Assessment of Intercostal Muscle Near-Infrared Spectroscopy for Estimating Respiratory Compensation Point in Trained Endurance Athletes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Design
2.3. Procedures
2.3.1. Cardiopulmonary Exercise Test
2.3.2. Measurement of SmO2
2.3.3. Skinfold
2.3.4. Data Analysis
2.3.5. Statistical Analysis
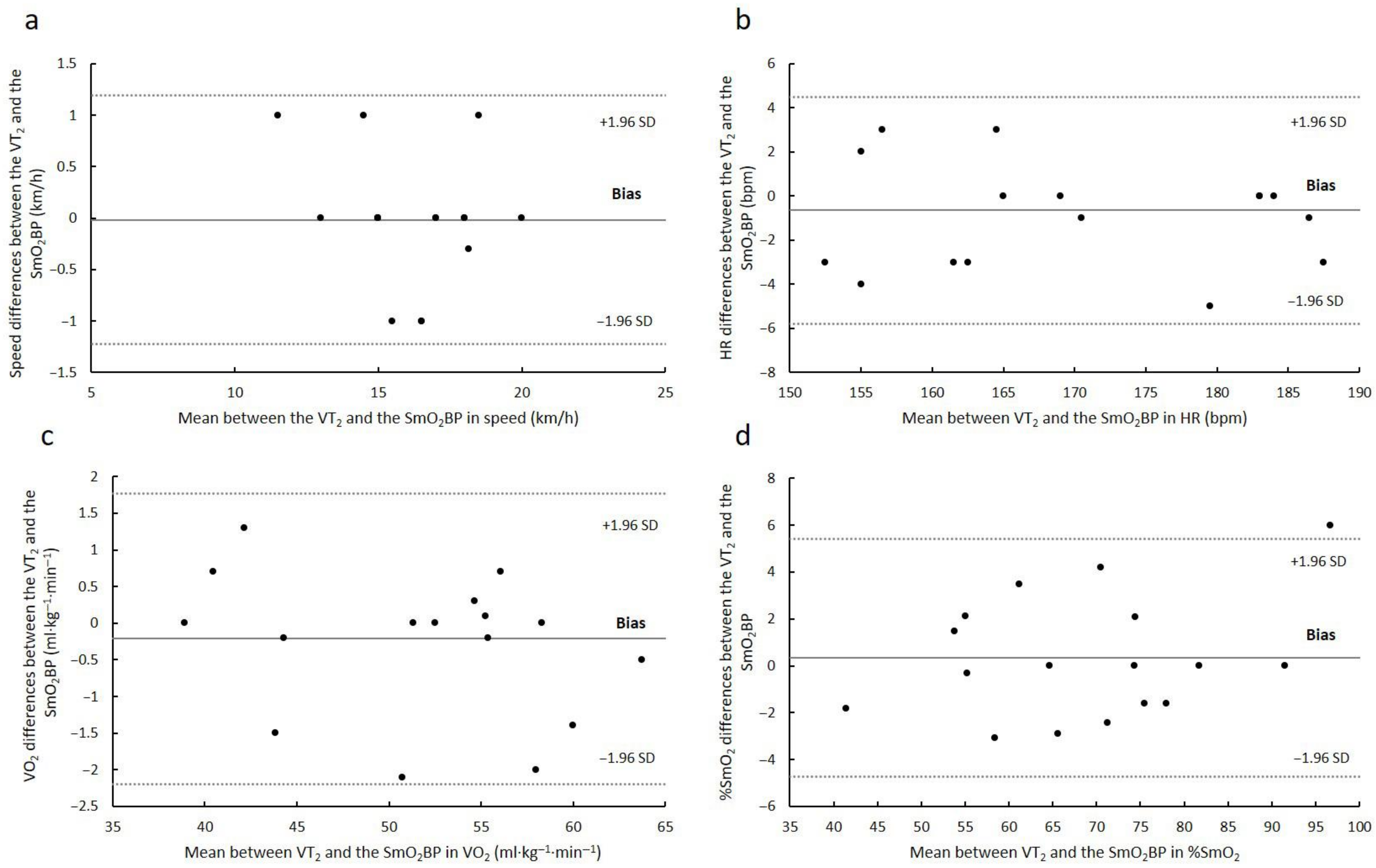
3. Results
4. Discussion
5. Conclusions
6. Practical Applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keir, D.A.; Iannetta, D.; Mattioni Maturana, F.; Kowalchuk, J.M.; Murias, J.M. Identification of non-invasive exercise thresholds: Methods, strategies, and an online app. Sports Med. 2022, 52, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol. 1986, 60, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Seiler, K.S.; Kjerland, G.O. Quantifying training intensity distribution in elite endurance athletes: Is there evidence for an “optimal” distribution? Scand. J. Med. Sci. Sports 2006, 16, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, K.; Whipp, B.J.; Koyl, S.N.; Beaver, W.L. Anaerobic threshold and respiratory gas exchange during exercise. J. Appl. Physiol. 1973, 35, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Amann, M.; Subudhi, A.W.; Walker, J.; Eisenman, P.; Shultz, B.; Foster, C. An evaluation of the predictive validity and reliability of ventilatory threshold. Med. Sci. Sports Exerc. 2004, 36, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Wilkerson, D.P.; Dimenna, F.J.; Jones, A.M. Influence of repeated sprint training on pulmonary O2 uptake and muscle deoxygenation kinetics in humans. J. Appl. Physiol. 2009, 106, 1875–1887. [Google Scholar] [CrossRef] [PubMed]
- Osmani, F.; Lago-Fuentes, C.; Alemany-Iturriaga, J.; Barcala-Furelos, M. The relationship of muscle oxygen saturation analyzer with other monitoring and quantification tools in a maximal incremental treadmill test. Front. Physiol. 2023, 14, 1155037. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, A.; Ammann, L.; Gächter, F.; Zibung, M.; Erlacher, D. Muscle oxygen saturation breakpoints reflect ventilatory thresholds in both cycling and running. J. Hum. Kin. 2022, 83, 87–97. [Google Scholar] [CrossRef]
- Perrey, S.; Ferrari, M. Muscle oximetry in sports science: A systematic review. Sports Med. 2018, 48, 597–616. [Google Scholar] [CrossRef]
- Boone, J.; Barstow, T.J.; Celie, B.; Prieur, F.; Bourgois, J. The interrelationship between muscle oxygenation, muscle activation, and pulmonary oxygen uptake to incremental ramp exercise: Influence of aerobic fitness. Appl. Physiol. Nutr. Metab. 2016, 41, 55–62. [Google Scholar] [CrossRef]
- Contreras-Briceno, F.; Espinosa-Ramirez, M.; Hevia, G.; Llambias, D.; Carrasco, M.; Cerda, F.; Lopez-Fuenzalida, A.; Garcia, P.; Gabrielli, L.; Viscor, G. Reliability of NIRS portable device for measuring intercostal muscles oxygenation during exercise. J. Sports Sci. 2019, 37, 2653–2659. [Google Scholar] [CrossRef]
- Crum, E.M.; O’Connor, W.J.; Van Loo, L.; Valckx, M.; Stannard, S.R. Validity and reliability of the Moxy oxygen monitor during incremental cycling exercise. Eur. J. Sport. Sci. 2017, 17, 1037–1043. [Google Scholar] [CrossRef]
- Espinosa-Ramirez, M.; Moya-Gallardo, E.; Araya-Roman, F.; Riquelme-Sanchez, S.; Rodriguez-Garcia, G.; Reid, W.D.; Viscor, G.; Araneda, O.F.; Gabrielli, L.; Contreras-Briceno, F. Sex-differences in the oxygenation levels of intercostal and vastus lateralis muscles during incremental exercise. Front. Physiol. 2021, 12, 738063. [Google Scholar] [CrossRef] [PubMed]
- Farzam, P.; Starkweather, Z.; Franceschini, M.A. Validation of a novel wearable, wireless technology to estimate oxygen levels and lactate threshold power in the exercising muscle. Physiol. Rep. 2018, 6, e13664. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Carranza, V.; Gonzalez-Mohino, F.; Turner, A.P.; Rodriguez-Barbero, S.; Gonzalez-Rave, J.M. Using a portable near-infrared spectroscopy device to estimate the second ventilatory threshold. Int. J. Sports Med. 2021, 42, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzis, I.; Athanasopoulos, D.; Habazettl, H.; Kuebler, W.M.; Wagner, H.; Roussos, C.; Wagner, P.D.; Zakynthinos, S. Intercostal muscle blood flow limitation in athletes during maximal exercise. J. Physiol. 2009, 587 Pt 14, 3665–3677. [Google Scholar] [CrossRef] [PubMed]
- Van Beekvelt, M.C.; Borghuis, M.S.; van Engelen, B.G.; Wevers, R.A.; Colier, W.N. Adipose tissue thickness affects in vivo quantitative near-IR spectroscopy in human skeletal muscle. Clin. Sci. 2001, 101, 21–28. [Google Scholar] [CrossRef]
- Ferrari, M.; Mottola, L.; Quaresima, V. Principles, techniques, and limitations of near infrared spectroscopy. Can. J. Appl. Physiol. 2004, 29, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, U.; Muller, P.H.; Schmulling, R.M. Determination of anaerobic threshold by the ventilation equivalent in normal individuals. Respiration 1979, 38, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.C.; Parmenter, M.A. Using near-infrared spectroscopy to determine maximal steady state exercise intensity. J. Strength Cond. Res. 2009, 23, 1833–1840. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Int. J. Nurs. Stud. 2010, 47, 931–936. [Google Scholar] [CrossRef]
- Moalla, W.; Dupont, G.; Berthoin, S.; Ahmaidi, S. Respiratory muscle deoxygenation and ventilatory threshold assessments using near infrared spectroscopy in children. Int. J. Sports Med. 2005, 26, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Austin, K.G.; Daigle, K.A.; Patterson, P.; Cowman, J.; Chelland, S.; Haymes, E.M. Reliability of near-infrared spectroscopy for determining muscle oxygen saturation during exercise. Res. Q. Exerc. Sport 2005, 76, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Celie, B.; Boone, J.; Van Coster, R.; Bourgois, J. Reliability of near infrared spectroscopy (NIRS) for measuring forearm oxygenation during incremental handgrip exercise. Eur. J. Appl. Physiol. 2012, 112, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Boone, J.; Vandekerckhove, K.; Coomans, I.; Prieur, F.; Bourgois, J.G. An integrated view on the oxygenation responses to incremental exercise at the brain, the locomotor and respiratory muscles. Eur. J. Appl. Physiol. 2016, 116, 2085–2102. [Google Scholar] [CrossRef] [PubMed]
- Barstow, T.J. Understanding near infrared spectroscopy and its application to skeletal muscle research. J. Appl. Physiol. 2019, 126, 1360–1376. [Google Scholar] [CrossRef]
| Variable | Test (n = 17) | Retest (n = 17) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RCP | SmO2BP | p | RCP | SmO2BP | p | |||||
| M | SD | M | SD | M | SD | M | SD | |||
| Speed (km·h−1) | 16.1 | 2.09 | 16.1 | 2.22 | 0.908 | 15.2 | 1.78 | 15.4 | 1.90 | 0.269 |
| O2 (ml·kg−1·min−1) | 52.5 | 8.36 | 52.7 | 8.42 | 0.401 | 49.8 | 5.97 | 49.8 | 7.07 | 0.930 |
| Percentage of O2max (%) | 88.5 | 4.10 | 88.8 | 4.08 | 0.447 | 88.4 | 5.37 | 88.3 | 5.32 | 0.891 |
| HR (bpm) | 170.8 | 13.63 | 171.5 | 13.33 | 0.324 | 167.1 | 12.77 | 168.2 | 13.45 | 0.263 |
| Percentage of HRmax (%) | 94.1 | 3.08 | 94.4 | 3.00 | 0.311 | 92.8 | 2.90 | 93.4 | 3.13 | 0.282 |
| SmO2 (%) | 68.9 | 14.57 | 68.6 | 13.81 | 0.607 | 62.3 | 11.22 | 63.0 | 11.12 | 0.098 |
| RER | 1.01 | 0.05 | 1.01 | 0.05 | 0.590 | 1.02 | 0.06 | 1.02 | 0.06 | 0.906 |
| ICC | Mean Differences | |||
|---|---|---|---|---|
| Variable | R | 95% CI | Mean Differences | 95% CI |
| Speed (km·h−1) | 0.859 | 0.65–0.94 | −0.78 | −1.34–(−0.22) |
| O2 (mL·kg·s−1) | 0.793 | 0.52–0.92 | −2.68 | −5.22–(−0.13) |
| HR (bpm) | 0.823 | 0.57–0.93 | −3.29 | −7.39–0.81 |
| SmO2 (%) | 0.600 | 0.18–0.83 | −5.60 | −11.36–0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Arenas, S.; Quero-Calero, C.D.; Abellan-Aynes, O.; Andreu-Caravaca, L.; Fernandez-Calero, M.; Manonelles, P.; Lopez-Plaza, D. Assessment of Intercostal Muscle Near-Infrared Spectroscopy for Estimating Respiratory Compensation Point in Trained Endurance Athletes. Sports 2023, 11, 212. https://doi.org/10.3390/sports11110212
Romero-Arenas S, Quero-Calero CD, Abellan-Aynes O, Andreu-Caravaca L, Fernandez-Calero M, Manonelles P, Lopez-Plaza D. Assessment of Intercostal Muscle Near-Infrared Spectroscopy for Estimating Respiratory Compensation Point in Trained Endurance Athletes. Sports. 2023; 11(11):212. https://doi.org/10.3390/sports11110212
Chicago/Turabian StyleRomero-Arenas, Salvador, Carmen Daniela Quero-Calero, Oriol Abellan-Aynes, Luis Andreu-Caravaca, Marta Fernandez-Calero, Pedro Manonelles, and Daniel Lopez-Plaza. 2023. "Assessment of Intercostal Muscle Near-Infrared Spectroscopy for Estimating Respiratory Compensation Point in Trained Endurance Athletes" Sports 11, no. 11: 212. https://doi.org/10.3390/sports11110212






