Periodized Aerobic Training between Thresholds Improves Submaximal Cardiorespiratory Parameters in Octogenarians
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
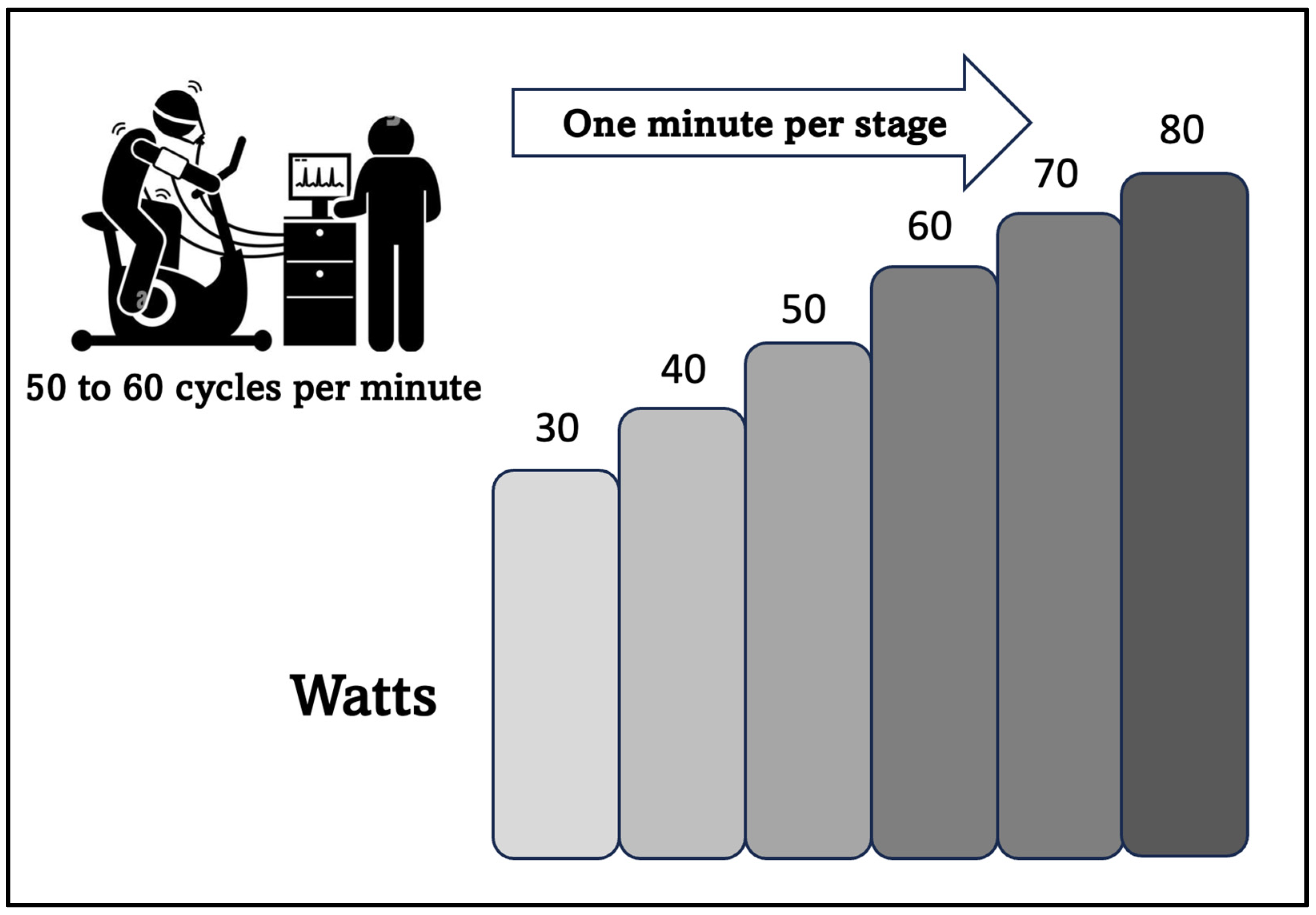
2.2. Submaximal CPET Test
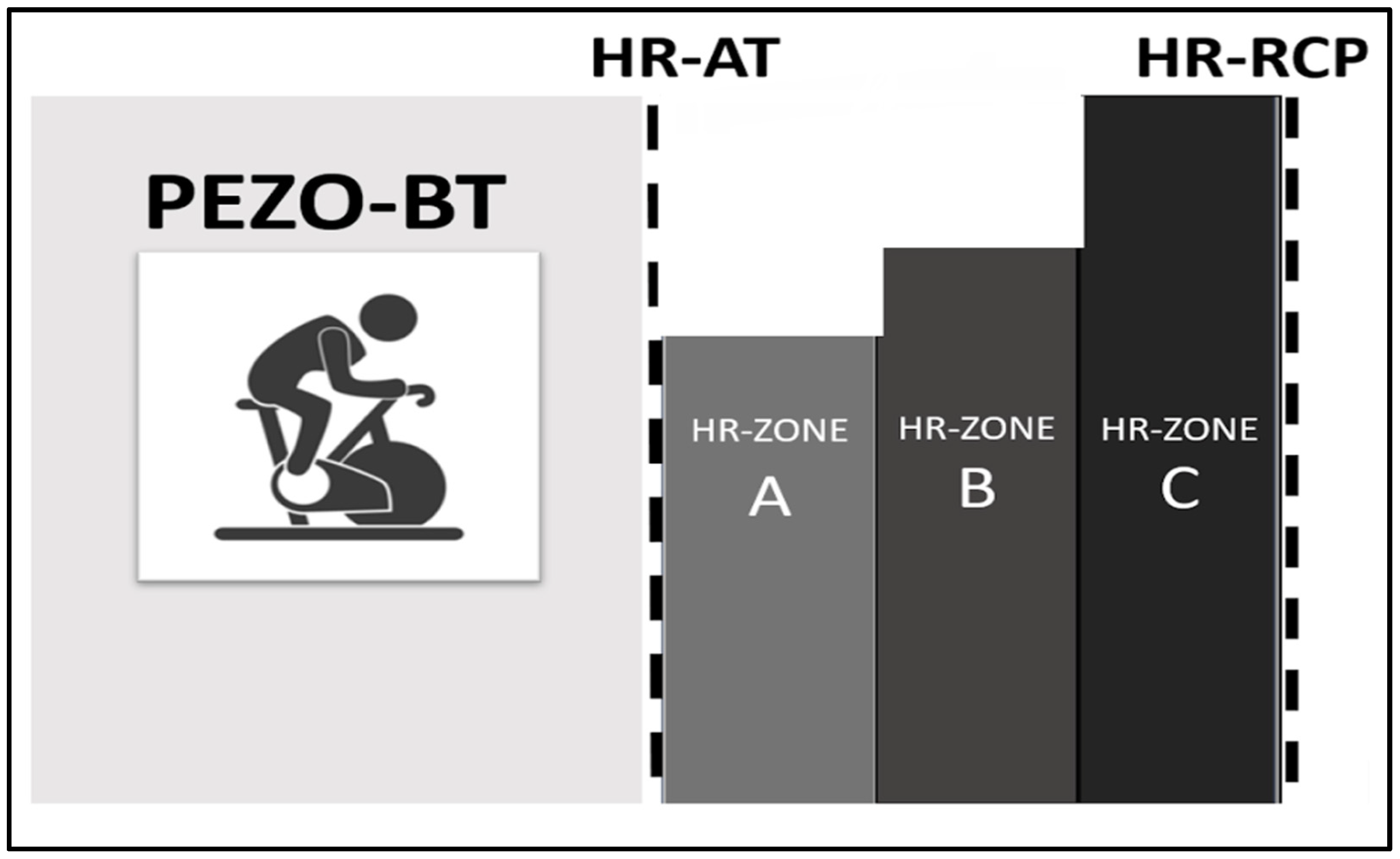
2.3. Training Zones
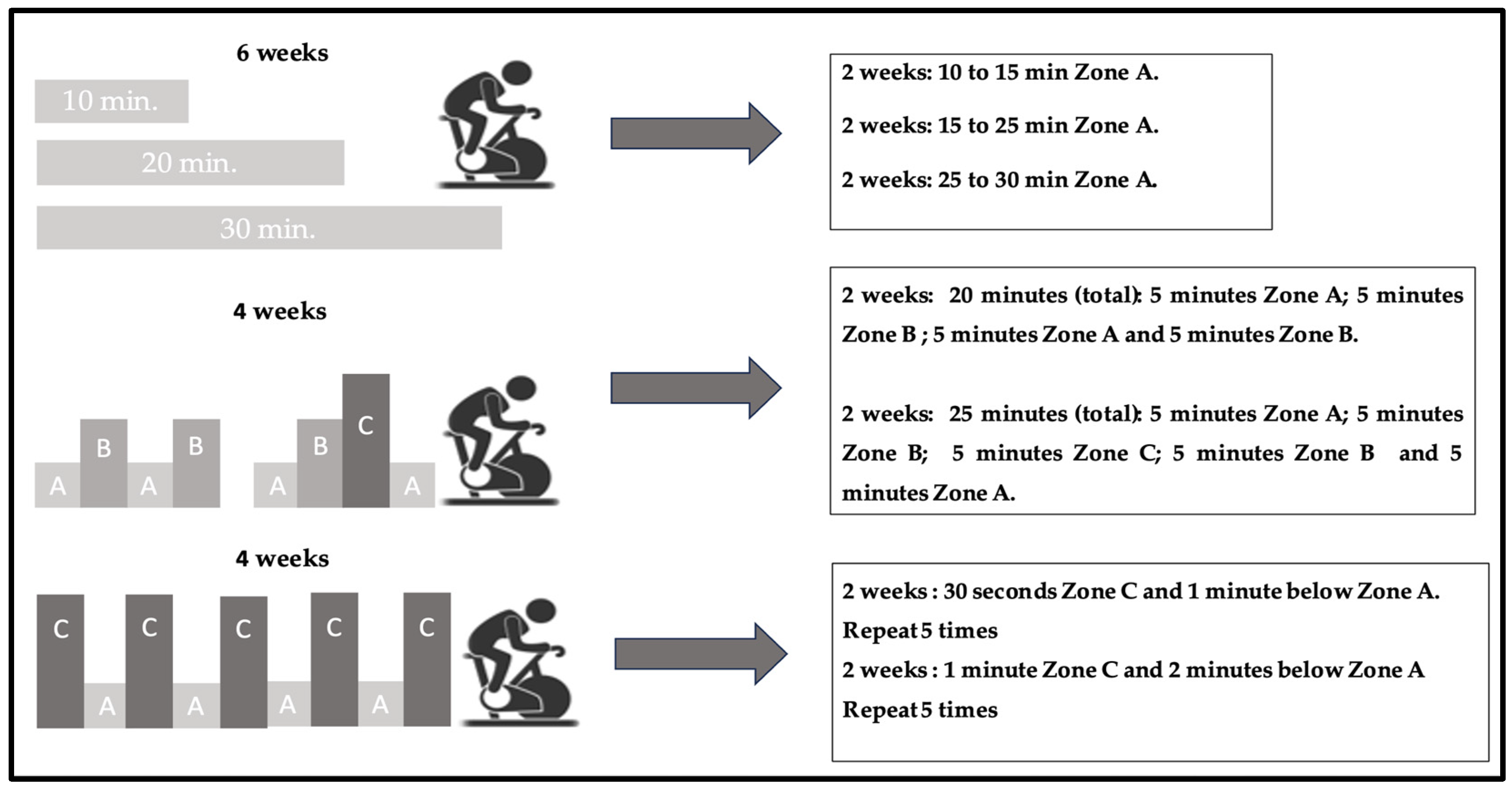
2.4. Training
2.5. Statistical Analysis
3. Results
3.1. Ergospirometry Submaximal Variables
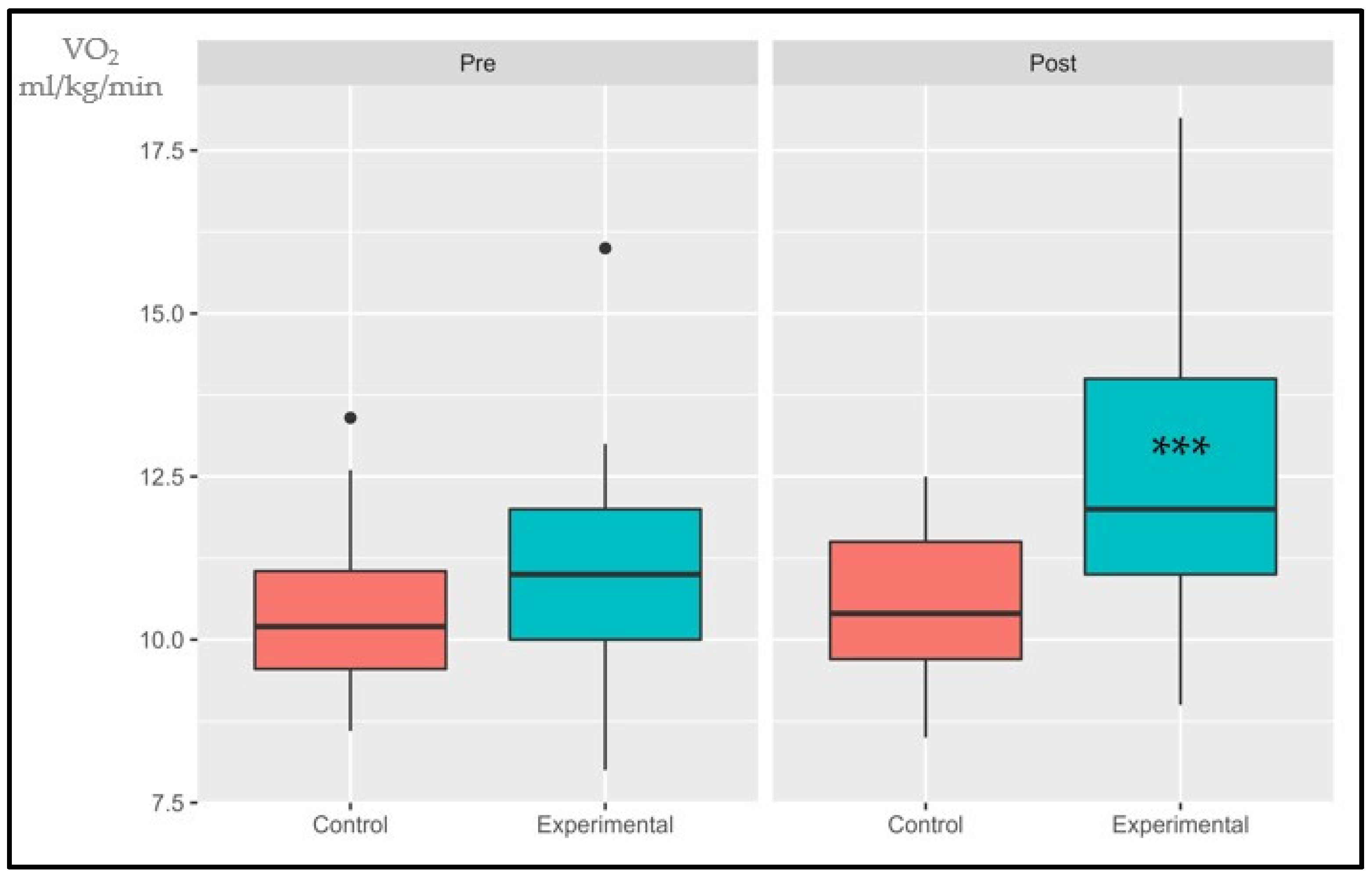
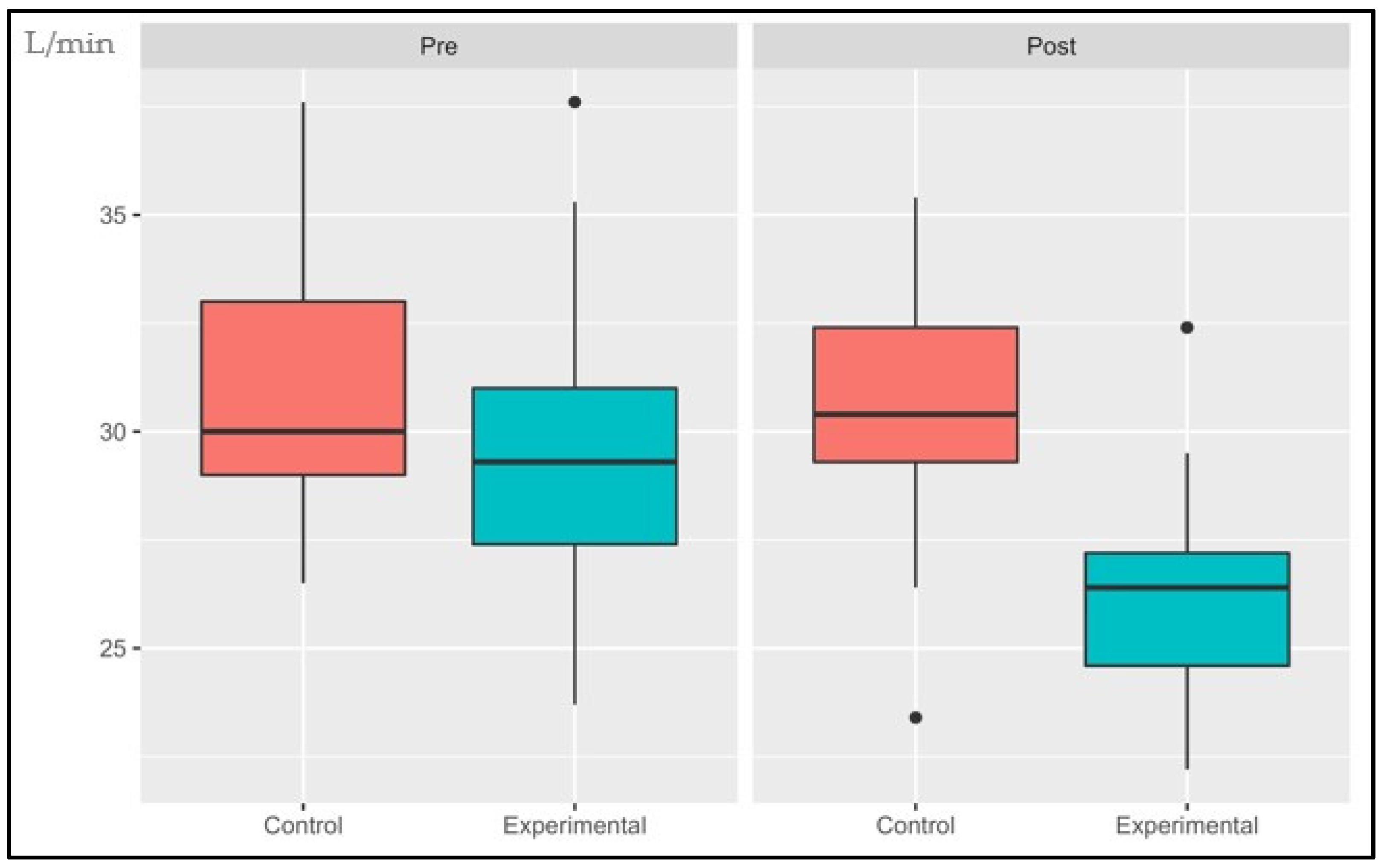
3.1.1. VO2AT
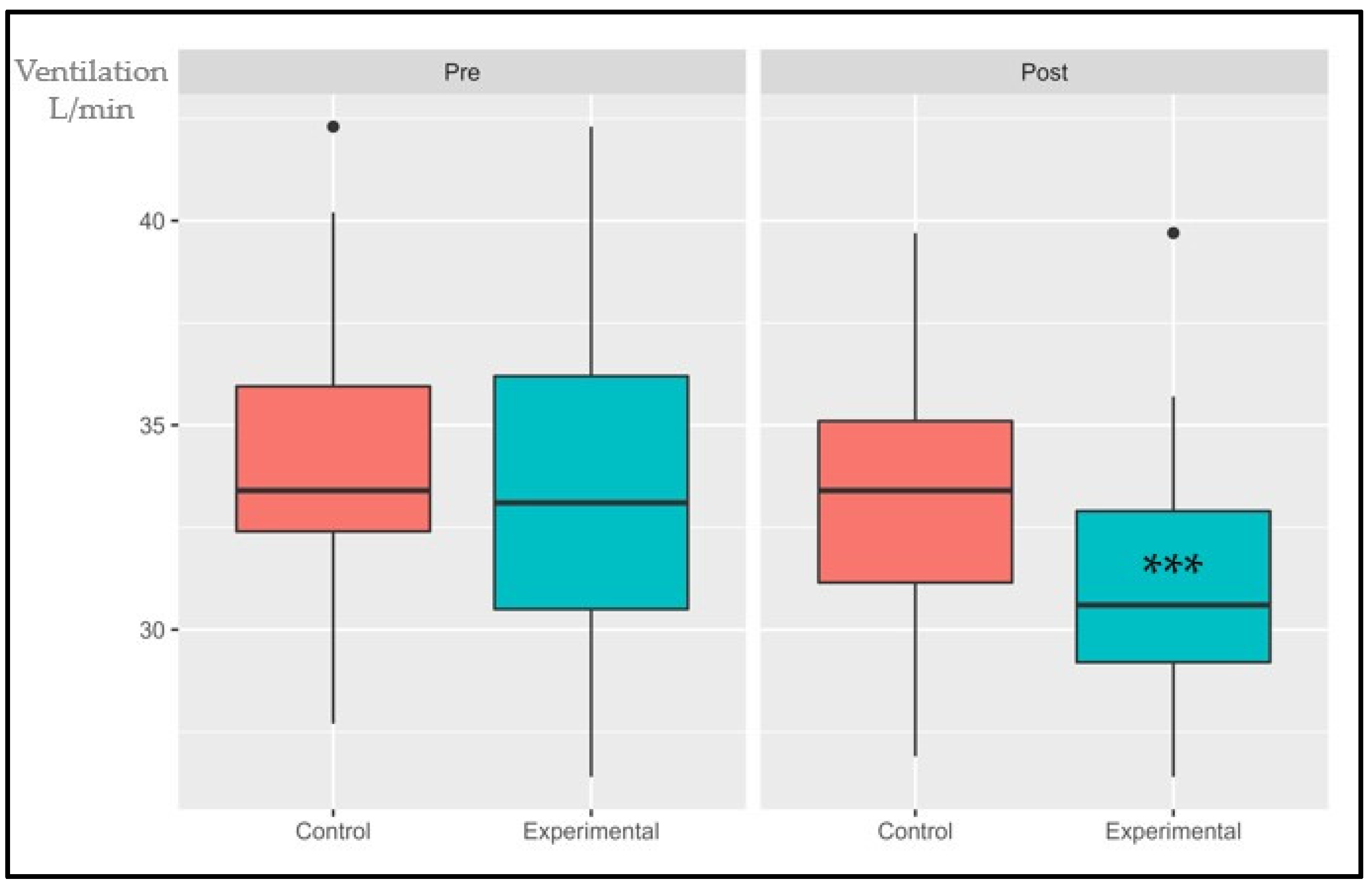
3.1.2. Ventilatory Class (VE/VCO2)
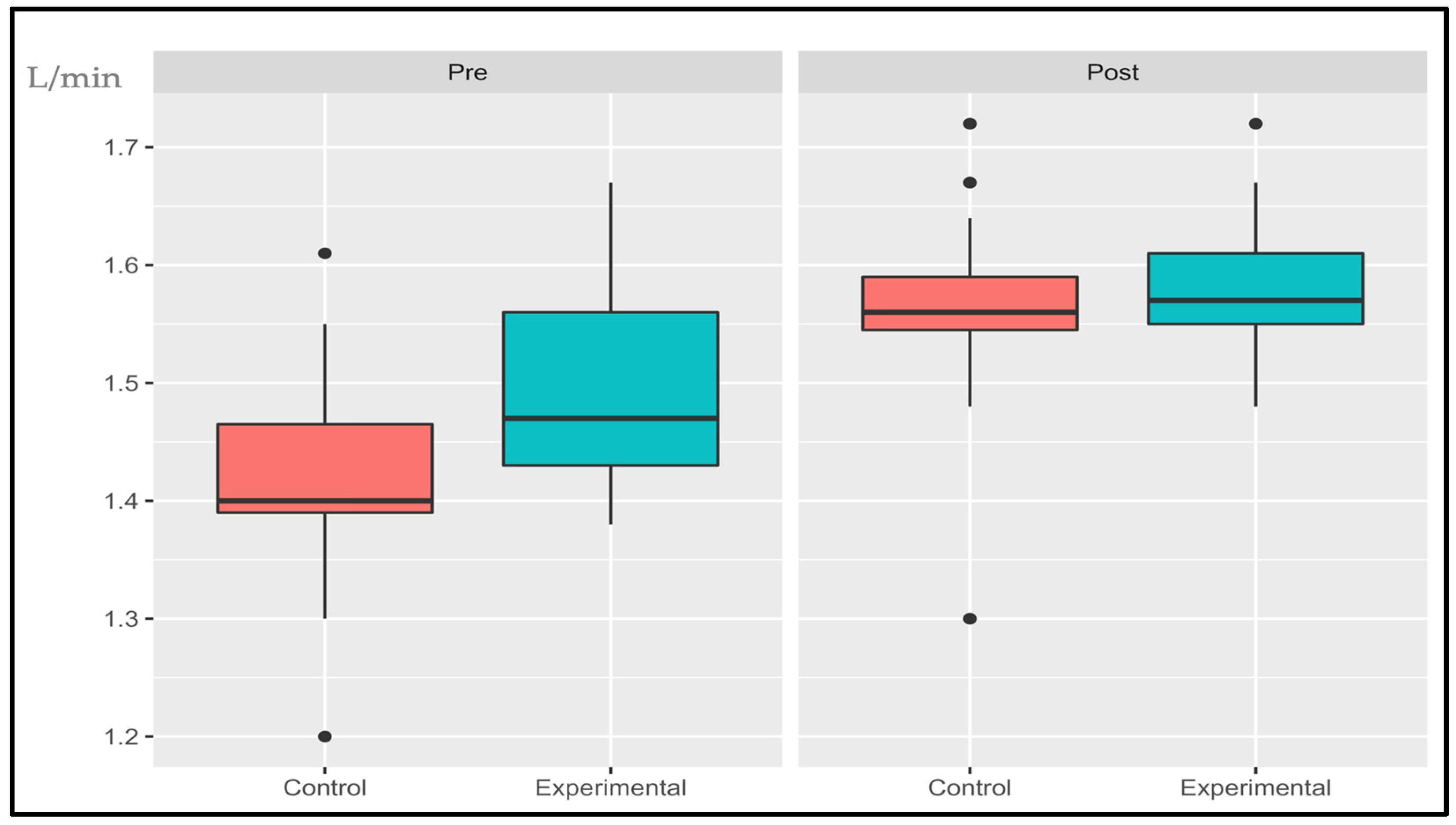
3.1.3. Oxygen Uptake Efficiency Slope (OUES)
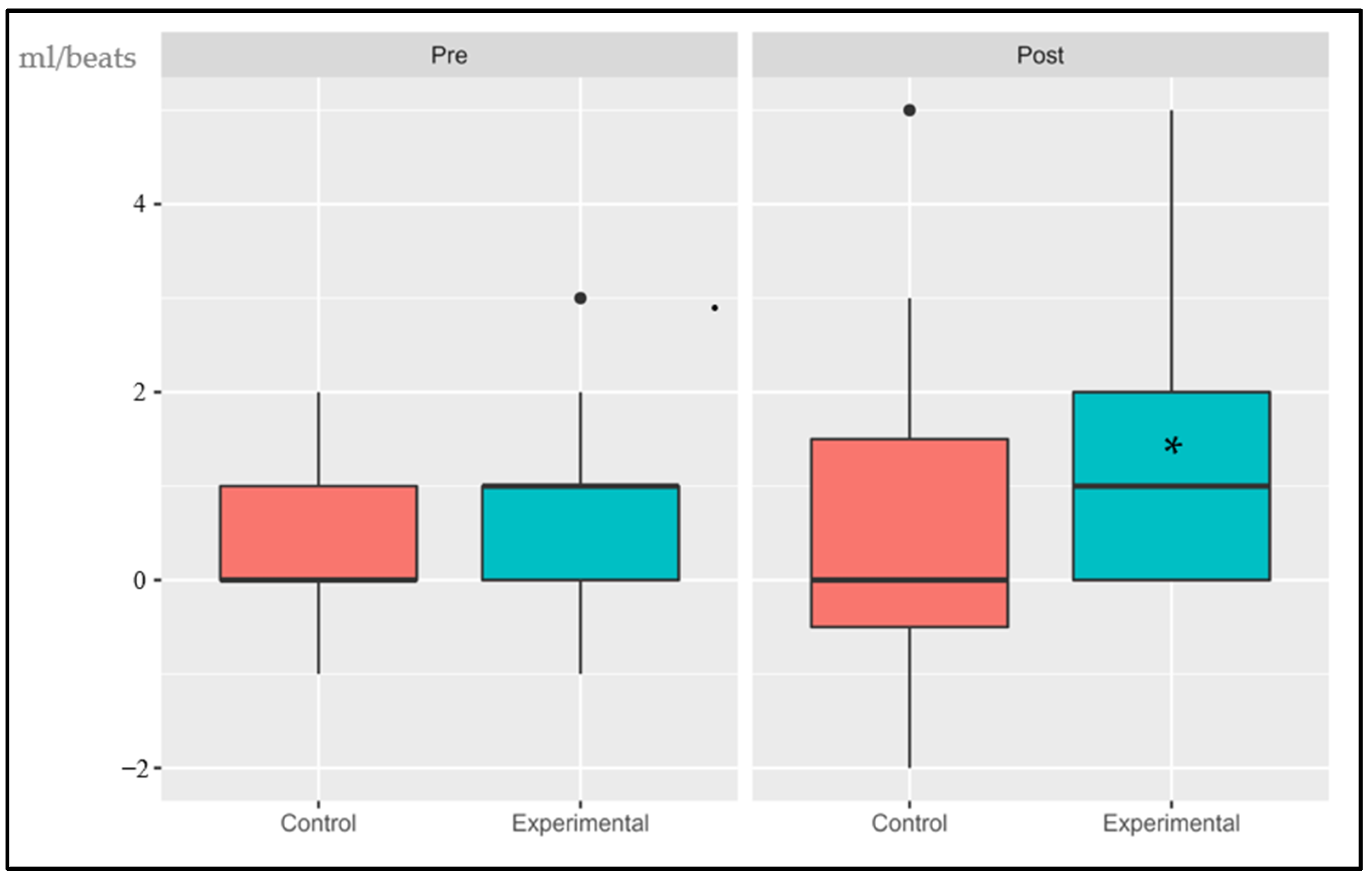
3.1.4. Delta Oxygen Pulse (∆VO2/HR RCP vs. AT)
3.1.5. Cardiopulmonary Optimal Point (COP)
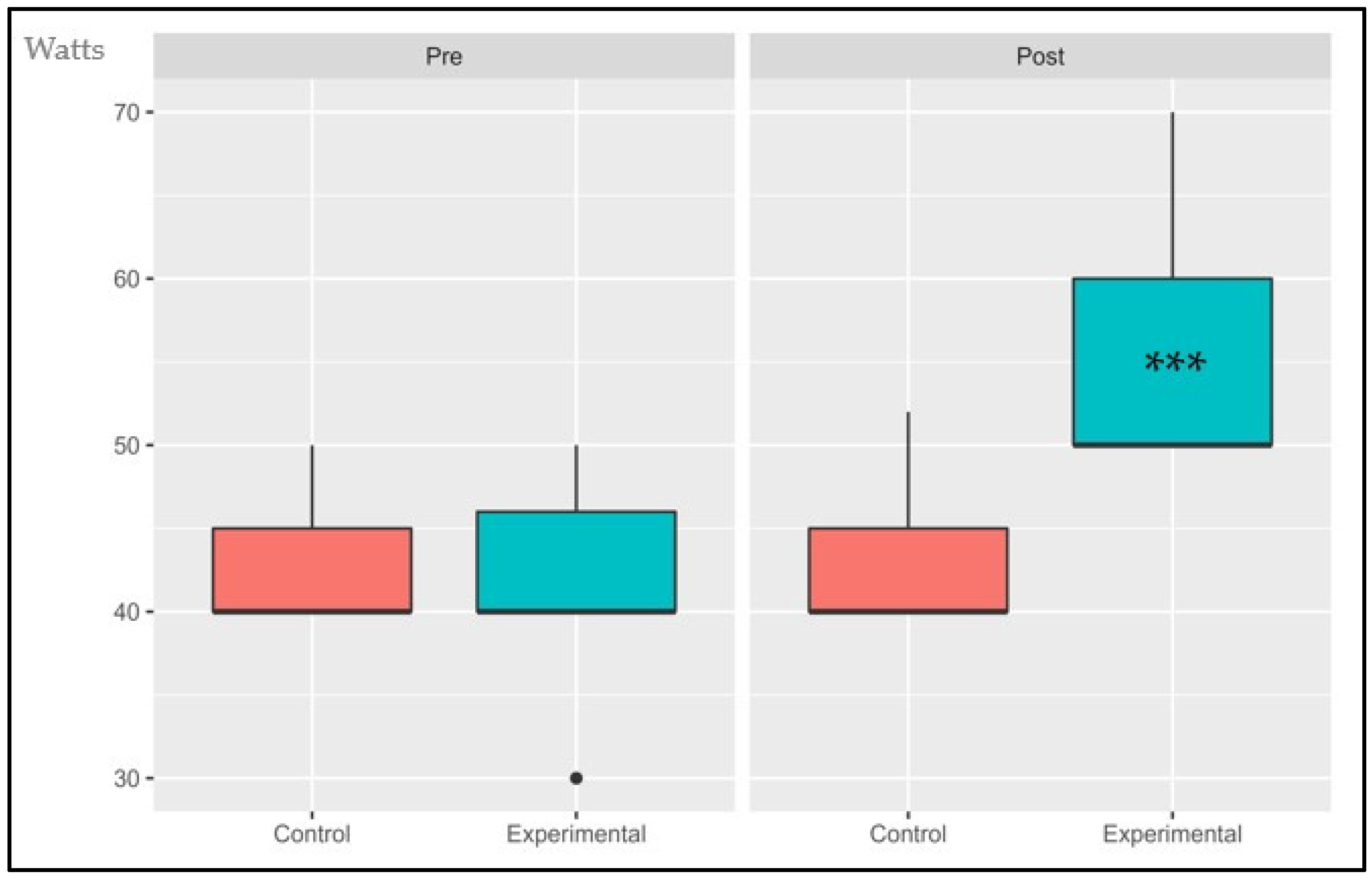
3.1.6. Power Output at AT (POAT)
3.2. Contingence Table
4. Discussion
5. Conclusions
6. Practical Applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Ageing 2019 (st/esa/ser. a/444); Department of Economic and Social Affairs: New York, NY, USA, 2020. [Google Scholar]
- Courel-Ibanez, J.; Buendia-Romero, A.; Pallares, J.G.; Garcia-Conesa, S.; Martinez-Cava, A.; Izquierdo, M. Impact of Tailored Multicomponent Exercise for Preventing Weakness and Falls on Nursing Home Residents’ Functional Capacity. J. Am. Med. Dir. Assoc. 2022, 23, 98–104.e103. [Google Scholar] [CrossRef]
- Valdivia, G.; Dominguez, A.L.A. Población de 80 y más años en Chile: Una visión preliminar desde el punto de vista epidemiológico. Revista Médica Clínica Las Condes 2012, 23, 5–12. [Google Scholar] [CrossRef]
- Departamento de Estadísticas e Información de Salud. Población ine por Grupo Etario. Available online: https://deis.minsal.cl/ (accessed on 25 December 2021).
- Izquierdo, M.; Duque, G.; Morley, J.E. Physical activity guidelines for older people: Knowledge gaps and future directions. Lancet Healthy Longev. 2021, 2, e380–e383. [Google Scholar] [CrossRef] [PubMed]
- Naruse, M.; Trappe, S.; Trappe, T.A. Human skeletal muscle-specific atrophy with aging: A comprehensive review. J. Appl. Physiol. 2023, 134, 900–914. [Google Scholar] [CrossRef] [PubMed]
- Cofre-Bolados, C.; Ferrari, G.; Valdivia-Moral, P.; Vidal-Diaz, F.; Ramirez-Velez, R.; Izquierdo-Redin, M. Sub Maximal Ergospirometry Parameters in Untrained Non-Frail Octogenarian Subjects. Medicina (Kaunas) 2022, 58, 378. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, A.I.; Moradell, A.; Navarrete-Villanueva, D.; Subias-Perie, J.; Perez-Gomez, J.; Ara, I.; Gonzalez-Gross, M.; Casajus, J.A.; Vicente-Rodriguez, G.; Gomez-Cabello, A. Effects of Multicomponent Training Followed by a Detraining Period on Frailty Level and Functional Capacity of Older Adults with or at Risk of Frailty: Results of 10-Month Quasi-Experimental Study. Int. J. Environ. Res. Public Health 2022, 19, 12417. [Google Scholar] [CrossRef] [PubMed]
- Cadore, E.L.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Idoate, F.; Millor, N.; Gomez, M.; Rodriguez-Manas, L.; Izquierdo, M. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age (Dordr.) 2014, 36, 773–785. [Google Scholar] [CrossRef]
- Trappe, S.; Hayes, E.; Galpin, A.; Kaminsky, L.; Jemiolo, B.; Fink, W.; Trappe, T.; Jansson, A.; Gustafsson, T.; Tesch, P. New records in aerobic power among octogenarian lifelong endurance athletes. J. Appl. Physiol. 2013, 114, 3–10. [Google Scholar] [CrossRef]
- Casas-Herrero, A.; Anton-Rodrigo, I.; Zambom-Ferraresi, F.; Saez de Asteasu, M.L.; Martinez-Velilla, N.; Elexpuru-Estomba, J.; Marin-Epelde, I.; Ramon-Espinoza, F.; Petidier-Torregrosa, R.; Sanchez-Sanchez, J.L.; et al. Effect of a multicomponent exercise programme (VIVIFRAIL) on functional capacity in frail community elders with cognitive decline: Study protocol for a randomized multicentre control trial. Trials 2019, 20, 362. [Google Scholar] [CrossRef]
- Janssens, J.P.; Pache, J.C.; Nicod, L.P. Physiological changes in respiratory function associated with ageing. Eur. Respir. J. 1999, 13, 197–205. [Google Scholar] [CrossRef]
- Petrella, M.; Aprahamian, I.; Mamoni, R.L.; de Vasconcellos Romanini, C.F.; Lima, N.A.; de Cassio Robello, E.; da Costa, D.L.; An, V.N.; Aguirre, B.N.; Galdeano, J.R.; et al. The effect of a multicomponent exercise protocol (VIVIFRAIL(c)) on inflammatory profile and physical performance of older adults with different frailty status: Study protocol for a randomized controlled trial. BMC Geriatr. 2021, 21, 83. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines on Physical Activity and Sedentary Behaviour: Web Annex: Evidence Profiles; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Izquierdo, M.; Merchant, R.A.; Morley, J.E.; Anker, S.D.; Aprahamian, I.; Arai, H.; Aubertin-Leheudre, M.; Bernabei, R.; Cadore, E.L.; Cesari, M.; et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef] [PubMed]
- Billat, L.V. Interval training for performance: A scientific and empirical practice. Special recommendations for middle- and long-distance running. Part II: Anaerobic interval training. Sports Med. 2001, 31, 75–90. [Google Scholar] [CrossRef]
- Billat, L.V. Interval training for performance: A scientific and empirical practice. Special recommendations for middle- and long-distance running. Part I: Aerobic interval training. Sports Med. 2001, 31, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Laursen, P.B.; Jenkins, D.G. The scientific basis for high-intensity interval training: Optimising training programmes and maximising performance in highly trained endurance athletes. Sports Med. 2002, 32, 53–73. [Google Scholar] [CrossRef]
- Buchheit, M.; Laursen, P.B. High-intensity interval training, solutions to the programming puzzle: Part II: Anaerobic energy, neuromuscular load and practical applications. Sports Med. 2013, 43, 927–954. [Google Scholar] [CrossRef]
- Buchheit, M.; Laursen, P.B. High-intensity interval training, solutions to the programming puzzle: Part I: Cardiopulmonary emphasis. Sports Med. 2013, 43, 313–338. [Google Scholar] [CrossRef]
- Rohmansyah, N.A.; Ka Praja, R.; Phanpheng, Y.; Hiruntrakul, A. High-Intensity Interval Training Versus Moderate-Intensity Continuous Training for Improving Physical Health in Elderly Women. Inquiry 2023, 60, 469580231172870. [Google Scholar] [CrossRef]
- Roxburgh, B.H.; Nolan, P.B.; Weatherwax, R.M.; Dalleck, L.C. Is moderate intensity exercise training combined with high intensity interval training more effective at improving cardiorespiratory fitness than moderate intensity exercise training alone? J. Sports Sci. Med. 2014, 13, 702. [Google Scholar]
- Kessler, H.S.; Sisson, S.B.; Short, K.R. The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Med. 2012, 42, 489–509. [Google Scholar] [CrossRef]
- Alvarez, C.; Ramirez-Campillo, R.; Martinez-Salazar, C.; Mancilla, R.; Flores-Opazo, M.; Cano-Montoya, J.; Ciolac, E.G. Low-Volume High-Intensity Interval Training as a Therapy for Type 2 Diabetes. Int. J. Sports Med. 2016, 37, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Tjønna, A.E.; Lee, S.J.; Rognmo, Ø.; Stølen, T.O.; Bye, A.; Haram, P.M.; Loennechen, J.P.l.; Al-Share, Q.Y.; Skogvoll, E.; Slørdahl, S.A. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: A pilot study. Circulation 2008, 118, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Cadore, E.L.; Saez de Asteasu, M.L.; Izquierdo, M. Multicomponent exercise and the hallmarks of frailty: Considerations on cognitive impairment and acute hospitalization. Exp. Gerontol. 2019, 122, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, K. High Intensity Interval Training in Elderly Could Improve Elderly Health Significantly. Int. J. Aging Health Mov. 2019, 1, 10–11. [Google Scholar]
- Older, P.; Hall, A. The role of cardiopulmonary exercise testing for preoperative evaluation of the elderly. Mortality 1996, 11, 10. [Google Scholar]
- West, M.; Jack, S.; Grocott, M. Perioperative cardiopulmonary exercise testing in the elderly. Best Pract. Res. Clin. Anaesthesiol. 2011, 25, 427–437. [Google Scholar] [CrossRef]
- Izquierdo, M. Multicomponent physical exercise program: Vivifrail. Nutr. Hosp. 2019, 36, 50–56. [Google Scholar] [CrossRef]
- Lambert, C.P.; Evans, W.J. Adaptations to aerobic and resistance exercise in the elderly. Rev. Endocr. Metab. Disord. 2005, 6, 137–143. [Google Scholar] [CrossRef]
- Gonzales, T.I.; Westgate, K.; Strain, T.; Hollidge, S.; Jeon, J.; Christensen, D.L.; Jensen, J.; Wareham, N.J.; Brage, S. Cardiorespiratory fitness assessment using risk-stratified exercise testing and dose–response relationships with disease outcomes. Sci. Rep. 2021, 11, 15315. [Google Scholar] [CrossRef]
- Sietsema, K.E.; Stringer, W.W.; Sue, D.Y.; Ward, S. Wasserman & Whipp’s Principles of Exercise Testing and Interpretation, 6th ed.; Lippincott Williams & Wilkins (LWW): Philadelphia, PA, USA, 2020. [Google Scholar]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Després, J.-P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef]
- Arena, R.; Myers, J.; Abella, J.; Peberdy, M.A.; Bensimhon, D.; Chase, P.; Guazzi, M. Development of a ventilatory classification system in patients with heart failure. Circulation 2007, 115, 2410–2417. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Arena, R.; Halle, M.; Piepoli, M.F.; Myers, J.; Lavie, C.J. 2016 focused update: Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur. Heart J. 2018, 39, 1144–1161. [Google Scholar] [CrossRef] [PubMed]
- Gitt, A.K.; Wasserman, K.; Kilkowski, C.; Kleemann, T.; Kilkowski, A.; Bangert, M.; Schneider, S.; Schwarz, A.; Senges, J. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation 2002, 106, 3079–3084. [Google Scholar] [CrossRef] [PubMed]
- Coeckelberghs, E.; Buys, R.; Goetschalckx, K.; Cornelissen, V.A.; Vanhees, L. Prognostic value of the oxygen uptake efficiency slope and other exercise variables in patients with coronary artery disease. Eur. J. Prev. Cardiol. 2016, 23, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.S.; Araújo, C.G.S. Cardiorespiratory optimal point during exercise testing as a predictor of all-cause mortality. Rev. Port. De Cardiol. 2017, 36, 261–269. [Google Scholar] [CrossRef]
- Dias Ferreira Reis, J.; Goncalves, A.; Bras, P.; Ferreira, V.; Viegas, J.; Rio, P.; Moreira, R.; Pereira Silva, T.; Timoteo, A.; Soares, R. Prognostic value of the cardiorespiratory optimal point during submaximal exercise testing. Eur. Heart J. 2020, 41, ehaa946.0957. [Google Scholar] [CrossRef]
- Baba, R.; Tsuyuki, K.; Yano, H.; Ninomiya, K.; Ebine, K. Robustness of the oxygen uptake efficiency slope to exercise intensity in patients with coronary artery disease. Nagoya J. Med. Sci. 2010, 72, 83–89. [Google Scholar]
- Barros, C.R.; Monte-Silva, K.; Sales, C.B.; Souza, R.P.; Santos, T.; Pessoa, M.; Viana, R.; Alcoforado, L.; Lima, A.M.J.; Fernandes, J.; et al. Oxygen uptake efficiency slope: A submaximal test evaluation tool that provides cardiopulmonary reserve data in individuals with Parkinson’s disease. Braz. J. Phys. Ther. 2021, 25, 641–647. [Google Scholar] [CrossRef]
- Silva, M.; Baptista, L.; Neves, R.; França, E.; Loureiro, H.; Rezende, M.; Ferreira, V.; Veríssimo, M.; Martins, R. High intensity interval training improves health-related quality of life in adults and older adults with diagnosed cardiovascular risk. J. Phys. Educ. Sport 2019, 19, 611–618. [Google Scholar]
- Ramírez-Vélez, R.; García-Alonso, N.; Legarra-Gorgoñón, G.; Oscoz-Ochandorena, S.; Oteiza, J.; Izquierdo, M. Ventilatory efficiency in response to maximal exercise in persistent COVID-19 syndrome patients: A cross-sectional study. Rev. Esp. Cardiol. 2023, 76, 206–209. [Google Scholar] [CrossRef]
- Rudd, A.; Khan, H.; Gamble, D.; Stephen, P.; Horgan, G.; Dawson, A.; Frenneaux, M.; Dawson, D.K. 76 Oxygen uptake efficiency slope—A valuable substitute for peak vo2? Heart 2022, 108, A56–A57. [Google Scholar] [CrossRef]
- Laukkanen, J.A.; Kunutsor, S.K.; Yates, T.; Willeit, P.; Kujala, U.M.; Khan, H.; Zaccardi, F. Prognostic relevance of cardiorespiratory fitness as assessed by submaximal exercise testing for all-cause mortality: A UK Biobank prospective study. Mayo Clin. Proc. 2020, 95, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.L.; Cotie, L.M.; Cole, C.A.; Harris, J.; Moran, B.; Scott, K.; Terada, T.; Buckley, J.P.; Pipe, A.L. Submaximal Exercise Testing in Cardiovascular Rehabilitation Settings (BEST Study). Front. Physiol. 2019, 10, 1517. [Google Scholar] [CrossRef]
- David, R.; Bassett, J. Scientific contributions of A. V. Hill: Exercise physiology pioneer. J. Appl. Physiol. 2002, 93, 1567–1582. [Google Scholar] [CrossRef]
- Meyer, T.; Lucia, A.; Earnest, C.P.; Kindermann, W. A conceptual framework for performance diagnosis and training prescription from submaximal gas exchange parameters--theory and application. Int. J. Sports Med. 2005, 26 (Suppl. S1), S38–S48. [Google Scholar] [CrossRef] [PubMed]
- Issurin, V.B. New horizons for the methodology and physiology of training periodization. Sports Med. 2010, 40, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Araújo, B.T.S.; Barros, A.; Nunes, D.T.X.; Remígio de Aguiar, M.I.; Mastroianni, V.W.; de Souza, J.A.F.; Fernades, J.; Campos, S.L.; Brandão, D.C.; Dornelas de Andrade, A. Effects of continuous aerobic training associated with resistance training on maximal and submaximal exercise tolerance, fatigue, and quality of life of patients post-COVID-19. Physiother. Res. Int. 2023, 28, e1972. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Agostoni, P.G. Monitoring gas exchange during a constant work rate exercise in patients with left ventricular dysfunction treated with carvedilol. Am. J. Cardiol. 2000, 85, 660–664. [Google Scholar] [CrossRef]
- Malhotra, R.; Bakken, K.; D’Elia, E.; Lewis, G.D. Cardiopulmonary Exercise Testing in Heart Failure. JACC Heart Fail. 2016, 4, 607–616. [Google Scholar] [CrossRef]
- Noonan, V.; Dean, E. Submaximal Exercise Testing: Clinical Application and Interpretation. Phys. Ther. 2000, 80, 782–807. [Google Scholar] [CrossRef]
- Sloth, M.; Sloth, D.; Overgaard, K.; Dalgas, U. Effects of sprint interval training on VO2max and aerobic exercise performance: A systematic review and meta-analysis. Scand. J. Med. Sci. Sports 2013, 23, e341–e352. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.P.; Shamim, W.; Davies, L.C.; Piepoli, M.F.; Ponikowski, P.; Anker, S.D.; Coats, A.J.S. Cardiopulmonary exercise testing for prognosis in chronic heart failure: Continuous and independent prognostic value from VE/VCO2slope and peak VO2. Eur. Heart J. 2000, 21, 154–161. [Google Scholar] [CrossRef] [PubMed]
- McGurk, S.P.; Blanksby, B.A.; Anderson, M.J. The relationship of hypercapnic ventilatory responses to age, gender and athleticism. Sports Med. 1995, 19, 173–183. [Google Scholar] [CrossRef]
- Moore, B.; Brubaker, P.H.; Stewart, K.P.; Kitzman, D.W. VE/VCO2 slope in older heart failure patients with normal versus reduced ejection fraction compared with age-matched healthy controls. J. Card. Fail. 2007, 13, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Louras, P.; Kamil-Rosenberg, S.; Myers, J.; Fairchild, J.K. Validation of the VE/VCO2 Slope as a Marker of Cognitive Performance in Older Adults. Arch. Phys. Med. Rehabil. 2022, 103, e158. [Google Scholar] [CrossRef]
- Lai, P.; Xue, J.-H.; Xie, M.-J.; Ye, J.-H.; Yang, N.; Zhong, Y.-M.; Liao, Y.-L. High-intensity and moderate-intensity interval training in heart failure with preserved ejection fraction: A meta-analysis of randomized controlled trials. Medicine 2023, 102, e33010. [Google Scholar] [CrossRef]
- Donelli da Silveira, A.; Beust de Lima, J.; da Silva Piardi, D.; dos Santos Macedo, D.; Zanini, M.; Nery, R.; Laukkanen, J.A.; Stein, R. High-intensity interval training is effective and superior to moderate continuous training in patients with heart failure with preserved ejection fraction: A randomized clinical trial. Eur. J. Prev. Cardiol. 2020, 27, 1733–1743. [Google Scholar] [CrossRef]
- Mueller, S.; Winzer, E.B.; Duvinage, A.; Gevaert, A.B.; Edelmann, F.; Haller, B.; Pieske-Kraigher, E.; Beckers, P.; Bobenko, A.; Hommel, J. Effect of high-intensity interval training, moderate continuous training, or guideline-based physical activity advice on peak oxygen consumption in patients with heart failure with preserved ejection fraction: A randomized clinical trial. JAMA 2021, 325, 542–551. [Google Scholar] [CrossRef]
- Chou, C.-H.; Fu, T.-C.; Tsai, H.-H.; Hsu, C.-C.; Wang, C.-H.; Wang, J.-S. High-intensity interval training enhances mitochondrial bioenergetics of platelets in patients with heart failure. Int. J. Cardiol. 2019, 274, 214–220. [Google Scholar] [CrossRef]
- Palermo, P.; Mapelli, M.; Campodonico, J.; Vignati, C.; Agostoni, P. P310 Cardiopulmonary Exercise Test in the Evaluation of a patient with Hfref: The Importance in the Analysis of Gas Kinetics. Eur. Heart J. Suppl. 2023, 25, D163–D164. [Google Scholar] [CrossRef]
- Oliveira, R.B.; Myers, J.; Araújo, C.G.; Abella, J.; Mandic, S.; Froelicher, V. Maximal exercise oxygen pulse as a predictor of mortality among male veterans referred for exercise testing. Eur. J. Cardiovasc. Prev. Rehabil. 2009, 26, 358–364. [Google Scholar] [CrossRef]
- Belardinelli, R.; Lacalaprice, F.; Carle, F.; Minnucci, A.; Cianci, G.; Perna, G.; D’Eusanio, G. Exercise-induced myocardial ischaemia detected by cardiopulmonary exercise testing. Eur. Heart J. 2003, 24, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, E.C.; Hollanda, R.; Vargas, J.P.; Silveira, C.W.; Lemos, A.L.; Hollanda, R.; Ribeiro, J.P. Flattening of oxygen pulse during exercise may detect extensive myocardial ischemia. Med. Sci. Sports Exerc. 2007, 39, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, A.; da Silva, C.L.; Souza, F.C.C.; Serra, S.; Marino, P.; Sl Lima, R. Clinical, scintigraphic, and angiographic predictors of oxygen pulse abnormality in patients undergoing cardiopulmonary exercise testing. Clin. Cardiol. 2017, 40, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.S.; Ricardo, D.R.; Araujo, C.G. Cardiorespiratory optimal point: A submaximal variable of the cardiopulmonary exercise testing. Arq. Bras. Cardiol. 2012, 99, 988–996. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Du, L.; Zhang, X.; Chen, K.; Ren, X.; Chen, S.; He, Q. Effect of high-intensity interval training on physical health in coronary artery disease patients: A meta-analysis of randomized controlled trials. J. Cardiovasc. Dev. Dis. 2021, 8, 158. [Google Scholar] [CrossRef]
- Pattyn, N.; Coeckelberghs, E.; Buys, R.; Cornelissen, V.A.; Vanhees, L. Aerobic interval training vs. moderate continuous training in coronary artery disease patients: A systematic review and meta-analysis. Sports Med. 2014, 44, 687–700. [Google Scholar] [CrossRef]
- Xie, B.; Yan, X.; Cai, X.; Li, J. Effects of High-Intensity Interval Training on Aerobic Capacity in Cardiac Patients: A Systematic Review with Meta-Analysis. BioMed Res. Int. 2017, 2017, 5420840. [Google Scholar] [CrossRef]
- Blackwell, J.E.M.; Gharahdaghi, N.; Brook, M.S.; Watanabe, S.; Boereboom, C.L.; Doleman, B.; Lund, J.N.; Wilkinson, D.J.; Smith, K.; Atherton, P.J.; et al. The physiological impact of high-intensity interval training in octogenarians with comorbidities. J. Cachexia Sarcopenia Muscle 2021, 12, 866–879. [Google Scholar] [CrossRef]
- Laukkanen, J.A.; Zaccardi, F.; Khan, H.; Kurl, S.; Rauramaa, R. Abstract 19920: Long-term Change in Cardiorespiratory Fitness and All-cause Mortality: A Follow-up Study. Circulation 2015, 132, A19920. [Google Scholar] [CrossRef]
- Boereboom, C.L.; Phillips, B.E.; Williams, J.P.; Lund, J.N. A 31-day time to surgery compliant exercise training programme improves aerobic health in the elderly. Tech. Coloproctol. 2016, 20, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-c.; Sui, X.; Artero, E.G.; Lee, I.-M.; Church, T.S.; McAuley, P.A.; Stanford, F.C.; Kohl, H.W.; Blair, S.N. Long-Term Effects of Changes in Cardiorespiratory Fitness and Body Mass Index on All-Cause and Cardiovascular Disease Mortality in Men. Circulation 2011, 124, 2483–2490. [Google Scholar] [CrossRef] [PubMed]
| Variable | Group | |
|---|---|---|
| Control (8M; 15F) | Intervention (4M; 21F) | |
| Age (y), mean ± SD | 83.4 ± 3.5 | 83.3 ± 3.4 |
| Weight (kg), mean ± SD | 64.9 ± 11.1 | 66.4 ± 13.7 |
| Height (m), mean ± SD | 1.58 ± 7.1 | 1.58 ± 7.4 |
| BMI (kg/m2), mean ± SD | 25.9 ± 7 | 26.4 ± 4.7 |
| Cardiometabolic disease, number (%) | 16 (68) | 18 (72) |
| Cardiometabolic drugs, number (%) | 14 (61) | 16 (64) |
| Group Differences | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Category | Control | χ2 | p | Intervention | χ2 | p | ||
| Pre Mean (SD) | Post Mean (SD) | Pre Mean (SD) | Post Mean (SD) | ||||||
| VO2AT (mL/kg/min) | <11 altered | 17 (73.9) | 16 (69.5) | 0 | 0.99 | 10 (40) | 4 (16) | 4.477 | 0.034 |
| ≥11 normal | 6 (26.1) | 7 (30.5) | 15 (60) | 21 (84) | |||||
| VE/VCO2 (L/min) | >34 altered | 9 (39) | 8 (34.7) | 0 | 0.99 | 9 (36) | 3 (12) | 3.315 | 0.069 |
| ≤34 normal | 14 (61) | 15 (65.3) | 16 (64) | 22 (88) | |||||
| OUES (mL/min) | <1550 altered | 21 (91.3) | 6 (26) | 0.01 | 0.971 | 15 (60) | 6 (24) | 3.299 | 0.069 |
| ≥1550 normal | 2 (8.7) | 17 (74) | 10 (40) | 19 (76) | |||||
| COP (L/min) | >30 altered | 11 (47.8) | 13 (56.5) | 0 | 0.99 | 11 (44) | 1 (4) | 0.015 | 0.902 |
| ≤30 normal | 12 (52.2) | 10 (43.5) | 14 (56) | 24 (96) | |||||
| ∆VO2/HR RCP vs. AT | <0 altered | 14 (61) | 13 (57) | 0.256 | 0.613 | 9 (36) | 7 (28) | 0 | 0.99 |
| ≥0 normal | 9 (39) | 10 (43) | 16 (64) | 18 (72) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cofre-Bolados, C.; Vidal, F.; Gutiérrez Espinoza, H.; Betancourt-Peters, I.; Orihuela, P.A.; Izquierdo, M. Periodized Aerobic Training between Thresholds Improves Submaximal Cardiorespiratory Parameters in Octogenarians. Sports 2023, 11, 219. https://doi.org/10.3390/sports11110219
Cofre-Bolados C, Vidal F, Gutiérrez Espinoza H, Betancourt-Peters I, Orihuela PA, Izquierdo M. Periodized Aerobic Training between Thresholds Improves Submaximal Cardiorespiratory Parameters in Octogenarians. Sports. 2023; 11(11):219. https://doi.org/10.3390/sports11110219
Chicago/Turabian StyleCofre-Bolados, Cristian, Félix Vidal, Héctor Gutiérrez Espinoza, Ignacio Betancourt-Peters, Pedro A. Orihuela, and Mikel Izquierdo. 2023. "Periodized Aerobic Training between Thresholds Improves Submaximal Cardiorespiratory Parameters in Octogenarians" Sports 11, no. 11: 219. https://doi.org/10.3390/sports11110219
APA StyleCofre-Bolados, C., Vidal, F., Gutiérrez Espinoza, H., Betancourt-Peters, I., Orihuela, P. A., & Izquierdo, M. (2023). Periodized Aerobic Training between Thresholds Improves Submaximal Cardiorespiratory Parameters in Octogenarians. Sports, 11(11), 219. https://doi.org/10.3390/sports11110219







