.; Lee, K.W.; Cho, G.J.; Hwang, T.G.; Baik, S.H.; Choi, D.S.; Kim, S.M. Effect of exercise training on A-FABP, lipocalin-2 and RBP4 levels in obese women. Clin. Endocrinol. 2009, 70, 569–574. [Google Scholar] [CrossRef]
- Khajehlandi, M.; Mohammadi, R. The Effect of Pilates Training on Body Composition, Lipid Profile, and Serum 25-Hydroxy Vitamin D Levels in Inactive Overweight Women. Zahedan J. Res. Med. Sci. 2021, 23, e100502. [Google Scholar] [CrossRef]
- Cauza, E.; Hanusch-Enserer, U.; Strasser, B.; Ludvik, B.; Metz-Schimmerl, S.; Pacini, G.; Wagner, O.; Georg, P.; Prager, R.; Kostner, K.; et al. The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch. Phys. Med. Rehabil. 2005, 86, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.I.; Owusu-Boakye, E.; Adegoke, B.O.; Adjei, D.N. Effects of aerobic exercise on selected physiological parameters and quality of life in patients with type 2 diabetes mellitus. Int. J. Gen. Med. 2011, 4, 723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albright, A.; Franz, M.; Hornsby, G.; Kriska, A.; Marrero, D.; Ullrich, I.; Verity, L.S. American College of Sports Medicine position stand. Exercise and type 2 diabetes. Med. Sci. Sports Exerc. 2000, 32, 1345–1360. [Google Scholar]
- Park, J.-S.; Holloszy, J.O.; Kim, K.; Koh, J.-H. Exercise training-induced PPARβ increases PGC-1α protein stability and improves insulin-induced glucose uptake in rodent muscles. Nutrients 2020, 12, 652. [Google Scholar] [CrossRef] [Green Version]
- Boudou, P.; Sobngwi, E.; Mauvais-Jarvis, F.; Vexiau, P.; Gautier, J. Absence of exercise-induced variations in adiponectin levels despite decreased abdominal adiposity and improved insulin sensitivity in type 2 diabetic men. Eur. J. Endocrinol. 2003, 149, 421–424. [Google Scholar] [CrossRef] [Green Version]
- Rezaeeshirazi, R. Aerobic versus resistance training: Leptin and metabolic parameters improvement in type 2 diabetes obese men. Res. Q. Exerc. Sport 2022, 93, 537–547. [Google Scholar] [CrossRef]
- Yokoyama, H.; Emoto, M.; Araki, T.; Fujiwara, S.; Motoyama, K.; Morioka, T.; Koyama, H.; Shoji, T.; Okuno, Y.; Nishizawa, Y. Effect of aerobic exercise on plasma adiponectin levels and insulin resistance in type 2 diabetes. Diabetes Care 2004, 27, 1756–1758. [Google Scholar] [CrossRef]
- Xu, A.; Chan, K.W.; Hoo, R.L.; Wang, Y.; Tan, K.C.; Zhang, J.; Chen, B.; Lam, M.C.; Tse, C.; Cooper, G.J.; et al. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J. Biol. Chem. 2005, 280, 18073–18080. [Google Scholar] [CrossRef] [Green Version]
- Xi, L.; Qian, Z.; Du, P.; Fu, J. Pharmacokinetic properties of crocin (crocetin digentiobiose ester) following oral administration in rats. Phytomedicine 2007, 14, 633–636. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Hwang, J.-H.; Hong, S.-J.; Hsu, H.-K. Enhancement of glucose uptake in 3T3-L1 adipocytes by Toona sinensis leaf extract. Kaohsiung J. Med. Sci. 2003, 19, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Mattei, L.; Francisqueti-Ferron, F.V.; Garcia, J.L.; Ferron, A.J.T.; de Almeida Silva, C.C.V.; Gregolin, C.S.; Nakandakare-Maia, E.T.; Silva, J.D.C.P.; Moreto, F.; Minatel, I.O.; et al. Antioxidant and anti-inflammatory properties of gamma-oryzanol attenuates insulin resistance by increasing GLUT-4 expression in skeletal muscle of obese animals. Mol. Cell. Endocrinol. 2021, 537, 111423. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Neergheen, V.S.; Bahorun, T.; Jen, L.-S. Free radicals, antioxidants and diabetes: Embryopathy, retinopathy, neuropathy, nephropathy and cardiovascular complications. Neuroembryol. Aging 2006, 4, 117–137. [Google Scholar] [CrossRef]
- Bouassida, A.; Chamari, K.; Zaouali, M.; Feki, Y.; Zbidi, A.; Tabka, Z. Review on leptin and adiponectin responses and adaptations to acute and chronic exercise. Br. J. Sports Med. 2010, 44, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Perrea, D.; Iliadis, F.; Angelopoulou, N.; Liapis, C.; Alevizos, M. Exercise reduces resistin and inflammatory cytokines in patients with type 2 diabetes. Diabetes Care 2007, 30, 719–721. [Google Scholar] [CrossRef] [Green Version]
- Gueugnon, C.; Mougin, F.; Simon-Rigaud, M.-L.; Regnard, J.; Nègre, V.; Dumoulin, G. Effects of an in-patient treatment program based on regular exercise and a balanced diet on high molecular weight adiponectin, resistin levels, and insulin resistance in adolescents with severe obesity. Appl. Physiol. Nutr. Metab. 2012, 37, 672–679. [Google Scholar] [CrossRef]
- Monzillo, L.U.; Hamdy, O.; Horton, E.S.; Ledbury, S.; Mullooly, C.; Jarema, C.; Porter, S.; Ovalle, K.; Moussa, A.; Mantzoros, C.S. Effect of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obes. Res. 2003, 11, 1048–1054. [Google Scholar] [CrossRef] [Green Version]
- Barnes, K.; Miner, J. Role of resistin in insulin sensitivity in rodents and humans. Curr. Protein Pept. Sci. 2009, 10, 96–107. [Google Scholar] [CrossRef]
- Lu, J.; Xiang, G.; Liu, M.; Mei, W.; Xiang, L.; Dong, J. Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis 2015, 243, 438–448. [Google Scholar] [CrossRef]
- Miyamoto-Mikami, E.; Sato, K.; Kurihara, T.; Hasegawa, N.; Fujie, S.; Fujita, S.; Sanada, K.; Hamaoka, T.; Tabata, I.; Iemitsu, M. Endurance training-induced increase in circulating irisin levels is associated with reduction of abdominal visceral fat in middle-aged and older adults. PLoS ONE 2015, 10, e0120354. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y.; Qi, L.; Zhang, M.; Wang, X.; Cui, T.; et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 2014, 63, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Kern, M.; Wells, J.A.; Stephens, J.M.; Elton, C.W.; Friedman, J.E.; Tapscott, E.B.; Pekala, P.H.; Dohm, G.L. Insulin responsiveness in skeletal muscle is determined by glucose transporter (Glut4) protein level. Biochem. J. 1990, 270, 397–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huertas, J.; Al Fazazi, S.; Hidalgo-Gutierrez, A.; López, L.; Casuso, R. Antioxidant effect of exercise: Exploring the role of the mitochondrial complex I superassembly. Redox Biol. 2017, 13, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Huo, F.; Liu, B.; Liu, J.; Chen, T.; Li, J.; Zhu, Z.; Lv, B. Crocin inhibits oxidative stress and pro-inflammatory response of microglial cells associated with diabetic retinopathy through the activation of PI3K/Akt signaling pathway. J. Mol. Neurosci. 2017, 61, 581–589. [Google Scholar] [CrossRef]
- Cai, J.; Yi, F.F.; Bian, Z.Y.; Shen, D.F.; Yang, L.; Yan, L.; Tang, Q.Z.; Yang, X.C.; Li, H. Crocetin protects against cardiac hypertrophy by blocking MEK-ERK1/2 signalling pathway. J. Cell. Mol. Med. 2009, 13, 909–925. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Kader, S.M. Aerobic versus resistance exercise training in modulation of insulin resistance, adipocytokines and inflammatory cytokine levels in obese type 2 diabetic patients. J. Adv. Res. 2011, 2, 179–183. [Google Scholar] [CrossRef] [Green Version]
- Conraads, V.M.; Beckers, P.; Bosmans, J.; De Clerck, L.S.; Stevens, W.J.; Vrints, C.J.; Brutsaert, D.L. Combined endurance/resistance training reduces plasma TNF-α receptor levels in patients with chronic heart failure and coronary artery disease. Eur. Heart J. 2002, 23, 1854–1860. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, F.C.; de Medeiros, A.I.; Nicioli, C.; Nunes, J.E.D.; Shiguemoto, G.E.; Prestes, J.; Verzola, R.M.M.; Baldissera, V.; de Andrade Perez, S.E. Circuit resistance training in sedentary women: Body composition and serum cytokine levels. Appl. Physiol. Nutr. Metab. 2009, 35, 163–171. [Google Scholar] [CrossRef]
- Thompson, D.; Walhin, J.P.; Batterham, A.M.; Stokes, K.A.; Cooper, A.R.; Andrews, R.C. Effect of diet or diet plus physical activity versus usual care on inflammatory markers in patients with newly diagnosed type 2 diabetes: The Early ACTivity in Diabetes (ACTID) randomized, controlled trial. J. Am. Heart Assoc. 2014, 3, e000828. [Google Scholar] [CrossRef] [Green Version]
- Arasteh, A.; Aliyev, A.; Khamnei, S.; Delazar, A.; Mesgari, M.; Mehmannavaz, Y. Crocus sativus on serum glucose, insulin and cholesterol levels in healthy male rats. J. Med. Plants Res. 2010, 4, 397–402. [Google Scholar]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Giaccio, M. Crocetin from saffron: An active component of an ancient spice. Crit. Rev. Food Sci. Nutr. 2004, 44, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Barzegari, A.; Mahdirejei, H.A. Effects of 8 weeks resistance training on plasma vaspin and lipid profile levels in adult men with type 2 diabetes. Casp. J. Intern. Med. 2014, 5, 103. [Google Scholar]
- Baba, C.S.; Alexander, G.; Kalyani, B.; Pandey, R.; Rastogi, S.; Pandey, A.; Choudhuri, G. Effect of exercise and dietary modification on serum aminotransferase levels in patients with nonalcoholic steatohepatitis. J. Gastroenterol. Hepatol. 2006, 21, 191–198. [Google Scholar] [CrossRef] [PubMed]






| Weeks | Stages of Exercise | Exercise Intensity | Duration per Session (Minute) | Type of Movement | |
|---|---|---|---|---|---|
| Weeks: 1 and 2: 3 sessions per week | warm-up | %55> | 10 | Jogging, combined hand-leg movements, stretching movements | |
| main part | aerobic | %55–60 | 10 | Combined hand-leg movements | |
| running | %60–65 | 20 | running | ||
| cool-down | %50> | 5 | Stretching large muscles | ||
| Weeks: 3, 4 and 5: 3 sessions per week | warm-up | %55> | 10 | Jogging, combined hand-leg movements, stretching movements | |
| main part | aerobic | %55–60 | 10 | Combined hand-leg movements | |
| running | %65–70 | 30 | running | ||
| cool-down | %50> | 5 | Stretching large muscles | ||
| Weeks: 6, 7 and 8: 3 sessions per week | warm-up | %55> | 10 | Jogging, combined hand-leg movements, stretching movements | |
| main part | aerobic | %55–60 | 10 | Combined hand-leg movements | |
| running | %70–75 | 40 | running | ||
| cool-down | %50> | 5 | Stretching large muscles | ||
| Variable | ST | PT | SS | P | |
|---|---|---|---|---|---|
| Age (year) | 51.5 ± 6.16 | 57.62 ± 6.81 | 54.12 ± 7.37 | 56.87 ± 5.11 | |
| Disease duration (year) | 4.5 ± 3.2 | 4.6 ± 2.8 | 3.8 ± 1.7 | 4.2 ± 3.0 | |
| Nutrient | Energy (calorie/day) | 1682.89 ± 135 | 1692.36 ± 166 | 1687.68 ± 149 | 1705.71 ± 125 |
| Carbohydrate (g/day) | 244.07 ± 62.48 | 225.93 ± 59.27 | 223.26 ± 44.76 | 252.84 ± 38.34 | |
| Protein (g/day) | 66.66 ± 14.33 | 73.53 ± 17.23 | 78.8 ± 13.39 | 80.00 ± 16.42 | |
| Fat (g/day) | 45.33 ± 12.78 | 48.33 ± 13.87 | 44.05 ± 14.11 | 46.08 ± 11.13 | |
| Fiber (g/day) | 12.75 ± 3.75 | 13.24 ± 4.45 | 12.92 ± 5.11 | 14.18 ± 4.21 | |
| Calcium (mg/day) | 262.92 ± 179.02 | 270.18 ± 201.61 | 268.87 ± 185.23 | 286.54 ± 178.11 | |
| Vitamin C (mg/day) | 55.69 ± 25.12 | 59.77 ± 26.24 | 57.26 ± 27.65 | 62.97 ± 29.44 | |
| Vitamin E (mg/day) | 2.5 ± 1.14 | 2.94 ± 1.46 | 2.87 ± 1.67 | 3.41 ± 2.47 | |
| Selenium (mg/day) | 42.90 ± 23.26 | 48.81 ± 21.67 | 47.89 ± 22.54 | 53.65 ± 24.11 | |
| Name of the Substance | Picrocrocin | Crocin | Safranal |
|---|---|---|---|
| Retention time (minute) | 14.8 | 16.2 | 26.1 |
| Saffron metabolites levels (mg/g) | 6.69 | 6.47 | 1.17 |
| Variables | Groups | Pre-Test | Post-Test | Follow-up | Cohen’s D |
|---|---|---|---|---|---|
| Body weight (kg) | ST | 80.98 ± 5.01 | 77.58 ± 6.37 ϕ | 78.12 ± 6.38 ϕ | 0.593 |
| PT | 81.87 ± 3.30 | 80.12 ± 3.47 | 80.73 ± 3.37 | 0.516 | |
| SS | 81.47 ± 6.91 | 79.56 ± 7.47 | 80.06 ± 7.62 | 0.265 | |
| P | 86.95 ± 5.90 | 87.18 ± 6.32 | 87.00 ± 6.14 | 0.037 | |
| Body fat (%) | ST | 31.98 ± 3.66 | 27.43 ± 2.41 ϕ | 28.23 ± 2.88 ϕ | 1.468 |
| PT | 33.12 ± 2.19 | 30.62 ± 1.84 | 31.42 ± 2.42 | 1.236 | |
| SS | 32.97 ± 3.16 | 30.87 ± 2.48 | 31.37 ± 3.20 | 0.739 | |
| P | 34.62 ± 3.06 | 35.02 ± 2.30 | 34.87 ± 4.01 | 0.147 | |
| BMI (kg/m2) | ST | 30.32 ± 2.42 | 28.97 ± 2.86 $,ϕ | 29.18 ± 2.88 ϕ | 0.509 |
| PT | 31.15 ± 1.50 | 30.40 ± 1.49 # | 30.63 ± 1.31 | 0.501 | |
| SS | 30.72 ± 3.56 | 30.1 ± 3.75 * | 30.18 ± 3.82 | 0.194 | |
| P | 34.03 ± 3.36 | 34.15 ± 3.57 | 34.07 ± 3.48 | 0.034 |
| Variable | Groups | Pre-Test | Post-Test | Follow-up | Cohen’s D |
|---|---|---|---|---|---|
| Adiponectin (ng/mL) | ST | 13.12 ± 4.18 | 27.17 ± 7.59 & | 23.97 ± 7.02 $ | 2.293 |
| PT | 11.22 ± 3.81 | 19.73 ± 4.39 * | 15.25 ± 3.77 | 2.070 | |
| SS | 12.03 ± 5.18 | 18.00 ± 5.92 # | 16.18 ± 5.46 | 1.073 | |
| P | 9.15 ± 3.37 | 9.50 ± 3.42 | 9.21 ± 3.17 | 0.102 | |
| Resistin (ng/mL) | ST | 12.20 ± 2.87 | 7.02 ± 2.04 $ | 8.02 ± 2.30 $ | 2.08 |
| PT | 14.85 ± 3.65 | 11.93 ± 2.85 | 14.00 ± 3.07 | 0.891 | |
| SS | 14.07 ± 2.91 | 12.05 ± 2.61 | 13.34 ± 2.66 | 0.730 | |
| P | 15.73 ± 5.80 | 15.85 ± 5.18 | 14.87 ± 5.67 | 0.021 | |
| TNF-α (pg/mL) | ST | 14.05 ± 3.41 | 9.56 ± 2.79 ϕ | 10.25 ± 2.81 ϕ | 1.585 |
| PT | 16.65 ± 4.85 | 15.23 ± 4.69 | 15.87 ± 4.61 | 0.299 | |
| SS | 16.43 ± 6.64 | 14.22 ± 4.52 | 14.62 ± 4.86 | 0.389 | |
| P | 18.10 ± 5.64 | 18.71 ± 5.58 | 18.47 ± 5.77 | 0.108 | |
| IL-6 (pg/mL) | ST | 9.98 ± 1.94 | 5.15 ± 1.34 $ | 7.01 ± 1.51 ϕ | 2.897 |
| PT | 11.52 ± 2.14 | 8.72 ± 2.15 * | 9.83 ± 1.64 | 1.305 | |
| SS | 10.93 ± 2.00 | 9.01 ± 1.52 # | 10.55 ± 1.88 | 1.080 | |
| P | 12.43 ± 3.79 | 12.56 ± 3.71 | 12.5 ± 4.09 | 0.034 | |
| Irisin (ng/dL) | ST | 171.05 ± 26.41 | 203.92 ± 8.81 ϕ | 198.75 ± 7.00 ϕ | 1.670 |
| PT | 166.12 ± 21.77 | 179.72 ± 24.62 | 176.37 ± 24.31 | 0.585 | |
| SS | 167.07 ± 19.28 | 177.8 ± 20.14 | 173.25 ± 23.3 | 0.544 | |
| P | 160.87 ± 17.74 | 157.15± 18.60 | 156.98 ± 18.07 | 0.204 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajabi, A.; Khajehlandi, M.; Siahkuhian, M.; Akbarnejad, A.; Khoramipour, K.; Suzuki, K. Effect of 8 Weeks Aerobic Training and Saffron Supplementation on Inflammation and Metabolism in Middle-Aged Obese Women with Type 2 Diabetes Mellitus. Sports 2022, 10, 167. https://doi.org/10.3390/sports10110167
Rajabi A, Khajehlandi M, Siahkuhian M, Akbarnejad A, Khoramipour K, Suzuki K. Effect of 8 Weeks Aerobic Training and Saffron Supplementation on Inflammation and Metabolism in Middle-Aged Obese Women with Type 2 Diabetes Mellitus. Sports. 2022; 10(11):167. https://doi.org/10.3390/sports10110167
Chicago/Turabian StyleRajabi, Ali, Mojdeh Khajehlandi, Marefat Siahkuhian, Ali Akbarnejad, Kayvan Khoramipour, and Katsuhiko Suzuki. 2022. "Effect of 8 Weeks Aerobic Training and Saffron Supplementation on Inflammation and Metabolism in Middle-Aged Obese Women with Type 2 Diabetes Mellitus" Sports 10, no. 11: 167. https://doi.org/10.3390/sports10110167
APA StyleRajabi, A., Khajehlandi, M., Siahkuhian, M., Akbarnejad, A., Khoramipour, K., & Suzuki, K. (2022). Effect of 8 Weeks Aerobic Training and Saffron Supplementation on Inflammation and Metabolism in Middle-Aged Obese Women with Type 2 Diabetes Mellitus. Sports, 10(11), 167. https://doi.org/10.3390/sports10110167







