COVID19 Pandemic and Physical Activity: An Observational Study on Sleep Quality and Anxiety
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Questionnaires
2.3. Data Analysis
3. Results
3.1. Participants’ Characteristics
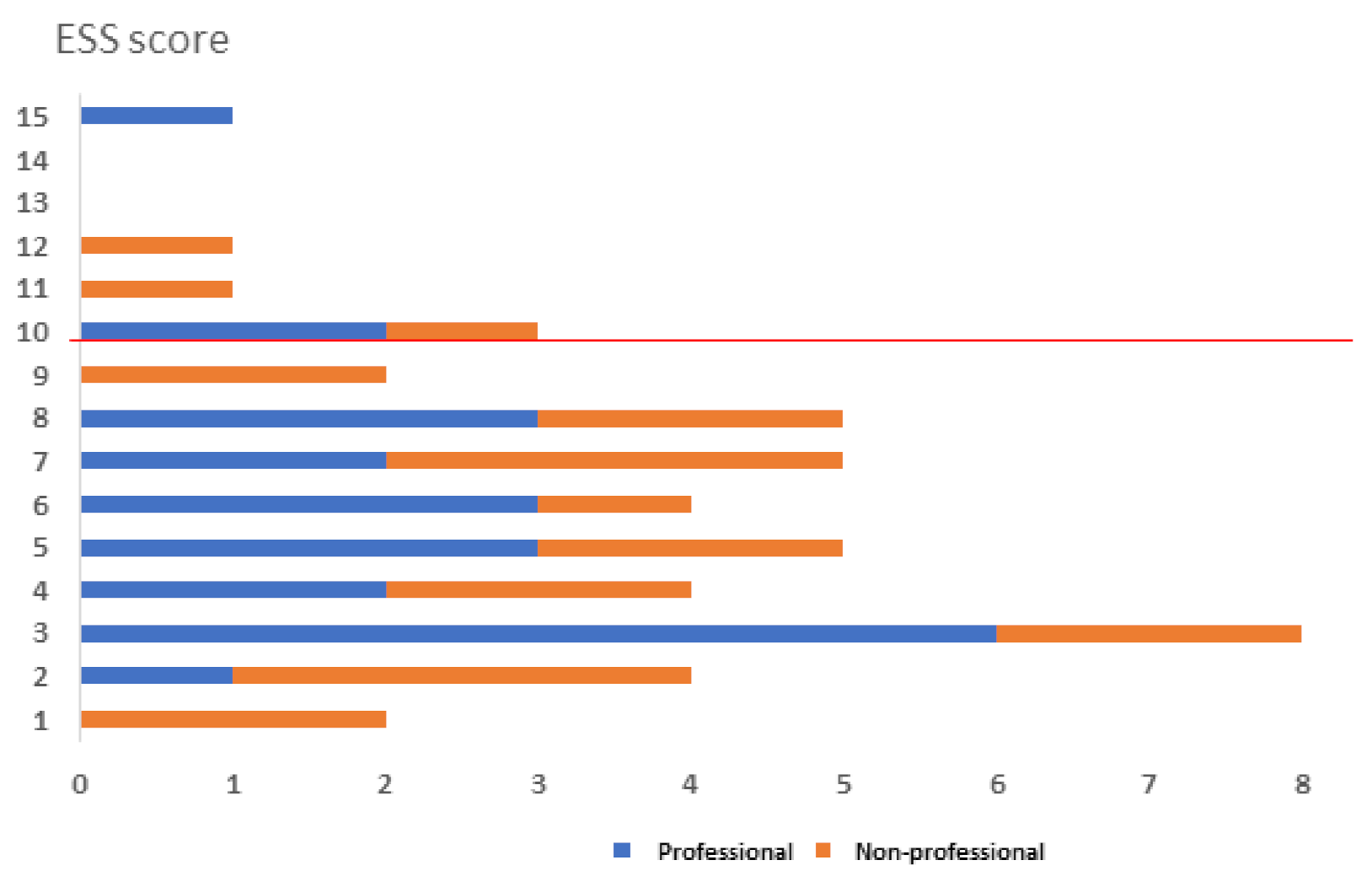
3.2. Anxiety Level and Quality of Sleep
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasch, B.; Born, J. About sleep’s role in memory. Physiol. Rev. 2013, 93, 681–766. [Google Scholar] [CrossRef] [PubMed]
- Lemola, S.; Räikkönen, K.; Scheier, M.F.; Matthews, K.A.; Pesonen, A.K.; Heinonen, K.; Lahti, J.; Komsi, N.; Paavonen, J.E.; Kajantie, E. Sleep quantity, quality and optimism in children. J. Sleep Res. 2011, 20 Pt 1, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Meijer, A.M.; Habekothe, H.T.; Van Den Wittenboer, G.L.H. Time in bed, quality of sleep and school functioning of children. J. Sleep Res. 2000, 9, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, A.C.; Muehlbach, M.J.; Schweitzer, P.K.; Walsh, J.K. Cognitive function following acute sleep restriction in children ages 10–14. Sleep 1998, 21, 861–868. [Google Scholar] [PubMed]
- Reutrakul, S.; Van Cauter, E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism 2018, 84, 56–66. [Google Scholar] [CrossRef]
- Touchette, E.; Petit, D.; Seguin, J.R.; Boivin, M.; Tremblay, R.E.; Montplaisir, J.Y. Associations between sleep duration patterns and behavioral/cognitive functioning at school entry. Sleep 2007, 30, 1213–1219. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Taggart, F.M.; Kandala, N.B.; Currie, A.; Peile, E.; Stranges, S.; Miller, M.A. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 2008, 31, 619–626. [Google Scholar] [CrossRef] [Green Version]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Katz, E.S.; Kheirandish-Gozal, L.; et al. National Sleep Foundation’s Sleep time duration recommendations: Methodology and results summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef]
- Palmer, C.A.; Alfano, C.A. Sleep and emotion regulation: An organizing, integrative review. Sleep Med. Rev. 2017, 31, 6–16. [Google Scholar] [CrossRef]
- Tempesta, D.; Socci, V.; De Gennaro, L.; Ferrara, M. Sleep and emotional processing. Sleep Med. Rev. 2018, 40, 183–195. [Google Scholar] [CrossRef]
- Roberts, R.E.; Duong, H.T. The prospective association between sleep deprivation and depression among adolescents. Sleep 2014, 37, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Halson, S.L.; Shaw, G.; Versey, N.; Miller, D.J.; Sargent, C.; Roach, G.D.; Nyman, L.; Carter, J.M.; Baar, K. Optimisation and Validation of a Nutritional Intervention to Enhance Sleep Quality and Quantity. Nutrients 2020, 12, 2579. [Google Scholar] [CrossRef]
- Reid, K.J.; Baron, K.G.; Lu, B.; Naylor, E.; Wolfe, L.; Zee, P.C. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med. 2010, 11, 934–940. [Google Scholar] [CrossRef] [Green Version]
- Cooney, G.; Dwan, K.; Mead, G. Exercise for depression. JAMA 2014, 311, 2432–2433. [Google Scholar] [CrossRef]
- Rebar, A.L.; Stanton, R.; Geard, D.; Short, C.; Duncan, M.J.; Vandelanotte, C. A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol. Rev. 2015, 9, 366–378. [Google Scholar] [CrossRef]
- Jayakody, K.; Gunadasa, S.; Hosker, C. Exercise for anxiety disorders: Systematic review. Br. J. Sports Med. 2014, 48, 187–196. [Google Scholar] [CrossRef]
- Mammen, G.; Faulkner, G. Physical activity and the prevention of depression: A systematic review of prospective studies. Am. J. Prev. Med. 2013, 45, 649–657. [Google Scholar] [CrossRef]
- Ghrouz, A.K.; Noohu, M.M.; Dilshad Manzar, M.; Warren Spence, D.; BaHammam, A.S.; Pandi-Perumal, S.R. Physical activity and sleep quality in relation to mental health among college students. Sleep Breath. 2019, 23, 627–634. [Google Scholar] [CrossRef]
- Romdhani, M.; Hammouda, O.; Chaabouni, Y.; Mahdouani, K.; Driss, T.; Chamari, K.; Souissi, N. Sleep deprivation affects post-lunch dip performances, biomarkers of muscle damage and antioxidant status. Biol. Sport 2019, 36, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Pfefferbaum, B.; North, C.S. Mental health and the COVID-19 pandemic. N. Engl. J. Med. 2020, 383, 510–512. [Google Scholar] [CrossRef]
- Xiong, J.; Lipsitz, O.; Nasri, F.; Lui, L.M.W.; Gill, H.; Phan, L.; Chen-Li, D.; Iacobucci, M.; Ho, R.; Majeed, A.; et al. Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J. Affect. Disord. 2020, 277, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Curcio, G.; Tempesta, D.; Scarlata, S.; Marzano, C.; Moroni, F.; Rossini, P.M.; Ferrara, M.; De Gennaro, L. Validity of the Italian version of the Pittsburgh Sleep Quality Index (PSQI). Neurol. Sci. 2013, 34, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Cavallera, G.M.; Boari, G. Validation of the Italian Version of the Morningness-Eveningness Questionnaire for Adolescents by A. Lancry and Th. Arbault. Med. Sci. Monit. 2015, 21, 2685–2693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [Green Version]
- Hind, K.; Gannon, L.; Brightmore, A.; Beck, B. Insights into relationships between body mass, composition and bone: Findings in elite rugby players. J. Clin. Densitom. 2015, 18, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Jurado-Fasoli, L.; De-la-O, A.; Molina-Hidalgo, C.; Migueles, J.H.; Castillo, M.J.; Amaro-Gahete, F.J. Exercise training improves sleep quality: A randomized controlled trial. Eur. J. Clin. Investig. 2020, 50, e13202. [Google Scholar] [CrossRef]
- Geneen, L.J.; Moore, R.A.; Clarke, C.; Martin, D.; Colvin, L.A.; Smith, B.H. Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 4, CD011279. [Google Scholar]
- Knufinke, M.; Nieuwenhuys, A.; Geurts, S.A.; Coenen, A.M.; Kompier, M.A. Self-reported sleep quantity, quality and sleep hygiene in elite athletes. J. Sleep Res. 2018, 27, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Wood, B.; Rea, M.S.; Plitnick, B.; Figueiro, M.G. Light level and duration of exposure determine the impact of self-luminous tablets on melatonin suppression. Appl. Ergon. 2013, 44, 237–240. [Google Scholar] [CrossRef]
- Romyn, G.; Robey, E.; Dimmock, J.A.; Halson, S.L.; Peeling, P. Sleep, anxiety and electronic device use by athletes in the training and competition environments. Eur. J. Sport Sci. 2016, 16, 301–308. [Google Scholar] [CrossRef]
- Caia, J.; Thornton, H.R.; Kelly, V.G.; Scott, T.J.; Halson, S.L.; Cupples, B.; Driller, M.W. Does self-perceived sleep reflect sleep estimated via activity monitors in professional rugby league athletes? J. Sports Sci. 2018, 36, 1492–1496. [Google Scholar] [CrossRef]
- Dunican, I.; Martin, D.; Halson, S.; Dawson, B.; Caldwell, J.A.; Jones, M.J.; Jones, M.J.; Eastwood, P. The effects of the removal of electronic devices for 48 hours on sleep in elite judo athletes. J. Strength Cond. Res. 2017, 31, 2832–2839. [Google Scholar] [CrossRef] [PubMed]
- Tayech, A.; Mejri, M.A.; Makhlouf, I.; Mathlouthi, A.; Behm, D.G.; Chaouachi, A. Second Wave of COVID-19 Global Pandemic and Athletes’ Confinement: Recommendations to Better Manage and Optimize the Modified Lifestyle. Int. J. Environ. Res. Public Health 2020, 17, 8385. [Google Scholar] [CrossRef]
- Chen, P.; Mao, L.; Nassis, G.P.; Harmer, P.; Ainsworth, B.E.; Li, F. Coronavirus disease (COVID-19): The need to maintain regular physical activity while taking precautions. J. Sport Health Sci. 2020, 9, 103–104. [Google Scholar] [CrossRef]
- Halabchi, F.; Mazaheri, R.; Sabeti, K.; Yunesian, M.; Alizadeh, Z.; Ahmadinejad, Z.; Aghili, S.M.; Tavakol, Z. Regular Sports Participation as a Potential Predictor of Better Clinical Outcome in Adult Patients with COVID-19: A Large Cross-Sectional Study. J. Phys. Act. Health 2021, 18, 8–12. [Google Scholar] [CrossRef]

| Non-Professionals | Professionals | p-Value * | |
|---|---|---|---|
| n, % | 21 (45.65) | 25 (54.35) | - |
| Sex, M (n, %) | 8 (32) | 17 (68) | 0.043 |
| Age Class (n, %) | |||
| 0.731 | |||
| 10–19 | 3 (42.86) | 4 (57.14) | |
| 20–29 | 9 (42.86) | 12 (57.14) | |
| 30–39 | 9 (52.94) | 8 (47.06) | |
| Occupation (n, %) | |||
| Student | 8 (40.00) | 12 (60.00) | 0.632 |
| Unemployed | 3 (60.00) | 2 (40.00) | |
| Employed | 9 (52.94) | 8 (47.06) | |
| Employed in sports | 1 (25.00) | 3 (75.00) | |
| Education Attainment (n, %) | |||
| Middle School | 0 (0.00) | 1 (100) | 0.716 |
| High School | 12 (42.86) | 16 (57.14) | |
| First level degree | 5 (55.56) | 4 (44.44) | |
| Second level degree | 4 (50.00) | 4 (50.00) | |
| Pathological history (n, %) | |||
| Sleep disorders | 3 (33.33) | 6 (66.67) | 0.408 |
| Cups of coffee a day (n, %) | |||
| 0 | 2 (22.22) | 7 (77.78) | 0.118 |
| 1 | 17 (56.67) | 13 (43.33) | |
| 2 | 2 (28.57) | 5 (71.43) | |
| Energy Drink, yes (n, %) | 5 (50.00) | 5 (50.00) | 0.755 |
| Use of sleep medications or supplements (n, %) | 1 (16.67) | 5 (83.33) | 0.141 |
| Non-Professionals (n: 21) | Professionals (n: 25) | p-Value | |
|---|---|---|---|
| PSQI (median, IQR) | 3 (2–3) | 5 (4–5) | 0.125 * |
| PSQI (n, %) | |||
| Poor sleep quality | 5 (31.25) | 11 (68.65) | 0.152 ** |
| Good sleep quality | 16 (53.33) | 14 (46.67) | |
| PSQI Single Scores (mean, ± sd) | |||
| duration of sleep *** | 0.43, ±0.81 | 0.28, ±0.54 | 0.6560 * |
| sleep disturbance *** | 1.00, ±0.45 | 1.08, ±0.57 | 0.5859 * |
| sleep latency *** | 1.00, ±01.05 | 1.56, ±0.87 | 0.0340 * |
| day dysfunction due to sleepiness *** | 1.00, ±0.63 | 1.16, ±0.62 | 0.2470 * |
| sleep efficiency *** | 0.62, ±0.86 | 0.56, ±0.58 | 0.8338 * |
| overall sleep quality *** | 0.19, ±0.51 | 0.32, ±0.80 | 0.7912 * |
| need meds to sleep *** | 0.14, ±0.35 | 0.08, ±0.28 | 0.4998 * |
| * Mann–Whitney Test | |||
| ** Chi-Squared/Fisher Exacta Test |
| Coef. | Std. Err. | t | p | [95% Conf. | Interval] | |
|---|---|---|---|---|---|---|
| Sex, F (ref: M) | 0.217 | 1.144 | 0.190 | 0.851 | −2.114 | 2.548 |
| Age | −0.028 | 0.099 | −0.280 | 0.779 | −0.230 | 0.174 |
| Occupation, ref: Student | ||||||
| Unemployed | 1.626 | 1.758 | 0.920 | 0.362 | −1.955 | 5.207 |
| Employed | 1.153 | 1.244 | 0.930 | 0.361 | −1.381 | 3.688 |
| Employed in sports | 0.843 | 1.836 | 0.460 | 0.649 | −2.896 | 4.583 |
| Cups of coffee a day, ref: 0 | ||||||
| 1 | −2.248 | 1.384 | −1.620 | 0.114 | −5.067 | 0.571 |
| 2 | −1.116 | 1.509 | −0.740 | 0.465 | −4.190 | 1.958 |
| Energy Drink, ref: no | 0.416 | 1.072 | 0.390 | 0.700 | −1.767 | 2.599 |
| Past alcohol or drug use. ref: never | ||||||
| Yes. Seldom | 0.432 | 1.464 | 0.300 | 0.770 | −2.550 | 3.415 |
| Yes. Often | 1.270 | 1.776 | 0.720 | 0.480 | −2.347 | 4.887 |
| Use of sleep medications or supplements; ref: no | 3.529 | 1.534 | 2.300 | 0.028 * | 0.403 | 6.654 |
| Sport Category, ref: Sedentary | 0.454 | 1.131 | −0.400 | 0.691 | −2.758 | 1.850 |
| Multivariate regression; Dependent variable: PSQI (continuous) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elce, A.; Daniele, A.; Loperto, I.; De Coppi, L.; Sangiorgio, A.; Vivona, A.; Sorrentino, C.; Iannaccone, S.; Martiniello, L.; Nigro, E. COVID19 Pandemic and Physical Activity: An Observational Study on Sleep Quality and Anxiety. Sports 2022, 10, 44. https://doi.org/10.3390/sports10030044
Elce A, Daniele A, Loperto I, De Coppi L, Sangiorgio A, Vivona A, Sorrentino C, Iannaccone S, Martiniello L, Nigro E. COVID19 Pandemic and Physical Activity: An Observational Study on Sleep Quality and Anxiety. Sports. 2022; 10(3):44. https://doi.org/10.3390/sports10030044
Chicago/Turabian StyleElce, Ausilia, Aurora Daniele, Ilaria Loperto, Lucia De Coppi, Armando Sangiorgio, Angelina Vivona, Clorinda Sorrentino, Simona Iannaccone, Lucia Martiniello, and Ersilia Nigro. 2022. "COVID19 Pandemic and Physical Activity: An Observational Study on Sleep Quality and Anxiety" Sports 10, no. 3: 44. https://doi.org/10.3390/sports10030044







