Effects of Exercise on Quality of Life in Subjects with Alzheimer’s Disease: Systematic Review with Meta-Analysis of Randomized Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
- Population: participants aged 18 years old or older, diagnosed with Alzheimer’s disease according to the criteria of a mild to moderate AD according to the criteria of the revised version of the DSM (Diagnostic and Statistical Manual) [25];
- Intervention: interventions based on exercise;
- Comparison: Alzheimer’s diseases participants who maintained their daily activities with standard care for Alzheimer disease;
- Outcomes: quality of life;
- Type of study: randomized controlled trial (RCT);
2.2. Study Identification
2.3. Data Extraction
2.4. Quality of Study and Risk of Bias
2.5. Data Synthesis and Analysis
3. Results
3.1. Results of the Systematic Literature Search
3.2. Study Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Association. Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2018, 14, 367–429. [Google Scholar] [CrossRef]
- WebMD. Alzheimers. Available online: https://www.webmd.com/alzheimers/default.htm (accessed on 27 December 2022).
- World Health Organization. Global Action Plan on the Public Health Response to Dementia 2017–2025; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Direção Geral da Saúde. Nutrição e Doença de Alzheimer. In Programa Nacional para a Promoção da Alimentação Saudável; Direção Geral da Saúde: Lisboa, Portugal, 2015. [Google Scholar]
- International, A.D.; Wimo, A.; Ali, G.-C.; Guerchet, M.; Prince, M.; Prina, M.; Wu, Y.-T. World Alzheimer Report 2015: The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends; Alzheimer’s Disease International (ADI): London, UK, 2015. [Google Scholar]
- Zhu, Q.-B.; Bao, A.-M.; Swaab, D. Activation of the Brain to Postpone Dementia: A Concept Originating from Postmortem Human Brain Studies. Neurosci. Bull. 2019, 35, 253–266. [Google Scholar] [CrossRef]
- Alzheimer’s Association. Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2022, 18, 700–789. [Google Scholar] [CrossRef]
- Georges, J.; Bintener, C.; Miller, O. Dementia in Europe Yearbook 2019: Estimating the Prevalence of Dementia in Europe, Alzheimer Europe. 2020, pp. 1–108. Available online: https://www.alzheimer-europe.org/resources/publications/dementia-europe-yearbook-2019-estimating-prevalence-dementia-europe (accessed on 27 December 2022).
- Colcombe, S.; Kramer, A.F. Fitness Effects on the Cognitive Function of Older Adults: A Meta-Analytic Study. Psychol. Sci. 2003, 14, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Heyn, P. The Effect of a Multisensory Exercise Program on Engagement, Behavior, and Selected Physiological Indexes in Persons with Dementia. Am. J. Alzheimers Dis. Other Demen. 2003, 18, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Heyn, P.; Abreu, B.C.; Ottenbacher, K.J. The Effects of Exercise Training on Elderly Persons with Cognitive Impairment and Dementia: A Meta-Analysis. Arch. Phys. Med. Rehabil. 2004, 85, 1694–1704. [Google Scholar] [CrossRef]
- Heyn, P.C.; Johnsons, K.E.; Kramer, A.F. Endurance and Strength Training Outcomes on Cognitively Impaired and Cognitively Intact Older Adults: A Meta-Analysis. J. Nutr. Health Aging 2008, 12, 401–409. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on Physical Activity 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Henskens, M.; Nauta, I.M.; van Eekeren, M.C.A.; Scherder, E.J.A. Effects of Physical Activity in Nursing Home Residents with Dementia: A Randomized Controlled Trial. Dement. Geriatr. Cogn. Disord. 2018, 46, 60–80. [Google Scholar] [CrossRef]
- WHO. Whoqol—Measuring Quality Of Life|The World Health Organization. Available online: https://www.who.int/tools/whoqol (accessed on 27 December 2022).
- Cámara-Calmaestra, R.; Martínez-Amat, A.; Aibar-Almazán, A.; Hita-Contreras, F.; de Miguel Hernando, N.; Achalandabaso-Ochoa, A. Effectiveness of Physical Exercise on Alzheimer’s disease. A Systematic Review. J. Prev. Alzheimers Dis. 2022, 9, 601–616. [Google Scholar] [CrossRef]
- Ojagbemi, A.; Akin-Ojagbemi, N. Exercise and Quality of Life in Dementia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Appl. Gerontol. 2019, 38, 27–48. [Google Scholar] [CrossRef]
- Du, Z.; Li, Y.; Li, Y.; Zhou, C.; Li, F.; Yang, X. Physical activity can improve cognition in patients with Alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. Clin. Inter. 2018, 13, 1593–1603. [Google Scholar] [CrossRef]
- Jia, R.X.; Liang, J.H.; Xu, Y.; Wang, Y.Q. Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: A meta-analysis. BMC Geriatr. 2019, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, L.; Wang, L.; Jin, X.; Zhang, H. Physical activity for executive function and activities of daily living in AD patients: A systematic review and meta-analysis. Front. Psychol. 2020, 11, 560461. [Google Scholar] [CrossRef] [PubMed]
- López-Ortiz, S.; Valenzuela, P.L.; Seisdedos, M.M.; Morales, J.S.; Vega, T.; Castillo-García, A.; Nisticò, R.; Mercuri, N.B.; Lista, S.; Lucia, A.; et al. Exercise interventions in Alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. Ageing Res. Rev. 2021, 72, 101479. [Google Scholar] [CrossRef]
- Enette, L.; Vogel, T.; Merle, S.; Valard-Guiguet, A.-G.; Ozier-Lafontaine, N.; Neviere, R.; Leuly-Joncart, C.; Fanon, J.L.; Lang, P.O. Effect of 9 Weeks Continuous vs. Interval Aerobic Training on Plasma BDNF Levels, Aerobic Fitness, Cognitive Capacity and Quality of Life among Seniors with Mild to Moderate Alzheimer’s Disease: A Randomized Controlled Trial. Eur. Rev. Aging Phys. Act. 2020, 17, 2. [Google Scholar] [CrossRef]
- Hoffmann, K.; Sobol, N.A.; Frederiksen, K.S.; Beyer, N.; Vogel, A.; Vestergaard, K.; Brændgaard, H.; Gottrup, H.; Lolk, A.; Wermuth, L.; et al. Moderate-to-High Intensity Physical Exercise in Patients with Alzheimer’s Disease: A Randomized Controlled Trial. J. Alzheimer’s Dis. 2015, 50, 443–453. Available online: https://pubmed.ncbi.nlm.nih.gov/26682695/ (accessed on 11 June 2021). [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; DSM-5; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. Te Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Lamb, S.E.; Sheehan, B.; Atherton, N.; Nichols, V.; Collins, H.; Mistry, D.; Dosanjh, S.; Slowther, A.M.; Khan, I.; Petrou, S.; et al. Dementia And Physical Activity (Dapa) Trial of Moderate to High Intensity Exercise Training for People with Dementia: Randomised Controlled Trial. BMJ 2018, 361, k1675. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Shan, C.-L.; Qing, H.; Wang, W.; Zhu, Y.; Yin, M.-M.; Machado, S.; Yuan, T.-F.; Wu, T. The Effects of Aerobic Exercise on Cognitive Function of Alzheimer’s Disease Patients. CNS Neurol. Disord. Drug Targets 2015, 14, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Yu, D.S.F. Effects of a Moderate-Intensity Aerobic Exercise Programme on the Cognitive Function and Quality of Life of Community-Dwelling Elderly People with Mild Cognitive Impairment: A Randomised Controlled Trial. Int. J. Nurs. Stud. 2019, 93, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Pitkälä, K.H.; Pöysti, M.M.; Laakkonen, M.; Tilvis, R.S.; Savikko, N.; Kautiainen, H.; Strandberg, T.E. Effects of the Finnish Alzheimer Disease Exercise Trial (FINALEX): A Randomized Controlled Trial. JAMA Intern. Med. 2013, 173, 894–901. [Google Scholar] [CrossRef]
- Abd El-Kader, S.M.; Al-Jiffri, O.H. Aerobic exercise improves quality of life, psychological well-being and systemic inflammation in subjects with Alzheimer’s disease. Afr. Health Sci. 2016, 16, 1045–1055. [Google Scholar] [CrossRef]
- Meng, Q.; Lin, M.S.; Tzeng, I.S. Relationship between Exercise and Alzheimer’s Disease: A Narrative Literature Review. Front. Neurosci. 2020, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; Merchant, R.A.; Morley, J.E.; Anker, S.D.; Aprahamian, I.; Arai, H.; Aubertin-Leheudre, M.; Bernabei, R.; Cadore, E.L. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Wang, Y.; Li, J.; Chang, J.; Jia, Q. Effect of Physical Exercise on Cognitive Function of Alzheimer’s Disease Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trial. Front. Psychiatry 2022, 13, 927128. [Google Scholar] [CrossRef]
- Lawton, M.P. Quality of Life in Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 1994, 8 (Suppl. S3), 138–150. [Google Scholar] [CrossRef]
- Jonker, C.; Gerritsen, D.L.; Bosboom, P.R.; van der Stehen, J.T. A Model for Quality of Life Measures in Patients with Dementia: Lawton’s next Step. Dement. Geriatr. Cogn. Disord. 2004, 18, 159–164. [Google Scholar] [CrossRef]
- Byrne-Davis, L.M.T.; Bennett, P.D.; Wilcock, G.K. How Are Quality of Life Ratings Made? Toward a Model of Quality of Life in People with Dementia. Qual. Life Res. 2006, 15, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Hoe, J.; Hancock, G.; Livingston, G.; Orrell, M. Quality of Life of People with Dementia in Residential Care Homes. Br. J. Psychiatry 2006, 188, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Karlawish, J.H.T.; Casarett, D.; Klocinski, J.; Clark, C.M. The Relationship between Caregivers’ Global Ratings of Alzheimer’s Disease Patients’ Quality of Life, Disease Severity, and the Caregiving Experience. J. Am. Geriatr. Soc. 2001, 49, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, R.G.; Gibbons, L.E.; McCurry, S.M.; Teri, L. Assessing Quality of Life in Older Adults with Cognitive Impairment. Psychosom. Med. 2002, 64, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Sands, L.P.; Ferreira, P.; Stewart, A.L.; Brod, M.; Yaffe, K. What Explains Differences between Dementia Patients’ and Their Caregivers’ Ratings of Patients’ Quality of Life? Am. J. Geriatr. Psychiatry 2004, 12, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Ready, R.E.; Ott, B.R.; Grace, J. Patient versus Informant Perspectives of Quality of Life in Mild Cognitive Impairment and Alzheimer’s Disease. Int. J. Geriat. Psychiatry 2004, 19, 256–265. [Google Scholar] [CrossRef]
- Thorgrimsen, L.; Selwood, A.; Spector, A.; Royan, L.; de Madariaga Lopez, M.; Woods, R.T.; Orrell, M. Whose Quality of Life Is It Anyway? The Validity and Reliability of the Quality of Life-Alzheimer’s Disease (Qol-Ad) Scale. Alzheimer Dis. Assoc. Disord. 2003, 17, 201–208. [Google Scholar] [CrossRef]

| Study | Country | Participants | Age (Years) (M ± SD) | Outcomes | Type of Intervention | Conclusions |
|---|---|---|---|---|---|---|
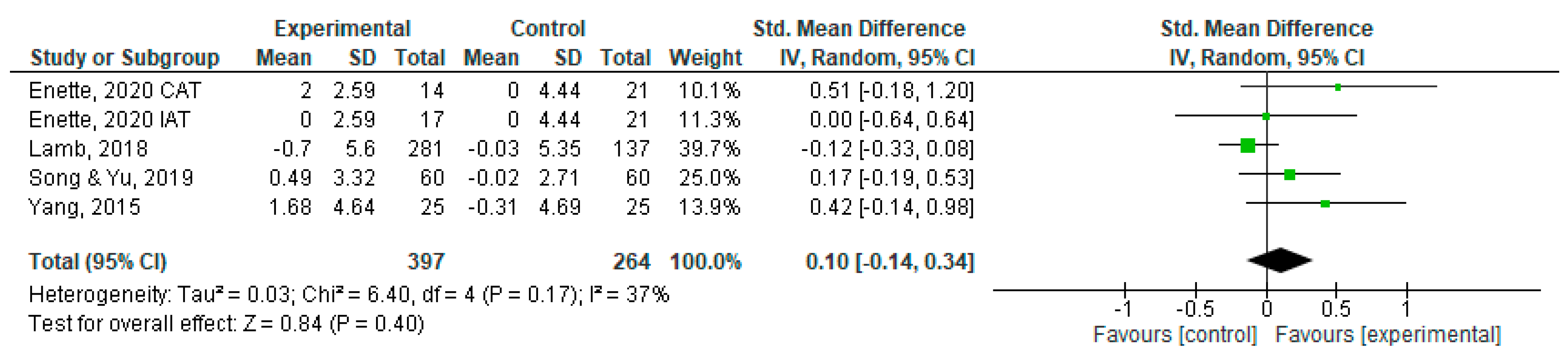
| Lamb et al., 2018 [29] | United States | Intervention group n = 281 (men:166/women:112); Control group n = 137 (men: 86/women: 51) | 76.9 (7.9) | ADAS—Cog; Qol-AD; EuroQol | Aerobic exercise, 25 min of moderate- to hard-intensity cycling, depending on tolerance level, and strength exercise, three sets of 20 repetitions at gym; duration 60 to 90 min per session; 2x per week; one hour of home exercises per week; 16 weeks. | Exercise improved short-term physical fitness, but this did not translate into improvements in health-related quality of life. |
| Song & Yu, 2019 [31] | China | Intervention group n = 60 (men: 48/women: 12; Control group n = 60 (men: 42/women:18) | 75.78 (6.28) | MoCa; Qol-AD | Aerobic Exercise; moderate intensity; duration 60 min; 3x per week; 16 weeks | Participants in IG had a significant improvement in health-related quality of life compared to CG. |
| Yang et al., 2015 [30] | China | Intervention Group n = 25 (men: 10/women:15); Control Group n = 25 (men:7/women:18) | 72.5 (10.6) | ADAS-Cog; MMSE; Qol-AD | Aerobic Exercise; initial time 25 to 30 min and after 1 week increased to 40 min; 3x per week; 12 weeks | Aerobic exercise could improve cognitive function, mental status, and quality of life in AD patients. |
| Enette et al., 2020 [22] | France | Intervention group n = 31 (CAT = 14 (men:3/women:11)/IAT = 17(men:6/women:11); Control group n = 21 (men:10/women:11) | 79 (24) | MMSE; Qol-AD | Aerobic Exercise; duration 30 min; 2x per week; 9 weeks | In the CAT group, there were improvements in quality of life compared to the other two groups (in mood and financially). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, M.; Correia, É.; Vitorino, A.; Rodrigues, J.; Cid, L.; Bento, T.; Antunes, R.; Monteiro, D.; Couto, N. Effects of Exercise on Quality of Life in Subjects with Alzheimer’s Disease: Systematic Review with Meta-Analysis of Randomized Clinical Trials. Sports 2023, 11, 149. https://doi.org/10.3390/sports11080149
Mendes M, Correia É, Vitorino A, Rodrigues J, Cid L, Bento T, Antunes R, Monteiro D, Couto N. Effects of Exercise on Quality of Life in Subjects with Alzheimer’s Disease: Systematic Review with Meta-Analysis of Randomized Clinical Trials. Sports. 2023; 11(8):149. https://doi.org/10.3390/sports11080149
Chicago/Turabian StyleMendes, Mariana, Érica Correia, Anabela Vitorino, José Rodrigues, Luís Cid, Teresa Bento, Raul Antunes, Diogo Monteiro, and Nuno Couto. 2023. "Effects of Exercise on Quality of Life in Subjects with Alzheimer’s Disease: Systematic Review with Meta-Analysis of Randomized Clinical Trials" Sports 11, no. 8: 149. https://doi.org/10.3390/sports11080149
APA StyleMendes, M., Correia, É., Vitorino, A., Rodrigues, J., Cid, L., Bento, T., Antunes, R., Monteiro, D., & Couto, N. (2023). Effects of Exercise on Quality of Life in Subjects with Alzheimer’s Disease: Systematic Review with Meta-Analysis of Randomized Clinical Trials. Sports, 11(8), 149. https://doi.org/10.3390/sports11080149










