The Oral Microbiome Profile of Water Polo Players Aged 16–20
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Subjects
2.2. Methods
2.2.1. Study Design
2.2.2. Microbial Analyses
2.2.3. Statistical Analyses
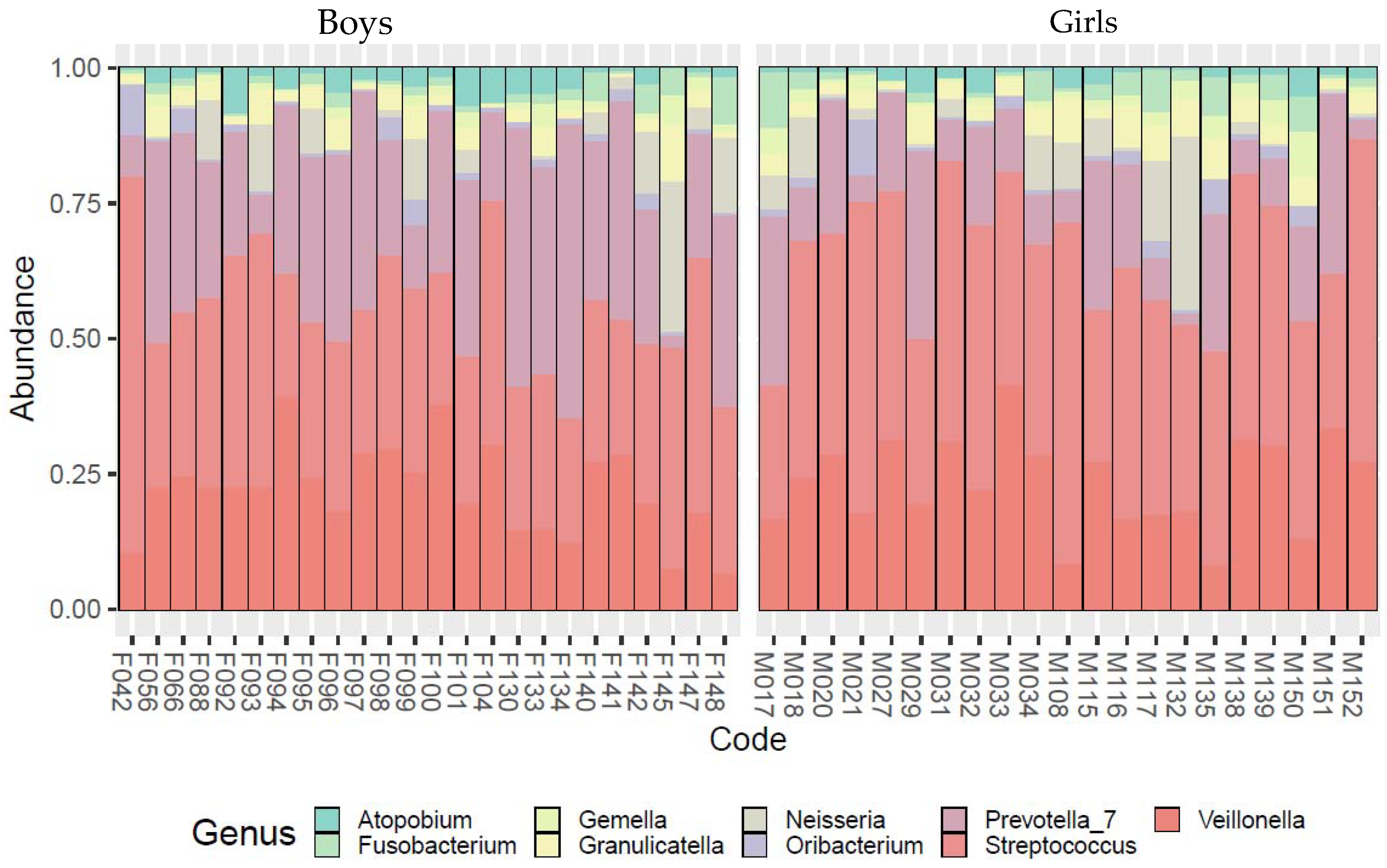
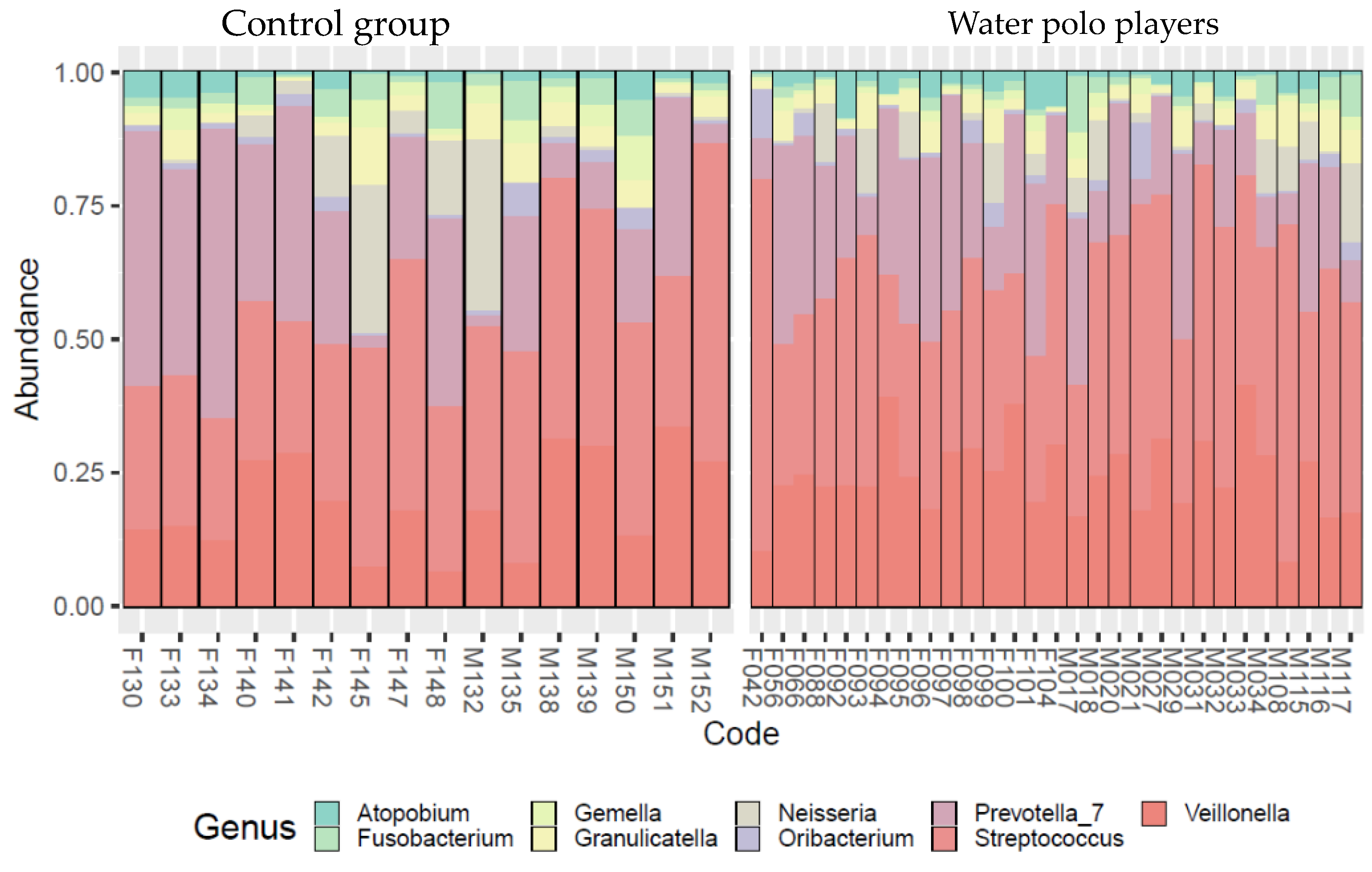
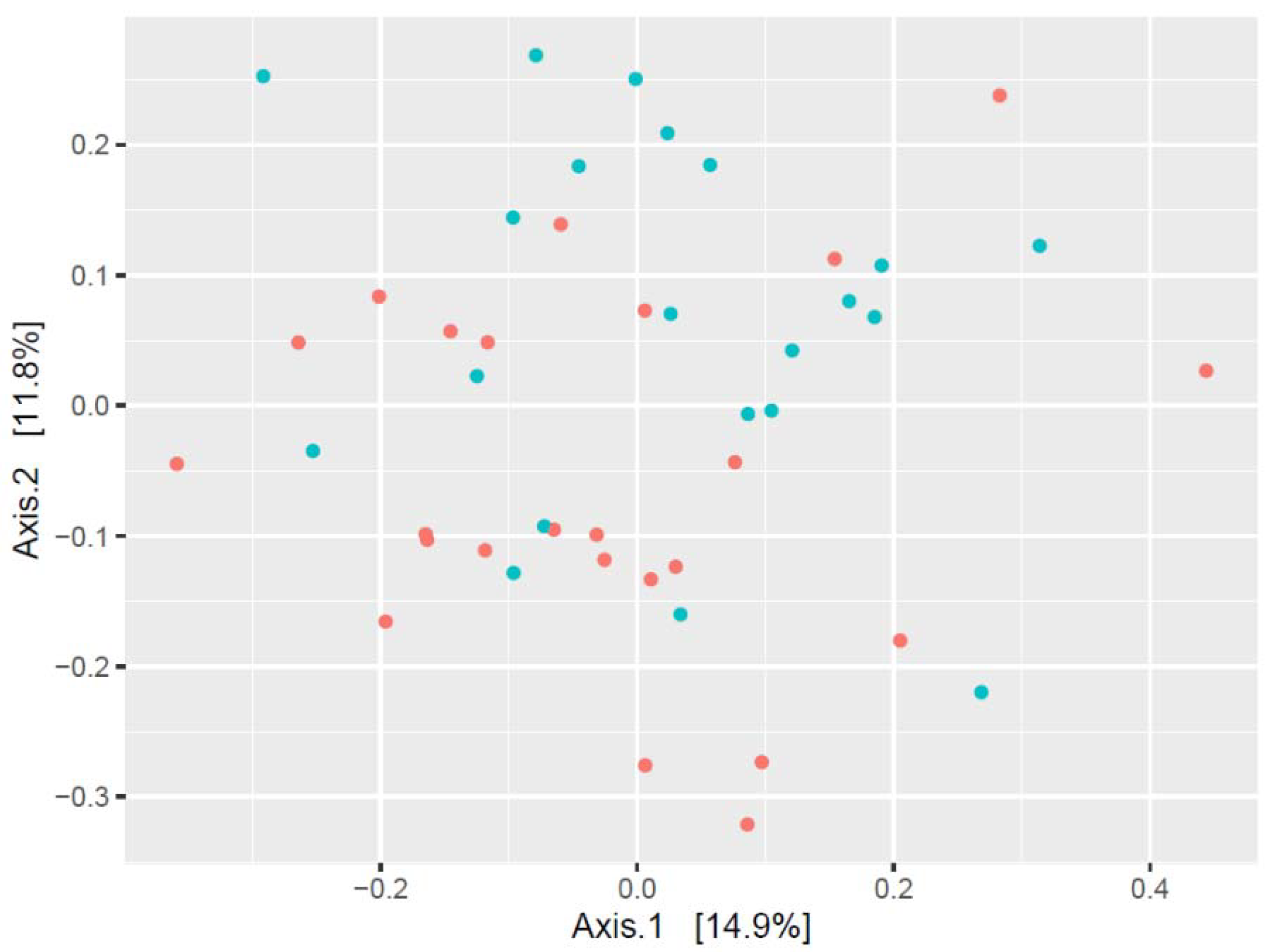
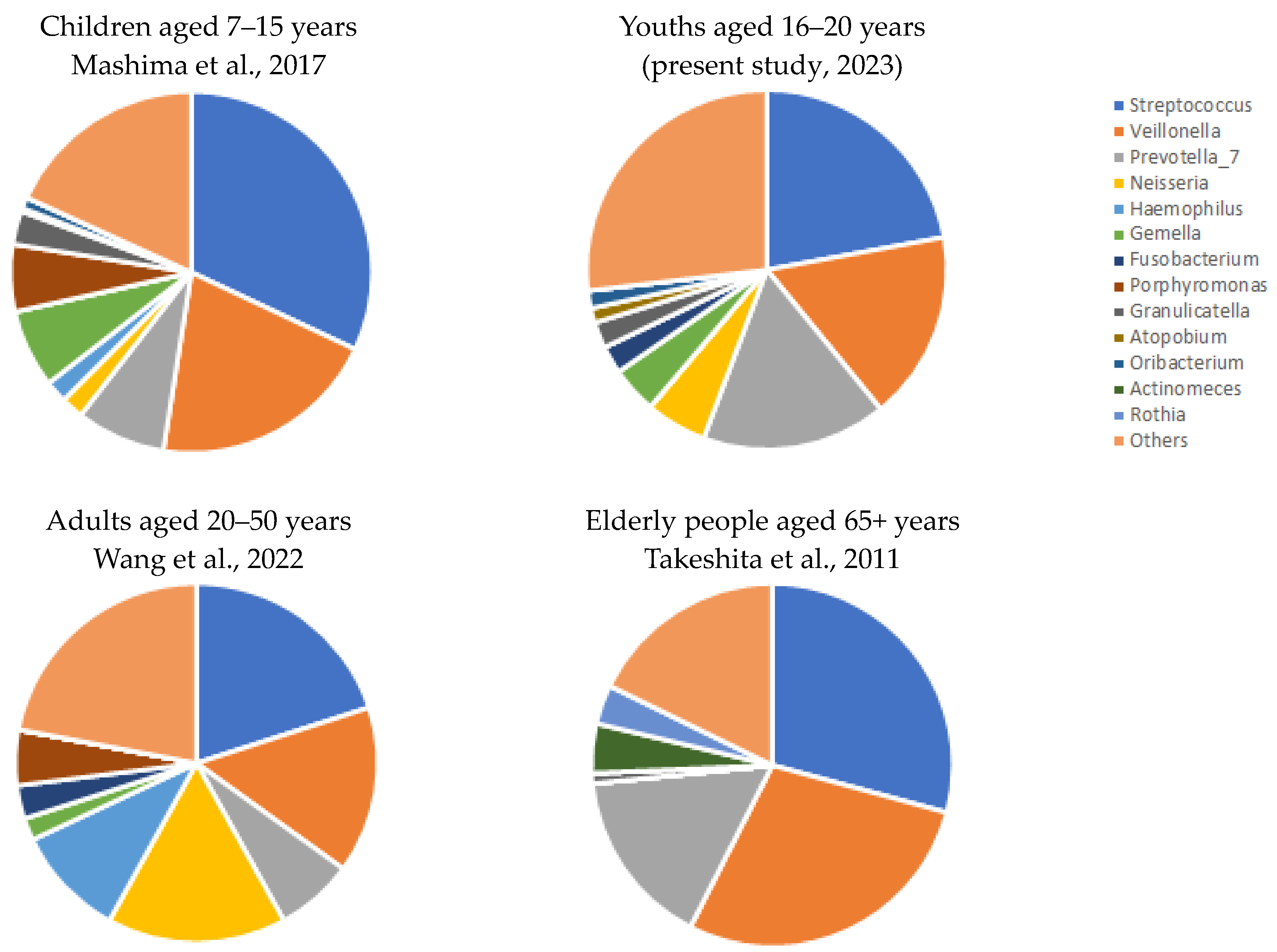
3. Results
4. Discussion
5. Conclusions
- The microbial profile of youths corresponded to the microbiome reported by previous studies during development of children and aging;
- Our data revealed a significant sexual dimorphism in the microbial community composition: females were found to develop streptococcal disease and tonsillitis more frequently than males, while males were found to have a higher abundance of pathogens of supragingival plaque, subgingival plaque, and a sexually transmitted disease, bacterial vaginosis;
- Water polo players’ oral microbiome differed from age-matched non-athletes;
- The dominant genera were present in both groups’ microbiome but in different proportions. Some genera were missing from the non-athlete age-peers’ microbiome, and those genera cause inflammations in the oral cavity, respiratory system, and vagina. This result suggests that regular water training can unfavorably alter the composition of the oral microbial community and can result in an increase in the abundance of inflammation-causing pathogens in particular, causing, unsurprisingly, oral infections.
6. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verma, D.; Garg, P.K.; Dubey, A.K. Insights into the human oral microbiome. Arch. Microbiol. 2018, 200, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.; Chan, Y.; Kheur, S.; Jin, L.; Watt, R.M.; Mattheos, N. Salivary microbiome in non-oral disease: A summary of evidence and commentary. Arch. Oral Biol. 2017, 83, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Su, L.; Wang, Y.; Deng, S. Improved high-throughput sequencing of the human oral microbiome: From Illumina to PacBio. Can. J. Infect. Dis. Med. Microbiol. 2020, in press. [Google Scholar] [CrossRef]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122. [Google Scholar] [CrossRef] [PubMed]
- Belstrom, D.; Holmstrup, P.; Nielsen, C.H.; Kirkby, N.; Twetman, S.; Heitmann, B.L.; Klepac-Ceraj, V.; Paster, B.J.; Fiehn, N.E. Bacterial profiles of saliva in relation to diet, lifestyle factors, and socioeconomic status. J. Oral Microbiol. 2014, 6, 23609. [Google Scholar] [CrossRef] [PubMed]
- Nasidze, I.; Li, J.; Quinque, D.; Tang, K.; Stoneking, M. Global diversity in the human salivary microbiome. Genome Res. 2009, 19, 636–643. [Google Scholar] [CrossRef]
- Takeshita, T.; Kageyama, S.; Furuta, M.; Tsuboi, H.; Takeuchi, K.; Shibata, Y.; Shimazaki, Y.; Akifusa, S.; Ninomiya, T.; Kiyohara, Y.; et al. Bacterial diversity in saliva and oral health-related conditions: The Hisayama Study. Sci. Rep. 2016, 6, 22164. [Google Scholar] [CrossRef]
- Wu, J.; Peters, B.A.; Dominianni, C.; Zhang, Y.; Pei, Z.; Yang, L.; Ma, Y.; Purdue, M.P.; Jacobs, E.J.; Gapstur, S.M.; et al. Cigarette smoking and the oral microbiome in a large study of American adults. ISME J. 2016, 10, 2435–2446. [Google Scholar] [CrossRef]
- Fan, X.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Freedman, N.D.; Alekseyenko, A.V.; Wu, J.; Yang, L.; Pei, Z.; et al. Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Microbiome 2018, 6, 1–15. [Google Scholar] [CrossRef]
- Raju, S.C.; Lagström, S.; Ellonen, P.; De Vos, W.M.; Eriksson, J.G.; Weiderpass, E.; Rounge, T.B. Gender-specific associations between saliva microbiota and body size. Front. Microbiol. 2019, 10, 767. [Google Scholar] [CrossRef]
- Renson, A.; Jones, H.E.; Beghini, F.; Segata, N.; Zolnik, C.P.; Usyk, M.; Moody, T.U.; Thorpe, L.; Burk, R.; Waldron, L.; et al. Sociodemographic variation in the oral microbiome. Ann. Epidemiol. 2019, 35, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cai, Q.; Zheng, W.; Steinwandel, M.; Blot, W.J.; Shu, X.O.; Long, J. Oral microbiome and obesity in a large study of low-income and African-American populations. J. Oral Microbiol. 2019, 11, 1650597. [Google Scholar] [CrossRef] [PubMed]
- Nearing, J.T.; DeClercq, V.; Van Limbergen, J.; Langille, M.G. Assessing the variation within the oral microbiome of healthy adults. Msphere 2020, 5, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Spence, L.; Brown, W.J.; Pyne, D.B.; Nissen, M.D.; Sloots, T.P.; McCormack, J.G.; Locke, A.S.; Fricker, P.A. Incidence, etiology, and symptomatology of upper respiratory illness in elite athletes. Med. Sci. Sports Exerc. 2007, 25, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, D.; Cosi, A.; Fulco, D.; D’Ercole, S. The impact of sport training on oral health in athletes. Dent. J. 2021, 9, 51–54. [Google Scholar] [CrossRef]
- De Oliveira, E.P.; Burini, R.C. The impact of physical exercise on the gastrointestinal tract. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 533–538. [Google Scholar] [CrossRef]
- Stromberg, J.D. Care of water polo players. Curr. Sports Med. Rep. 2017, 16, 363–369. [Google Scholar] [CrossRef]
- Strauss, R.H.; Lanese, R.R.; Leizman, D.J. Illness and absence among wrestlers, swimmers, and gymnasts at a large university. Am. J. Sports Med. 1988, 16, 653–655. [Google Scholar] [CrossRef]
- Prien, A.; Mountjoy, M.; Miller, J.; Boyd, K.; van den Hoogenband, C.; Gerrard, D.; Cherif, M.Y.; Lu, Y.; Nanousis, K.; Liscano, E.I.; et al. Injury and illness in aquatic sport: How high is the risk? A comparison of results from three FINA World Championships. Br. J. Sports Med. 2017, 51, 277–282. [Google Scholar] [CrossRef]
- Shields, J.M.; Hill, V.R.; Arrowood, M.J.; Beach, M.J. Inactivation of Cryptosporidium parvum under chlorinated recreational water conditions. J. Water Health 2008, 6, 513–520. [Google Scholar] [CrossRef]
- Hlavsa, M.C.; Roberts, V.A.; Kahler, A.M.; Hilborn, E.D.; Mecher, T.R.; Beach, M.J.; Wade, T.J.; Yoder, J.S. Outbreaks of illness associated with recreational water—United States, 2011–2012. Morb. Mortal. Wkly. Rep. 2015, 64, 668. [Google Scholar] [CrossRef]
- Perkins, A.; Trimmier, M. Recreational waterborne illnesses: Recognition, treatment, and prevention. Am. Fam. Phys. 2017, 95, 554–560. [Google Scholar]
- Yu-Rin, K. Analysis of the Effect of Daily Water Intake on Oral Health: Result from Seven Waves of a Population-Based Panel Study. Water 2021, 13, 2016. [Google Scholar] [CrossRef]
- Popkin, B.M.; D’Anci, K.E.; Rosenberg, I.H. Water, hydration, and health. Nutr. Rev. 2010, 68, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Perrier, E.; Vergne, S.; Klein, A.; Poupin, M.; Rondeau, P.; Le Bellego, L.; Armstrong, L.E.; Lang, F.; Stookey, J.; Tack, I. Hydration biomarkers in free-living adults with different levels of habitual fluid consumption. Br. J. Nutr. 2013, 109, 1678–1687. [Google Scholar] [CrossRef] [PubMed]
- Vanhaecke, T.; Bretin, O.; Poirel, M.; Tap, J. Drinking Water Source and Intake Are Associated with Distinct Gut Microbiota Signatures in US and UK Populations. J. Nutr. 2022, 152, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.R.; Broad, E.M.; Riley, M.D.; Burke, L.M. Body mass changes and voluntary fluid intakes of elite level water polo players and swimmers. J. Sci. Med. Sport 2002, 5, 183–193. [Google Scholar] [CrossRef]
- Kameoka, S.; Motooka, D.; Watanabe, S.; Kubo, R.; Jung, N.; Midorikawa, Y.; Shinozaki, N.O.; Sawai, Y.; Takeda, A.K.; Nakamura, S. Benchmark of 16S rRNA gene amplicon sequencing using Japanese gut microbiome data from the V1–V2 and V3–V4 primer sets. BMC Genom. 2021, 22, 1–10. [Google Scholar] [CrossRef]
- Anesti, V.; McDonald, I.R.; Ramaswamy, M.; Wade, W.G.; Kelly, D.P.; Wood, A.P. Isolation and molecular detection of methylotrophic bacteria occurring in the human mouth. Environ. Microbiol. 2005, 7, 1227–1238. [Google Scholar] [CrossRef]
- Bernardi, S.; Karygianni, L.; Filippi, A.; Anderson, A.C.; Zürcher, A.; Hellwig, E.; Vach, K.; Macchiarelli, G.; Al-Ahmad, A. Combining culture and culture-independent methods reveals new microbial composition of halitosis patients’ tongue biofilm. Microbiologyopen 2020, 9, e958. [Google Scholar] [CrossRef]
- Wade, W.G. Resilience of the oral microbiome. Periodontology 2021, 86, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.N. Microbiological changes of the ageing oral cavity. Arch. Oral Biol. 2018, 96, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, F.; Gu, H.; Wei, X.; Zhang, K.; Zhou, Y.; Luo, H. Comparative evaluation of the salivary and buccal mucosal microbiota by 16S rRNA sequencing for forensic investigations. Front. Microbiol. 2022, 13, 777882. [Google Scholar] [CrossRef] [PubMed]
- Mashima, I.; Theodorea, C.F.; Thaweboon, B.; Thaweboon, S.; Scannapieco, F.A.; Nakazawa, F. Exploring the salivary microbiome of children stratified by the oral hygiene index. PLoS ONE 2017, 12, e0185274. [Google Scholar] [CrossRef]
- Takeshita, T.; Yasui, M.; Tomioka, M.; Nakano, Y.; Shimazaki, Y.; Yamashita, Y. Enteral tube feeding alters the oral indigenous microbiota in elderly adults. Appl. Environ. Microbiol. 2011, 77, 6739–6745. [Google Scholar] [CrossRef]
- Bomholt, C.; Glaub, A.; Gravermann, K.; Albersmeier, A.; Brinkrolf, K.; Ruckert, C.; Tauch, A. Whole-genome sequence of the clinical strain Corynebacterium argentoratense DSM 44202, isolated from a human throat specimen. Genome Announc. 2013, 1, 10–11. [Google Scholar] [CrossRef]
- Bao, K.; Li, X.; Poveda, L.; Qi, W.; Selevsek, N.; Gumus, P.; Emingil, G.; Grossmann, J.; Diaz, P.I.; Hajishengallis, G.; et al. Proteome and microbiome mapping of human gingival tissue in health and disease. Front. Cell. Infect. Microbiol. 2020, 10, 588155. [Google Scholar] [CrossRef]
- Monteiro, H.F.; Zhou, Z.; Gomes, M.S.; Peixoto, P.M.; Bonsaglia, E.C.; Canisso, I.F.; Weimer, B.C.; Lima, F.S. Rumen and lower gut microbiomes relationship with feed efficiency and production traits throughout the lactation of Holstein dairy cows. Sci. Rep. 2022, 12, 4904. [Google Scholar] [CrossRef]
- Marrazzo, J.M.; Thomas, K.K.; Fiedler, T.L.; Ringwood, K.; Fredricks, D.N. Relationship of specific vaginal bacteria and bacterial vaginosis treatment failure in women who have sex with women. Ann. Intern. Med. 2008, 149, 20–28. [Google Scholar] [CrossRef]
- Naginyte, M.; Do, T.; Meade, J.; Devine, D.A.; Marsh, P.D. Enrichment, of periodontal pathogens from the biofilms of healthy adults. Sci. Rep. 2019, 9, 5491. [Google Scholar] [CrossRef]
- D’Ercole, S.; Tieri, M.; Martinelli, D.; Tripodi, D. The effect of swimming on oral health status: Competitive versus non-competitive athletes. J. Appl. Oral Sci. 2016, 24, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Rowland, S.N.; Chessor, R.; French, G.; Robinson, G.P.; O’Donnell, E.; James, L.J.; Bailey, S.J. Oral nitrate reduction is not impaired after training in chlorinated swimming pool water in elite swimmers. Appl. Physiol. Nutr. Metabol. 2021, 46, 86–89. [Google Scholar] [CrossRef] [PubMed]

| Bacterial Genus | % |
|---|---|
| Atopobium | 1.3 |
| Fusobacterium | 2.5 |
| Gemella | 4.2 |
| Granulicatella | 2.4 |
| Neisseria | 5.5 |
| Oribacterium | 1.6 |
| Prevotella_7 | 16.6 |
| Streptococcus | 22.1 |
| Veillonella | 17.0 |
| Df | Sums Sqs | Mean Sqs | F Model | R2 | p | |
|---|---|---|---|---|---|---|
| Sex | 1 | 0.308 | 0.308 | 1.883 | 0.040 | 0.016 |
| Water polo players vs. control group | 1 | 0.466 | 0.466 | 2.850 | 0.061 | <0.001 |
| Residuals | 42 | 6.861 | 0.163 | NA | 0.899 | NA |
| Total | 44 | 7.634 | NA | NA | 1.000 | NA |
| Bacterium Genus | M–F | Effect Size | W–C | Effect Size |
|---|---|---|---|---|
| Atopobium | 0.041 | 0.5 (medium) | 0.248 | 0.2 (small) |
| Fusobacterium | 0.029 | 0.6 (large) | 0.919 | 0.2 (small) |
| Gemella | 0.025 | 0.6 (large) | 0.040 | 0.6 (large) |
| Granulicatella | 0.262 | 0.3 (medium) | 0.836 | 0.2 (small) |
| Neisseria | 0.722 | 0.1 (small) | 0.760 | 0.2 (small) |
| Oribacterium | 0.709 | 0.1 (small) | 0.494 | 0.2 (small) |
| Prevotella_7 | 0.001 | 1.0 (very large) | 0.510 | 0.1 (small) |
| Streptococcus | 0.002 | 0.8 (large) | 0.426 | 0.1 (small) |
| Veillonella | 0.257 | 0.3 (medium) | 0.046 | 0.3 (medium) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalabiska, I.; Annar, D.; Keki, Z.; Borbas, Z.; Bhattoa, H.P.; Zsakai, A. The Oral Microbiome Profile of Water Polo Players Aged 16–20. Sports 2023, 11, 216. https://doi.org/10.3390/sports11110216
Kalabiska I, Annar D, Keki Z, Borbas Z, Bhattoa HP, Zsakai A. The Oral Microbiome Profile of Water Polo Players Aged 16–20. Sports. 2023; 11(11):216. https://doi.org/10.3390/sports11110216
Chicago/Turabian StyleKalabiska, Irina, Dorina Annar, Zsuzsa Keki, Zoltan Borbas, Harjit Pal Bhattoa, and Annamaria Zsakai. 2023. "The Oral Microbiome Profile of Water Polo Players Aged 16–20" Sports 11, no. 11: 216. https://doi.org/10.3390/sports11110216
APA StyleKalabiska, I., Annar, D., Keki, Z., Borbas, Z., Bhattoa, H. P., & Zsakai, A. (2023). The Oral Microbiome Profile of Water Polo Players Aged 16–20. Sports, 11(11), 216. https://doi.org/10.3390/sports11110216







