The Neuromuscular Fatigue-Induced Loss of Muscle Force Control
Abstract
:1. Introduction
2. Measurement and Quantification of Force Control during Neuromuscular Fatigue
3. Neuromuscular Fatigue-Induced Changes in Force Control
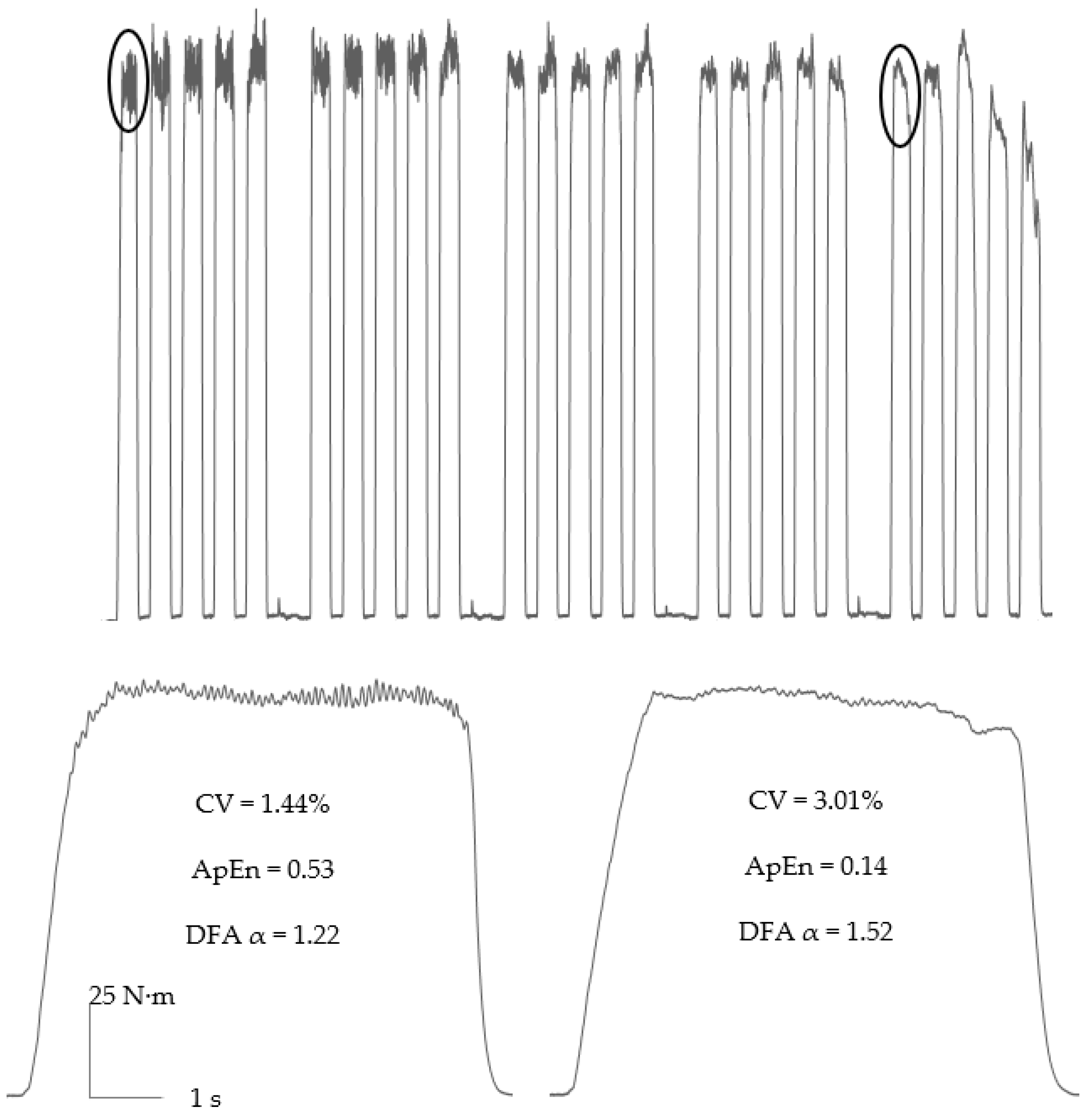
3.1. Contraction Type
3.2. Contraction Intensity
3.3. Sex Differences
3.4. Age Differences
3.5. Ergogenic Aids
4. Mechanistic Basis of the Neuromuscular Fatigue-Induced Loss of Muscle Force Control
4.1. Afferent Feedback
4.2. Neuromodulatory Pathways
4.3. Cortical and Reticulospinal Pathways
5. Performance Implications of the Neuromuscular Fatigue-Induced Loss of Muscle Force Control
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gandevia, S.C. Some central and peripheral factors affecting human motoneuronal output in neuromuscular fatigue. Sports Med. 1992, 13, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Enoka, R.M.; Duchateau, J. Translating fatigue to human performance. Med. Sci. Sports Exerc. 2016, 48, 2228–2238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Phys. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enoka, R.M.; Christou, E.A.; Hunter, S.K.; Kornatz, K.W.; Semmer, J.G.; Taylor, A.M.; Tracy, B.L. Mechanisms that contribute to differences in motor performance between young and old adults. J. Electromyogr. Kinesiol. 2003, 13, 1–12. [Google Scholar] [CrossRef]
- Woodworth, R.S. The accuracy of voluntary movement. Pschol. Rev. 1899, 3, 1–114. [Google Scholar]
- Fitts, P.M. The information capacity of the human motor system in controlling the amplitude of movement. J. Exp. Psychol. 1954, 47, 381–391. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, R.A.; Zelaznik, H.; Hawkins, B.; Frank, J.S.; Quinn, J.T.J. Motor-output variability: A theory for the accuracy of rapid motor acts. Psychol. Rev. 1979, 86, 415–451. [Google Scholar] [CrossRef]
- Slifkin, A.B.; Newell, K.M. Noise, information transmission, and force variability. J. Exp. Psychol. Hum. Percept. Perform. 1999, 25, 837–851. [Google Scholar] [CrossRef]
- Farina, D.; Negro, F.; Muceli, S.; Enoka, R.M. Principles of motor unit physiology evolve with advances in technology. Physiology 2016, 31, 83–94. [Google Scholar] [CrossRef]
- Binet, L. The laws of tremor. Lancet 1920, 195, 265–266. [Google Scholar] [CrossRef]
- Bousfield, W.A. The influence of fatigue on tremor. J. Exp. Psychol. 1932, 15, 104–107. [Google Scholar] [CrossRef]
- Furness, P.; Jessop, J.; Lippold, O.C. Long-lasting increases in the tremor of human hand muscles following brief, strong effort. J. Physiol. 1977, 265, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.K.; Enoka, R.M. Sex differences in the fatigability of arm muscles depends on absolute force during isometric contractions. J. Appl. Physiol. 2001, 91, 2686–2694. [Google Scholar] [CrossRef] [PubMed]
- Pethick, J.; Winter, S.L.; Burnley, M. Fatigue reduces the complexity of knee extensor torque fluctuations during maximal and submaximal intermittent isometric contractions in man. J. Physiol. 2015, 593, 2085–2096. [Google Scholar] [CrossRef]
- Pethick, J.; Winter, S.L.; Burnley, M. Relationship between muscle metabolic rate and muscle torque complexity during fatiguing intermittent isometric contractions in humans. Phys. Rep. 2019, 7, e14240. [Google Scholar] [CrossRef]
- Chatain, C.; Gruet, M.; Vallier, J.M.; Ramdani, S. Effects of nonstationarity on muscle force signals regularity during a fatiguing motor task. IEEE Trans. Neural. Syst. Rehabil. Eng. 2019, 28, 228–237. [Google Scholar] [CrossRef]
- Castronovo, A.M.; Negro, F.; Conforto, S.; Farina, D. The proportion of common synaptic input to motor neurons increases with an increase in net excitatory input. J. Appl. Physiol. 2015, 119, 1337–1346. [Google Scholar] [CrossRef]
- Pethick, J.; Winter, S.L.; Burnley, M. Loss of knee extensor torque complexity during fatiguing isometric muscle contractions occurs exclusively above the critical torque. Am. J. Physiol. 2016, 310, R1144–R1153. [Google Scholar] [CrossRef] [Green Version]
- Harris, C.M.; Wolpert, D.M. Signal-dependent noise determines motor planning. Nature 1998, 394, 780–784. [Google Scholar] [CrossRef]
- Davis, L.A.; Allen, S.P.; Hamilton, L.D.; Grabowski, A.M.; Enoka, R.M. Differences in postural sway among healthy adults are associated with the ability to perform steady contractions with leg muscles. Exp. Brain Res. 2020, 238, 487–497. [Google Scholar] [CrossRef]
- Mear, E.; Gladwell, V.; Pethick, J. Knee extensor force control as a predictor of dynamic balance. SportRxiv 2022. [Google Scholar] [CrossRef]
- Pethick, J.; Winter, S.L.; Burnley, M. Effects of ipsilateral and contralateral fatigue and muscle blood flow occlusion on the complexity of knee-extensor torque output in humans. Exp. Physiol. 2018, 103, 956–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pethick, J.; Piasecki, M. Alterations in muscle force control with aging: Is there a modulatory effect of lifelong physical activity? Front. Sports Act. Living 2022, 4, 817770. [Google Scholar] [CrossRef] [PubMed]
- Pethick, J.; Taylor, M.J.D.; Harridge, S.D.R. Aging and skeletal muscle force control: Current perspectives and future directions. Scand. J. Med. Sci. Sports 2022, 32, 1430–1443. [Google Scholar] [CrossRef]
- Pethick, J.; Winter, S.L.; Burnley, M. Physiological complexity: Influence of ageing, disease and neuromuscular fatigue on muscle force and torque fluctuations. Exp. Physiol. 2021, 106, 2046–2059. [Google Scholar] [CrossRef]
- Seely, A.J.; Macklem, P.T. Complex systems and the technology of variability analysis. Crit. Care 2004, 8, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Ebenbichler, G.R.; Kollmitzer, J.; Erim, Z.; Löscher, W.N.; Kerschan, K.; Posch, M.; Nowotny, T.; Kranzl, A.; Wöber, W.; Bochdansky, T. Load-dependence of fatigue related changes in tremor around 10 Hz. Clin. Neurophysiol. 2000, 111, 106–111. [Google Scholar] [CrossRef]
- Semmler, J.G.; Kutzscher, D.V.; Enoka, R.M. Limb immobilization alters muscle activation patterns during a fatiguing isometric contraction. Muscle Nerve 2000, 23, 1381–1392. [Google Scholar] [CrossRef]
- Gottlieb, S.; Lippold, O.C. The 4-6 HZ tremor during sustained contraction in normal human subjects. J. Physiol. 1983, 336, 499–509. [Google Scholar] [CrossRef]
- Löscher, W.N.; Cresswell, A.G.; Thorstensson, A. Electromyographic responses of the human triceps surae and force tremor during sustained sub-maximal isometric plantar flexion. Acta Physiol. Scand. 1994, 152, 73–82. [Google Scholar] [CrossRef]
- Löscher, W.N.; Cresswell, A.G.; Thorstensson, A. Central fatigue during a long-lasting submaximal contraction of the triceps surae. Exp. Brain Res. 1996, 108, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S.M. Approximate entropy as a measure of system complexity. Proc. Natl. Acad. Sci. USA 1991, 88, 2297–2301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberger, A.L.; Amaral, L.A.; Hausdorff, J.M.; Ivanov, P.C.; Peng, C.K.; Stanley, H.E. Fractal dynamics in physiology: Alterations with disease and ageing. Proc. Natl. Acad. Sci. USA 2002, 99, 2466–2472. [Google Scholar] [CrossRef] [Green Version]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, C.K.; Buldyrev, S.V.; Havlin, S.; Simons, M.; Stanley, H.E.; Goldberger, A.L. Mosaic organization of DNA nucleotides. Phys. Rev. E 1994, 49, 1685–1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vøllestad, K. Measurement of human muscle fatigue. J. Neurosci. Methods 1997, 74, 219–227. [Google Scholar] [CrossRef]
- Pethick, J.; Winter, S.L.; Burnley, M. Fatigue reduces the complexity of knee extensor torque during fatiguing sustained isometric contractions. Eur. J. Sport Sci. 2019, 19, 1349–1358. [Google Scholar] [CrossRef]
- Ye, X.; Beck, T.W.; Wages, N.P. Influence of dynamic exercise on force steadiness and common drive. J. Musculoskelet. Neuronal. Interact. 2014, 14, 377–386. [Google Scholar]
- Ye, X.; Beck, T.W.; Wages, N.P. Acute effects of concentric vs. eccentric exercise on force steadiness and electromyographic responses of the forearm flexors. J. Strength Cond. Res. 2015, 29, 604–611. [Google Scholar] [CrossRef]
- Lavender, A.P.; Nosaka, K. Changes in fluctuation of isometric force following eccentric and concentric exercise of the elbow flexors. Eur. J. Appl. Physiol. 2006, 96, 235–240. [Google Scholar] [CrossRef]
- Hunter, S.K.; Enoka, R.M. Changes in muscle activation can prolong the endurance time of a submaximal isometric contraction in humans. J. Appl. Physiol. 2003, 94, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Montecinos, C.; Calatayud, J.; Iturriaga, C.; Bustos, C.; Mena, B.; España-Romero, V.; Carpes, F.P. Influence of a self-regulated cognitive dual task on time to failure and complexity of submaximal isometric force control. Eur. J. Appl. Physiol. 2018, 118, 2021–2027. [Google Scholar] [CrossRef] [PubMed]
- McPhee, J.S.; Maden-Wilkinson, T.M.; Narici, M.V.; Jones, D.A.; Degens, H. Knee extensor fatigue resistance of young and older men and women performing sustained and brief intermittent isometric contractions. Muscle Nerve 2014, 50, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, J.J.; Smith, K.A.; Minahan, C.L. Sex differences in muscle activity emerge during sustained low-intensity contractions but not during intermittent low-intensity contractions. Phys. Rep. 2020, 87, e14398. [Google Scholar] [CrossRef] [Green Version]
- Sjøgaard, G.; Savard, G.; Juel, G. Muscle blood flow during isometric activity and its relation to muscle fatigue. Eur. J. Appl. Physiol. 1988, 57, 327–335. [Google Scholar] [CrossRef] [PubMed]
- De Ruiter, C.J.; Goudsmit, F.J.; Van Tricht, J.A.; De Haan, A. The isometric torque at which knee-extensor muscle reoxygenation stops. Med. Sci. Sports Exerc. 2007, 39, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Pethick, J.; Winter, S.L.; Burnley, M. Caffeine ingestion attenuates fatigue-induced loss of muscle torque complexity. Med. Sci. Sports Exerc. 2018, 50, 236–245. [Google Scholar] [CrossRef] [Green Version]
- Ansdell, P.; Thomas, K.; Howatson, G.; Hunter, S.; Goodall, S. Contraction intensity and sex differences in knee-extensor fatigability. J. Electromyogr. Kinesiol. 2017, 37, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Madeleine, P.; Jørgensen, L.; Søgaard, K.; Arendt-Nielsen, L.; Sjøgaard, G. Development of muscle fatigue as assessed by electromyography and mechanomyography during continuous and intermittent low-force contractions: Effects of the feedback mode. Eur. J. Appl. Physiol. 2002, 87, 28–37. [Google Scholar] [CrossRef]
- Semmler, J.G.; Tucker, K.J.; Allen, T.J.; Proske, U. Eccentric exercise increases EMG amplitude and force fluctuations during submaximal contractions of the elbow flexor muscles. J. Appl. Physiol. 2007, 103, 979–989. [Google Scholar] [CrossRef] [Green Version]
- Pethick, J.; Whiteaway, K.; Winter, S.L.; Burnley, M. Prolonged depression of knee-extensor torque complexity following eccentric exercise. Exp. Physiol. 2019, 104, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.B.; Arampatzis, A.; Duda, G.; Heller, M.O.; Taylor, W.R. Effect of fatigue on force fluctuations in knee extensors in young adults. Philos. Trans. R. Soc. A 2010, 368, 2783–2798. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Casanova, N.; Gomes, J.S.; Pezarat-Correia, P.; Freitas, S.; Vaz, J.R. Changes in torque complexity and maximal torque after a fatiguing exercise protocol. Sports Biomech. 2022. [Google Scholar] [CrossRef]
- Pethick, J.; Winter, S.L.; Burnley, M. Physiological evidence that the critical torque is a phase transition, not a threshold. Med. Sci. Sports Exerc. 2020, 52, 2390–2401. [Google Scholar] [CrossRef] [PubMed]
- Burnley, M.; Vanhatalo, A.; Jones, A.M. Distinct profiles of neuromuscular fatigue during muscle contractions below and above the critical torque in humans. J. Appl. Physiol. 2012, 113, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Burnley, M.; Jones, A.M. Power-duration relationship: Physiology, fatigue, and the limits of human performance. Eur. J. Sport Sci. 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Yoon, T.; Schlinder-Delap, B.; Griffith, E.E.; Hunter, S.K. Mechanisms of fatigue differ after low- and high-force fatiguing contractions in men and women. Muscle Nerve 2007, 36, 515–524. [Google Scholar] [CrossRef]
- Hendrix, C.R.; Housh, T.J.; Johnson, G.O.; Mielke, M.; Camic, C.L.; Zuniga, J.M.; Schmidt, R.J. Comparison of critical force to EMG fatigue thresholds during isometric leg extension. Med. Sci. Sports Exerc. 2009, 41, 956–964. [Google Scholar] [CrossRef]
- Monod, H.; Scherrer, J. The work capacity of a synergic muscular group. Ergonomics 1965, 8, 329–338. [Google Scholar] [CrossRef]
- Guo, Y.; Jones, E.J.; Inns, T.B.; Ely, I.A.; Stashuk, D.W.; Wilkinson, D.J.; Smith, K.; Piasecki, J.; Phillips, B.E.; Atherton, P.J.; et al. Neuromuscular recruitment strategies of the vastus lateralis according to sex. Acta Physiol. Scand. 2022, 235, e13803. [Google Scholar] [CrossRef]
- Inglis, J.G.; Gabriel, D.A. Sex differences in the modulation of the motor unit discharge rate leads to reduced force steadiness. Appl. Physiol. Nutr. Metab. 2021, 46, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.K. Sex differences in human fatigability: Mechanisms and insight to physiological responses. Acta Physiol. Scand. 2014, 210, 768–789. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.K.; Rhee, J. Revealing sex differences during upper and lower extremity neuromuscular fatigue in older adults through a neuroergonomics approach. Front. Neuroergonomics 2021, 2, 663368. [Google Scholar] [CrossRef]
- Forrest, S.M.; Challis, J.H.; Winter, S.L. The effect of signal acquisition and processing choices on ApEn values: Towards a “gold standard” for distinguishing effort levels from isometric records. Med. Eng. Phys. 2014, 36, 676–683. [Google Scholar] [CrossRef]
- Tenan, M.S.; Hackney, A.C.; Griffin, L. Maximal force and tremor changes across the menstrual cycle. Eur. J. Appl. Physiol. 2016, 116, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.J.; Adams, L.F.; Schmidt, P.F.; Rubinow, D.R.; Wassermann, E.M. Effects of ovarian hormones on human cortical excitability. Ann. Neurol. 2002, 51, 599–603. [Google Scholar] [CrossRef]
- Hunter, S.K.; Pereira, H.M.; Keenan, K.G. The aging neuromuscular system and motor performance. J. Appl. Physiol. 2016, 121, 982–995. [Google Scholar] [CrossRef] [Green Version]
- Hunter, S.K.; Critchlow, A.; Enoka, R.M. Muscle endurance is greater for old men compared with strength-matched young men. J. Appl. Physiol. 2005, 99, 890–897. [Google Scholar] [CrossRef] [Green Version]
- Griffith, E.E.; Yoon, T.; Hunter, S.K. Age and load compliance alter time to task failure for a submaximal fatiguing contraction with the lower leg. J. Appl. Physiol. 2010, 108, 1510–1519. [Google Scholar] [CrossRef] [Green Version]
- Justice, J.N.; Mani, D.; Pierpoint, L.A.; Enoka, R.M. Fatigability of the dorsiflexors and associations among multiple domains of motor function in young and old adults. Exp. Gerontol. 2014, 55, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Rhee, J.; Mehta, R.K.; Srinivasan, D. Neuromuscular control and performance differences associated with gender and obesity in fatiguing tasks performed by older adults. Front. Physiol. 2018, 9, 800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, M.D.; Dunne, A.; Bosworth, M.; Willems, M.E. Effect of New Zealand Blackcurrant extract on force steadiness of the quadriceps femoris muscle during sustained submaximal isometric contraction. J. Funct. Morphol. Kinesiol. 2022, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Pethick, J.; Casselton, C.; Winter, S.L.; Burnley, M. Ischemic preconditioning blunts loss of knee extensor torque complexity with fatigue. Med. Sci. Sports Exerc. 2021, 53, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Negro, F. Common synaptic input to motor neurons, motor unit synchronization, and force control. Exerc. Sport Sci. Rev. 2015, 43, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Negro, F.; Dideriksen, J.L. The effective neural drive to muscles in the common synaptic input to motor neurons. J. Physiol. 2014, 592, 3427–3441. [Google Scholar] [CrossRef] [PubMed]
- Negro, F.; Holobar, A.; Farina, D. Fluctuations in isometric muscle force can be described by one linear projection of low-frequency components of motor unit discharge rates. J. Physiol. 2009, 587, 5925–5938. [Google Scholar] [CrossRef]
- Thompson, C.K.; Negro, F.; Johnson, M.D.; Holmes, M.R.; McPherson, L.M.; Powers, R.K.; Farina, D.; Heckman, C.J. Robust and accurate decoding of motoneuron behaviour and prediction of the resulting force output. J. Physiol. 2018, 596, 2643–2659. [Google Scholar] [CrossRef] [Green Version]
- Rossato, J.; Tucker, K.; Avrillon, S.; Lacourpaille, L.; Holobar, A.; Hug, F. Less common synaptic input between muscles from the same group allows for more flexible coordination strategies during a fatiguing task. J. Neurophysiol. 2022, 127, 421–433. [Google Scholar] [CrossRef]
- Enoka, R.M.; Farina, D. Force steadiness: From motor units to voluntary actions. Physiology 2021, 36, 114–130. [Google Scholar] [CrossRef]
- Jones, A.M.; Wilkerson, D.P.; DiMenna, F.; Fulford, J.; Poole, D.C. Muscle metabolic responses to exercise above and below the “critical power” assessed using 31P-MRS. Am. J. Physiol. 2008, 294, R585–R593. [Google Scholar]
- Hureau, T.J.; Romer, L.M.; Amann, M. The “sensory tolerance limit”: A hypothetical construct determining exercise performance? Eur. J. Sport Sci. 2018, 18, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, D.S.; McNeil, C.J.; Gandevia, S.C.; Taylor, J.L. Firing of antagonist small-diameter muscle afferents reduces voluntary activation and torque of elbow flexors. J. Physiol. 2013, 591, 3591–3604. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Valdes, E.; Negro, F.; Falla, D.; Dideriksen, J.L.; Heckman, C.J.; Farina, D. Inability to increase the neural drive to muscle is associated with task failure during submaximal contractions. J. Neurophysiol. 2020, 124, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.J.; Bangsbo, J.; Renaud, J.M. Muscle K+, Na+, and Cl disturbances and Na+-K+ inactivation: Implications for fatigue. J. Appl. Physiol. 2008, 104, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Macefield, G.; Hagbarth, K.E.; Gorman, R.; Gandevia, S.C.; Burke, D. Decline in spindle support to alpha-motoneurones during sustained voluntary contractions. J. Physiol. 1991, 440, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Amann, M.; Duchateau, J.; Meeusen, R.; Rice, C.L. Neural contributions to muscle fatigue: From the brain to the muscle and back again. Med. Sci. Sports Exerc. 2016, 48, 2294–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enoka, R.M.; Duchateau, J. Muscle fatigue: What, why and how it influences muscle function. J. Physiol. 2008, 586, 11–23. [Google Scholar] [CrossRef]
- Germer, C.M.; Del Vecchio, A.; Negro, F.; Farina, D.; Elias, L.A. Neurophysiological correlates of force control improvement introduced by sinusoidal vibrotactile stimulation. J. Neural. Eng. 2020, 17, 016043. [Google Scholar] [CrossRef]
- Taylor, J.L.; Butler, J.E.; Gandevia, S.C. Changes in muscle afferents, motoneurons and motor drive during muscle fatigue. Eur. J. Appl. Physiol. 2000, 83, 106–115. [Google Scholar] [CrossRef]
- Johnson, M.D.; Heckman, C.J. Gain control mechanisms in spinal motoneurons. Front. Neural. Circuits 2014, 8, 81. [Google Scholar] [CrossRef] [Green Version]
- Thorstensen, J.R.; Taylor, J.L.; Tucker, M.G.; Kavanagh, J.J. Enhanced serotonin availability amplifies fatigue perception and modulates the TMS-induced silent period during sustained low-intensity elbow flexions. J. Physiol. 2020, 598, 2685–2701. [Google Scholar] [CrossRef] [PubMed]
- Heckman, C.J.; Enoka, R.M. Motor unit. Compr. Physiol. 2012, 2, 2629–2682. [Google Scholar] [PubMed]
- Henderson, T.T.; Thorstensen, J.R.; Morrison, S.; Tucker, M.G.; Kavanagh, J.J. Physiological tremor is suppressed and force steadiness is enhanced with increased availability of serotonin regardless of muscle fatigue. J. Neurophysiol. 2022, 127, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Foley, T.E.; Fleshner, M. Neuroplasticity of dopamine circuits after exercise: Implications for central fatigue. Neuromolcular Med. 2008, 10, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Thorstensen, J.R.; Tucker, M.G.; Kavanagh, J.J. Antagonism of the D2 receptor enhances tremor but reduces voluntary muscle activation in humans. Neuropharmacology 2018, 141, 343–352. [Google Scholar] [CrossRef] [Green Version]
- Gruet, M.; Temesi, J.; Rupp, T.; Levy, P.; Millet, G.Y.; Verges, S. Stimulation of the motor cortex and corticospinal tract to assess human muscle fatigue. Neuroscience 2013, 231, 384–399. [Google Scholar] [CrossRef]
- Brasil-Neto, J.P.; Pascual-Leone, A.; Valls-Solé, J.; Cammarota, A.; Cohen, L.G.; Hallett, M. Postexercise depression of motor evoked potentials: A measure of central nervous system fatigue. Exp. Brain Res. 1993, 93, 181–184. [Google Scholar] [CrossRef]
- Milanović, S.; Filipović, S.R.; Blesić, S.; Ilić, T.V.; Dhanasekaran, S.; Ljubisavljević, M. Paired-associative stimulation can modulate muscle fatigue induced motor cortex excitability changes. Behav. Brain Res. 2011, 223, 30–35. [Google Scholar] [CrossRef]
- Škarabot, J.; Mesquita, R.N.; Brownstein, C.G.; Ansdell, P. Myths and methodologies: How loud is the story told by the transcranial magnetic stimulation-evoked silent period? Exp. Physiol. 2019, 104, 635–642. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Sasaki, A.; Masugi, Y.; Milosevic, M.; Nakazawa, K. Changes in corticospinal excitability during bilateral and unilateral lower-limb force control tasks. Exp. Brain Res. 2020, 238, 1977–1987. [Google Scholar] [CrossRef]
- Prentice, S.D.; Drew, T. Contributions of the reticulospinal system to the postural adjustments occurring during voluntary gait modifications. J. Neurophysiol. 2001, 85, 679–698. [Google Scholar] [CrossRef] [PubMed]
- Cortes, N.; Onate, J.; Morrison, S. Differential effects of fatigue on movement variability. Gait Posture 2014, 39, 888–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meardon, S.A.; Hamill, J.; Derrick, T.R. Running injury and stride time variability over a prolonged run. Gait Posture 2011, 33, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Forestier, N.; Nougier, V. The effects of muscular fatigue on the coordination of a multijoint movement in humans. Neurosci. Lett. 1998, 252, 187–190. [Google Scholar] [CrossRef]
- Buzzi, U.H.; Stergiou, N.; Kurz, M.J.; Hageman, P.A.; Heidel, J. Nonlinear dynamics indicates aging affects variability during gait. Clin. Biomech. 2003, 18, 435–443. [Google Scholar] [CrossRef]
- Twomey, R.; Aboodarda, S.J.; Kruger, R.; Culos-Reed, S.N.; Temesi, J.; Millet, G.Y. Neuromuscular fatigue during exercise: Methodological considerations, etiology and potential role in chronic fatigue. Clin. Neurophysiol. 2017, 47, 95–110. [Google Scholar] [CrossRef]
- Kouzaki, M.; Shinohara, M. Steadiness in plantar flexor muscles and its relation to postural sway in young and elderly adults. Muscle Nerve 2010, 42, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Almuklass, A.M.; Price, R.C.; Gould, J.R.; Enoka, R.M. Force steadiness as a predictor of time to complete a pegboard test of dexterity in young men and women. J. Appl. Physiol. 2016, 120, 1410–1417. [Google Scholar] [CrossRef] [Green Version]
- Seynnes, O.; Hue, O.A.; Garrandes, F.; Colson, S.S.; Bernard, P.L.; Legros, P.; Singh, M.A.F. Force steadiness in the lower extremities as an independent predictor of functional performance in older women. J. Aging Phys. Act. 2005, 13, 395–408. [Google Scholar] [CrossRef]
- Newell, K.M. What are fundamental motor skills and what is fundamental about them? J. Mot. Learn. Dev. 2020, 8, 280–314. [Google Scholar] [CrossRef]
- Amann, M.; Dempsey, J.A. Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. J. Physiol. 2008, 586, 161–173. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pethick, J.; Tallent, J. The Neuromuscular Fatigue-Induced Loss of Muscle Force Control. Sports 2022, 10, 184. https://doi.org/10.3390/sports10110184
Pethick J, Tallent J. The Neuromuscular Fatigue-Induced Loss of Muscle Force Control. Sports. 2022; 10(11):184. https://doi.org/10.3390/sports10110184
Chicago/Turabian StylePethick, Jamie, and Jamie Tallent. 2022. "The Neuromuscular Fatigue-Induced Loss of Muscle Force Control" Sports 10, no. 11: 184. https://doi.org/10.3390/sports10110184
APA StylePethick, J., & Tallent, J. (2022). The Neuromuscular Fatigue-Induced Loss of Muscle Force Control. Sports, 10(11), 184. https://doi.org/10.3390/sports10110184




