Influence of Practice Periodization and Sleep Duration on Oxidative Stress in High School Judo Athletes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
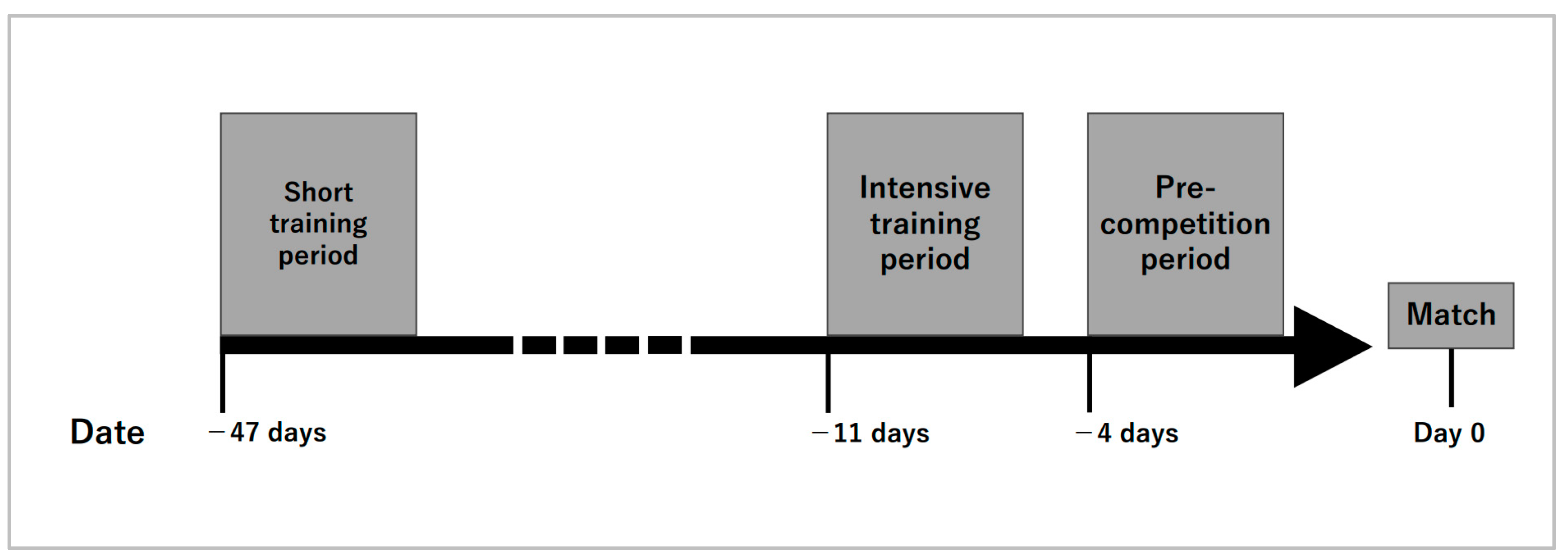
2.2. Measurement Period and Measurement Items
2.3. Statistical Processing
3. Results
3.1. Body Composition of Subjects during Each Petiod
3.2. Physical Activity Record
3.2.1. Practice Content and Intensity
3.2.2. Sleep Times
3.2.3. Physical Activity
3.3. Oxidative Stress and Antioxidant Capacity in Blood
3.4. Relationship between Sleep Duration and Oxidative Stress
3.5. Relationship between d-ROMs and BAP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forman, H.J.; Maiorino, M. Signaling functions of reactive oxygen species. Biochemistry 2010, 49, 835–842. [Google Scholar] [CrossRef]
- Morris, G.; Gevezova, M. Redox regulation of the immune response. Cell. Mol. Immunol. 2022, 19, 1079–1101. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Koyama, K. Exercise-induced oxidative stress: A tool for “hormesis” and “adaptive response”. J. Phys. Fit. Sports Med. 2014, 3, 115–120. [Google Scholar] [CrossRef]
- Merry, T.L.; Ristow, M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef]
- Schoenfeld, B.J. Potential mechanisms for a role of metabolic stress in hypertrophic adaptations to resistance training. Sports Med. 2013, 43, 179–194. [Google Scholar] [CrossRef]
- Suzuki, K. Chronic Inflammation as an Immunological Abnormality and Effectiveness of Exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef]
- Cheng, A.J.; Jude, B. Intramuscular mechanisms of overtraining. Redox Biol. 2020, 35, 101480. [Google Scholar] [CrossRef]
- Craven, J.; McCartney, D. Effects of Acute Sleep Loss on Physical Performance: A Systematic and Meta-Analytical Review. Sports Med. 2022, 52, 2669–2690. [Google Scholar] [CrossRef]
- Milewski, M.D.; Skaggs, D.L. Chronic lack of sleep is associated with increased sports injuries in adolescent athletes. J. Pediatr. Orthop. 2014, 34, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Guide to Development Sports Activities. Available online: https://www.japan-sports.or.jp/Portals/0/data/supoken/doc/ltld/ltld_guide_20210331.pdf (accessed on 5 May 2023).
- Athletics Canada Long Term Athlete Development. Available online: https://athletics.ca/wp-content/uploads/2015/01/LTAD_EN.pdf (accessed on 5 May 2023).
- Nishimaki, M. Effects of Rapid Weight Loss on Menstrual Disorders, Body Water and Electrolyte Control, and Oxidative Stress for Weight Class Athletes. Ph.D. Thesis, Waseda University Graduate School, Tokorozawa, Japan, July 2017. [Google Scholar]
- Development of a Sports Trauma Surveillance System in Japan. Available online: https://www.japan-sports.or.jp/Portals/0/data/supoken/doc/studiesreports/2001_2020/H2401.pdf (accessed on 6 July 2023).
- Pocecco, E.; Ruedl, G. Injuries in judo: A systematic literature review including suggestions for prevention. Br. J. Sports Med. 2013, 47, 1139–1143. [Google Scholar] [CrossRef]
- Japanese Olympic Committee. Available online: https://www.joc.or.jp/olympic/federations/index.html (accessed on 5 July 2023).
- WISMERLL Co., Ltd. Available online: https://wismerlleng.com/productsservices/redox-libra/ (accessed on 5 July 2023).
- Seki, Y. Evaluation of total oxidative stress by d-ROMs testing. J. Anal. Bio-Sci. 2009, 32, 301–306. [Google Scholar]
- Haga, S.; Asami, T. Oxygen consumption and heart rate of female judo athletes during practice. Res. J. Budo 1975, 7, 27–33. [Google Scholar] [CrossRef]
- Lakicevic, N.; Roklicer, R. Effects of Rapid Weight Loss on Judo Athletes: A Systematic Review. Nutrients 2020, 12, 1220. [Google Scholar] [CrossRef] [PubMed]
- Torres-Luque, G.; Hernández-García, R. Physical and Physiological Characteristics of Judo Athletes: An Update. Sports 2016, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, Y.; Morimura, T. Oxidative stress associated with rapid weight reduction decreases circulating adiponectin concentrations. Endocr. J. 2010, 57, 339–345. [Google Scholar] [CrossRef]
- King, M.A.; Clanton, T.L. Hyperthermia, dehydration, and osmotic stress: Unconventional sources of exercise-induced reactive oxygen species. Am. J. Physiol. Regul. Integr. Comp. Phys. 2016, 310, R105–R114. [Google Scholar] [CrossRef]
- Hayashi, T. Conversion of psychological stress into cellular stress response: Roles of the sigma-1 receptor in the process. Psychiatry Clin. Neurosci. 2015, 69, 179–191. [Google Scholar] [CrossRef]
- Daniela, M.; Catalina, L. Effects of Exercise Training on the Autonomic Nervous System with a Focus on Anti-Inflammatory and Antioxidants Effects. Antioxidants 2022, 11, 350. [Google Scholar] [CrossRef]
- Hisamitsu, N.; Hlaing, T. The effect of small island stress on the blood fluidity and reactive oxygen metabolite level in rats. J. Showa Univ. Soc. 2017, 77, 156–161. [Google Scholar] [CrossRef]
- Sugiura, K.; Akama, T. Fluctuations in oxidative stress during training for competitions in ball games. Bull. Fac. Hum. Dev. Cult. Fukushima Univ. 2022, 35, 131–138. [Google Scholar]
- Michailidis, Y.; Jamurtas, A.Z. Sampling time is crucial for measurement of aerobic exercise-induced oxidative stress. Med. Sci. Sports Exerc. 2007, 39, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- McBride, J.M.; Kraemer, W.J. Effect of resistance exercise on free radical production. Med. Sci. Sports Exerc. 1998, 30, 67–72. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Goldfarb, A.H. Effects of acute aerobic and anaerobic exercise on blood markers of oxidative stress. J. Strength Cond. Res. 2005, 19, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Wang, X. Effects of anaerobic exercise and aerobic exercise on biomarkers of oxidative stress. Environ. Health Prev. Med. 2007, 12, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Fisher-Wellman, K.; Bloomer, R.J. Acute exercise and oxidative stress: A 30 year history. Dyn Med 2009, 8, 1. [Google Scholar] [CrossRef]
- Goto, C.; Higashi, Y. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: Role of endothelium-dependent nitric oxide and oxidative stress. Circulation 2003, 108, 530–535. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Davis, P.G. Plasma protein carbonyl response to increasing exercise duration in aerobically trained men and women. Int. J. Sports Med. 2007, 28, 21–25. [Google Scholar] [CrossRef]
- Tokinoya, K.; Ishikura, K. Oxidative stress in the blood after full marathon in amateur runner: Assessment using d-ROM and BAP test. J. Sport Phys. Educ. Cent. Univ. Tsukuba 2021, 43, 13–20. [Google Scholar]
- Okamura, K.; Doi, T. Effect of repeated exercise on urinary 8-hydroxy-deoxyguanosine excretion in humans. Free Radic. Res. 1997, 26, 507–514. [Google Scholar] [CrossRef]
- Nagashima, M. Effect of endurance exercise on oxidative stress and antioxidant vitamin levels in trained cyclists. Jpn. J. Phys. Fit. Sports Med. 2011, 60, 279–286. [Google Scholar]
- Powers, S.K.; Criswell, D. Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am. J. Physiol. 1994, 266, R375–R380. [Google Scholar] [CrossRef]
- Powers, S.K.; Goldstein, E. Exercise Training and Skeletal Muscle Antioxidant Enzymes: An Update. Antioxidants 2022, 12, 39. [Google Scholar] [CrossRef]
- Lungato, L.; Marques, M.S. Sleep deprivation alters gene expression and antioxidant enzyme activity in mice splenocytes. Scand. J. Immunol. 2013, 77, 195–199. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef]
- Wei, T.; Li, C. Association between night-shift work and level of melatonin: Systematic review and meta-analysis. Sleep Med. 2020, 75, 502–509. [Google Scholar] [CrossRef]
- Malav, S.T.; Dana, H. Short-term sleep deprivation leads to decreased systemic redox metabolites and altered epigenetic status. PLoS ONE 2017, 12, e0181978. [Google Scholar] [CrossRef]
- Gulec, M.; Ozkol, H. Oxidative stress in patients with primary insomnia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 37, 247–251. [Google Scholar] [CrossRef]
- Chen, S.; Xie, Y. Sleep deprivation and recovery sleep affect healthy male resident’s pain sensitivity and oxidative stress markers: The medial prefrontal cortex may play a role in sleep deprivation model. Front. Mol. Neurosci. 2022, 15, 937468. [Google Scholar] [CrossRef]
- Matsuda, T.; Ogata, H. Effects of the menstrual cycle on oxidative stress and antioxidant response to high-intensity intermittent exercise until exhaustion in healthy women. J. Sports Med. Phys. Fit. 2020, 60, 1335–1341. [Google Scholar] [CrossRef]



| Measurement Period | Body Weight (kg) | Body Fat Percentage (%) |
|---|---|---|
| short training period | 66.9 ± 13.6 | 26.2 ± 5.4 |
| intensive training period | 67.0 ± 14.7 | 26.6 ± 5.1 |
| pre-competition period | 66.0 ± 14.7 | 26.2 ± 5.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, E.; Hayashida, H. Influence of Practice Periodization and Sleep Duration on Oxidative Stress in High School Judo Athletes. Sports 2023, 11, 163. https://doi.org/10.3390/sports11090163
Yoshida E, Hayashida H. Influence of Practice Periodization and Sleep Duration on Oxidative Stress in High School Judo Athletes. Sports. 2023; 11(9):163. https://doi.org/10.3390/sports11090163
Chicago/Turabian StyleYoshida, Ena, and Harumi Hayashida. 2023. "Influence of Practice Periodization and Sleep Duration on Oxidative Stress in High School Judo Athletes" Sports 11, no. 9: 163. https://doi.org/10.3390/sports11090163
APA StyleYoshida, E., & Hayashida, H. (2023). Influence of Practice Periodization and Sleep Duration on Oxidative Stress in High School Judo Athletes. Sports, 11(9), 163. https://doi.org/10.3390/sports11090163





