Impact of a Three-Month Training Break on Swimming Performance in Athletes with Intellectual Disability
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
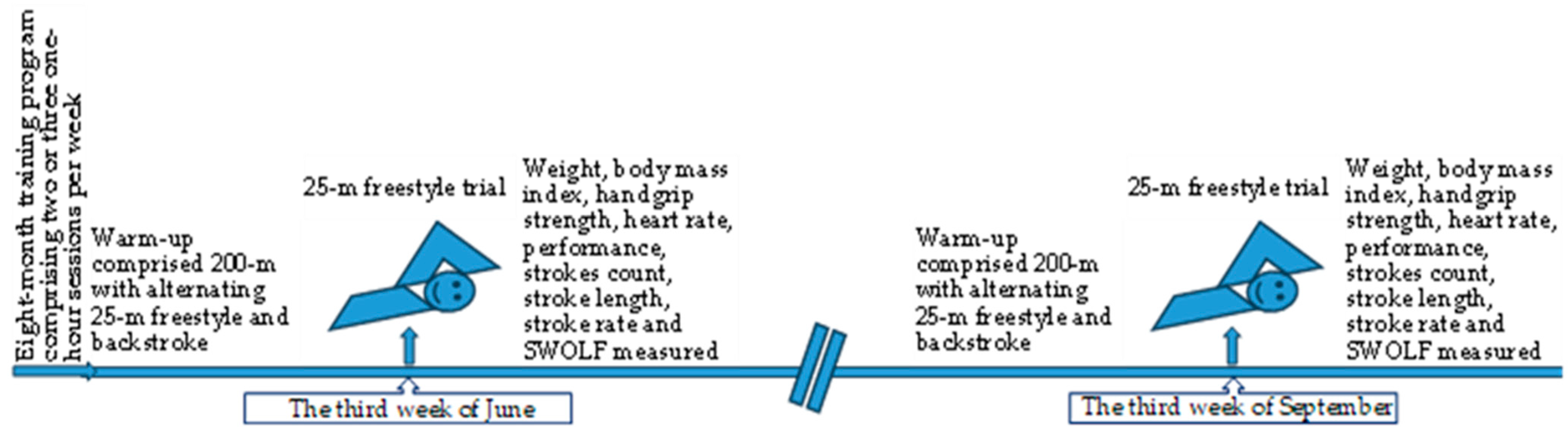
2.2. Measurement Procedure
2.3. Anthropometrics and Performance Parameters
2.4. Kinematic Analysis
2.5. Statistical Analysis
3. Results
3.1. Anthropometric Parameters
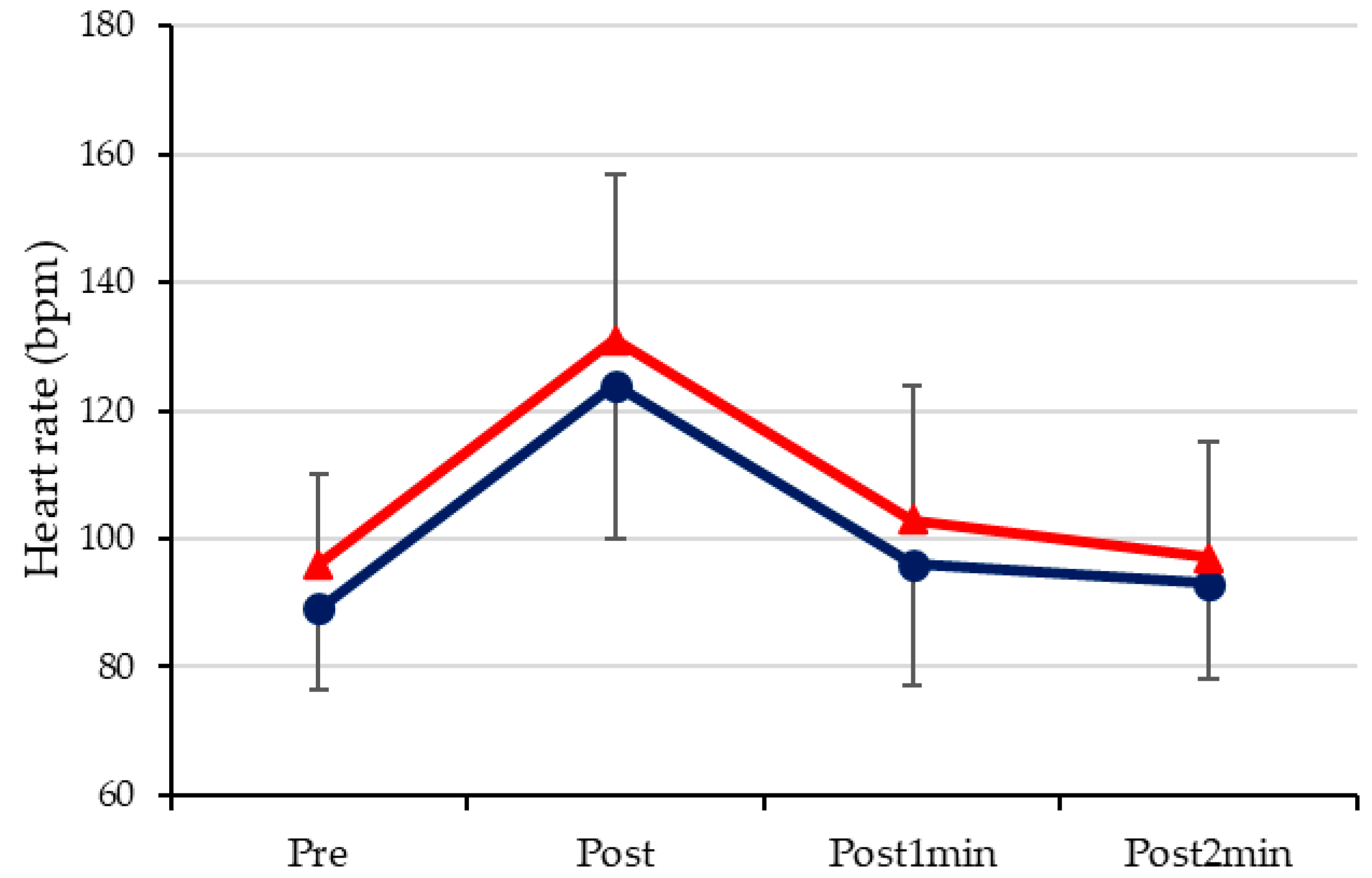
3.2. Heart Rate
3.3. Performance and Kinematic Parameters
4. Discussion
4.1. Anthropometric Parameters
4.2. Heart Rate
4.3. Performance and Kinematic Parameters
4.4. Muscular Strength
4.5. Limitations
5. Conclusions
Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schalock, R.L.; Luckasson, R.; Tassé, M.J. An overview of intellectual disability: Definition, diagnosis, classification, and systems of supports. Am. J. Intellect. Dev. Disabil. 2021, 126, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Schalock, R.L.; Borthwick-Duffy, S.A.; Bradley, V.J.; Buntinx, W.H.; Coulter, D.L.; Craig, E.M.; Yeager, M.H. Intellectual Disability: Definition, Classification, and Systems of Supports; American Association on Intellectual and Developmental Disabilities: Washington, DC, USA, 2010. [Google Scholar]
- Schalock, R.L.; Luckasson, R. A Systematic Approach to Subgroup Classification in Intellectual Disability. Intellect. Dev. Disabil. 2015, 53, 358–366. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Press: Washington, DC, USA, 2013; pp. 31–86. [Google Scholar]
- Shapiro, B.K.; O’Neill, M.E. Developmental delay and intellectual disability. In Nelson Textbook of Pediatrics, 21st ed.; Kliegman, R.M., St Geme, J.W., Blum, N.J., Shah, S.S., Tasker, R.C., Wilson, K.M., Eds.; Elsevier: Philadelphia, PA, USA, 2020; Chapter 53. [Google Scholar]
- Boer, P.H. Effects of detraining on anthropometry, aerobic capacity and functional ability in adults with Down syndrome. J. Appl. Res. Intellect. Disabil. 2018, 31 (Suppl. 1), 144–150. [Google Scholar] [CrossRef] [PubMed]
- van de Vliet, P.; Rintala, P.; Fröjd, K.; Verellen, J.; van Houtte, S.; Daly, D.J.; Vanlandewijck, Y.C. Physical fitness profile of elite athletes with intellectual disability. Scand. J. Med. Sci. Sports 2006, 16, 417–425. [Google Scholar] [CrossRef]
- Fernhall, B.; Pitetti, K.H. Limitations to physical work capacity in individuals with mental retardation. Clin. Exerc. Physiol. 2001, 3, 176–185. [Google Scholar]
- Giagazoglou, P.; Arabatzi, F.; Dipla, K.; Liga, M.; Kellis, E. Effect of a hippotherapy intervention program on static balance and strength in adolescents with intellectual disabilities. Res. Dev. Disabil. 2012, 33, 2265–2270. [Google Scholar] [CrossRef]
- Horvat, M.; Pitetti, K.H.; Croce, R. Isokinetic torque, average power, and flexion/extension ratios in nondisabled adults and adults with mental retardation. J. Orthop. Sports Phys. Ther. 1997, 25, 395–399. [Google Scholar] [CrossRef]
- Lahtinen, U.; Rintala, P.; Malin, A. Physical performance of individuals with intellectual disability: A 30 year follow-up. Adapt. Phys. Act. Q. 2007, 24, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Fernhall, B.; McCubbin, J.A.; Pitetti, K.H.; Rintala, P.; Rimmer, J.H.; Millar, A.L.; De Silva, A. Prediction of maximal heart rate in individuals with mental retardation. Med. Sci. Sports Exerc. 2001, 33, 1655–1660. [Google Scholar] [CrossRef]
- Declerck, M.; Feys, H.; Daly, D. Benefits of swimming for children with cerebral palsy: A pilot study. Serb. J. Sports Sci. 2013, 7, 57–69. [Google Scholar]
- Yılmaz, I.; Ergu, N.; Konukman, F.; Agbuğa, B.; Zorba, E.; Cimen, Z. The effects of water exercises and swimming on physical fitness of children with mental retardation. J. Hum. Kinet. 2009, 21, 105–111. [Google Scholar]
- Casey, A.F.; Rasmussen, R.; Mackenzie, S.J.; Glenn, J. Dual-energy x-ray absorptiometry to measure the influence of a 16-week community-based swim training program on body fat in children and adolescents with intellectual disabilities. Arch. Phys. Med. Rehabil. 2010, 91, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Fernhall, B.; Pitetti, K.H.; Vukovich, M.D.; Stubbs, N.; Hensen, T.; Winnick, J.P.; Short, F.X. Validation of cardiovascular fitness field tests in children with mental retardation. Am. J. Ment. Retard. 1998, 102, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Marques-Aleixo, I.; Querido, A.; Figueiredo, P.; Vilas-Boas, J.P.; Corredeira, R.; Daly, D.; Fernandes, R.J. Intracyclic velocity variation and arm coordination assessment in swimmers with Down syndrome. Adapt. Phys. Act. Q. 2013, 30, 70–84. [Google Scholar] [CrossRef]
- Einarsson, I.P.; Johannsson, E.; Daly, D. Between and within race changes in race parameters in swimmers with intellectual disabilities. In Proceedings of the 1st International Scientific Conference of Aquatic Space Activities; Nomura, T., Ungerechts, B.E., Eds.; University of Tsukuba: Ibaraki, Japan, 2008; pp. 153–158. [Google Scholar]
- Mujika, I.; Padilla, S. Detraining: Loss of training-induced physiological and performance adaptations. Part II: Long term insufficient training stimulus. Sports Med. 2000, 30, 145–154. [Google Scholar] [CrossRef]
- Nugroho, S.; Or, M. The Detraining Effects of Complete Inactivity. Doctoral Dissertation, Sport Science Faculty Yogyakarta State University, Yogyakarta, Indonesia, 2005. Available online: https://staffnew.uny.ac.id/upload/132319845/penelitian/DETRAINING++EFFECTS+OF+COMPLETE+INACTIVITY.pdf (accessed on 30 May 2024).
- Lemmer, J.T.; Ivey, F.M.; Ryan, A.S.; Martel, G.F.; Hurlbut, D.E.; Metter, J.E.; Fozard, J.L.; Fleg, J.L.; Hurley, B.F. Effect of strength training on resting metabolic rate and physical activity: Age and gender comparisons. Med. Sci. Sports Exerc. 2001, 33, 532–541. [Google Scholar] [CrossRef]
- Boer, P.H. The effect of 8 weeks of freestyle swim training on the functional fitness of adults with Down syndrome. J. Intellect. Disabil. Res. 2020, 64, 770–781. [Google Scholar] [CrossRef]
- Casey, A.F.; Emes, C. The effects of swim training on respiratory aspects of speech production in adolescents with down syndrome. Adapt. Phys. Act. Q. 2011, 28, 326–341. [Google Scholar] [CrossRef]
- Daly, D.; Einarsson, I.; Van de Vliet, P.; Vanlandewijck, Y. Freestyle race success in swimmers with intellectual disability. Portuguese J. Sports Sci. 2006, 6, 294–296. [Google Scholar]
- Bosquet, L.; Mujika, I. Detraining. In Endurance training: Science and Practice; Iñigo Mujika, S.L.U., Ed.; Iñigo Mujika: Vitoria-Gasteiz, Spain, 2012; pp. 99–106. [Google Scholar]
- EAOM-AmeA. Available online: https://www.eaom-amea.gr (accessed on 16 January 2024).
- Papadimitriou, K.; Papadimitriou, N.; Gourgoulis, V.; Barkoukis, V.; Loupos, D. Assessment of Young Swimmers’ Technique with Tec Pa Tool. Cent. Eur. J. Sport Sci. Med. 2021, 34, 39–51. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Loupos, D. The Effect of an Alternative Swimming Learning Program on Skills, Technique, Performance, and Salivary Cortisol Concentration at Primary School Ages Novice Swimmers. Healthcare 2021, 9, 1234. [Google Scholar] [CrossRef] [PubMed]
- López-Plaza, D.; Quero-Calero, C.D.; Alacid, F.; Abellán-Aynés, O. Stroke Steadiness as a Determinant Factor of Performance in 100 m Freestyle in Young Swimmers. Sports 2024, 12, 107. [Google Scholar] [CrossRef]
- Madou, T.; Vanluyten, K.; Martens, J.; Iserbyt, P. Assessment and Prediction of Swimming Performance Using the SWOLF Index. Int. J. Kin. High Educ. 2021, 7, 76–85. [Google Scholar] [CrossRef]
- Perego, P.; Andreoni, G.; Sironi, R.; Lenzi, S.; Santambrogio, G. Wearable device for swim assessment: A new ecologic approach for communication and analysis. EAI Endorsed Trans. Internet of Things 2015, 2, 147–150. [Google Scholar] [CrossRef]
- Ludbrook, J. Should we use one-sided or two-sided P values in tests of significance? Clin. Exp. Pharmacol. Physiol. 2013, 40, 357–361. [Google Scholar] [CrossRef]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut Off Points. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Jacinto, M.; Monteiro, D.; Antunes, R.; Ferreira, J.P.; Matos, R.; Campos, M.J. Effects of exercise on body mass index and waist circumference of individuals with intellectual and developmental disabilities: A systematic review with meta-analysis. Front. Physiol. 2023, 14, 1236379. [Google Scholar] [CrossRef]
- Jacob, U.S.; Pillay, J.; Johnson, E.; Omoya, O.T.; Adedokun, A.P. A systematic review of physical activity: Benefits and needs for maintenance of quality of life among adults with intellectual disability. Front. Sports Act. Living 2023, 5, 1184946. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, T.; Nagi, L.F.; Iqbal, S.P.; Razzaq, S.; Hassnain, S.; Khan, S.; Shahid, N. Relationship Between Physical Inactivity and Obesity in the Urban Slums of Lahore. Cureus 2022, 14, e23719. [Google Scholar] [CrossRef]
- Zwack, C.C.; McDonald, R.; Tursunalieva, A.; Lambert, G.W.; Lambert, E.A. Exploration of diet, physical activity, health knowledge and the cardiometabolic profile of young adults with intellectual disability. J. Intellect. Disabil. Res. 2022, 66, 517–532. [Google Scholar] [CrossRef]
- Ptomey, L.T.; Walpitage, D.L.; Mohseni, M.; Dreyer Gillette, M.L.; Davis, A.M.; Forseth, B.; Dean, E.E.; Waitman, L.R. Weight status and associated comorbidities in children and adults with Down syndrome, autism spectrum disorder and intellectual and developmental disabilities. J. Intellect. Disabil. Res. 2020, 64, 725–737. [Google Scholar] [CrossRef]
- Ryan, J.; McCallion, P.; McCarron, M.; Luus, R.; Burke, E.A. Overweight/obesity and chronic health conditions in older people with intellectual disability in Ireland. J. Intellect. Disabil. Res. 2021, 65, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
- Zacca, R.; Toubekis, A.; Freitas, L.; Silva, A.F.; Azevedo, R.; Vilas-Boas, J.P.; Pyne, D.B.; Castro, F.A.S.; Fernandes, R.J. Effects of detraining in age-group swimmers performance, energetics and kinematics. J. Sports Sci. 2019, 37, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Coyle, E.F.; Martin, W.H.; Sinacore, D.R.; Joyner, M.J.; Hagberg, J.M.; Holloszy, J.O. Time course of loss of adaptations after stopping prolonged intense endurance training. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 57, 1857–1864. [Google Scholar] [CrossRef]
- Pace, M.; Bricout, V.A. Low heart rate response of children with autism spectrum disorders in comparison to controls during physical exercise. Physiol. Behavl. 2015, 141, 63–68. [Google Scholar] [CrossRef]
- Esco, M.R.; Flatt, A.A. Ultra-short-term heart rate variability indexes at rest and post-exercise in athletes: Evaluating the agreement with accepted recommendations. J. Sports Sci. Med. 2014, 13, 535–541. [Google Scholar]
- Font-Farré, M.; Farche, A.C.S.; de Medeiros Takahashi, A.C.; Guerra-Balic, M.; Figueroa, A.; Oviedo, G.R. Cardiac autonomic modulation response before, during, and after submaximal exercise in older adults with intellectual disability. Front. Physiol. 2021, 12, 702418. [Google Scholar] [CrossRef]
- Zhou, K. Design of Training Load Monitoring and Adjustment Algorithm for Athletes: Based on Heart Rate Variability and Body Index Data. J. Electr. Syst. 2024, 20, 1600–1611. [Google Scholar] [CrossRef]
- Moreira, M.F.; Morais, J.E.; Marinho, D.A.; Silva, A.J.; Barbosa, T.M.; Costa, M.J. Growth influences biomechanical profile of talented swimmers during the summer break. Sports Biomech. 2014, 13, 62–74. [Google Scholar] [CrossRef]
- Głyk, W.; Hołub, M.; Karpiński, J.; Rejdych, W.; Sadowski, W.; Trybus, A.; Baron, J.; Rydzik, Ł.; Ambroży, T.; Stanula, A. Effects of a 12-Week Detraining Period on Physical Capacity, Power and Speed in Elite Swimmers. Int. J. Environ. Res. Public Health 2022, 19, 4594. [Google Scholar] [CrossRef]
- Barden, J.M.; Kell, R.T. Relationships between stroke parameters and critical swimming speed in a sprint interval training set. J. Sports Sci. 2009, 27, 227–235. [Google Scholar] [CrossRef]
- Hellard, P.; Avalos, M.; Hausswirth, C.; Pyne, D.; Toussaint, J.F.; Mujika, I. Identifying Optimal Overload and Taper in Elite Swimmers over Time. J. Sports Sci. Med. 2013, 12, 668–678. [Google Scholar] [PubMed]
- Tsalis, G.; Toubekis, A.G.; Michailidou, D.; Gourgoulis, V.; Douda, H.; Tokmakidis, S.P. Physiological responses and stroke-parameter changes during interval swimming in different age-group female swimmers. J. Strength Cond. Res. 2012, 26, 3312–3319. [Google Scholar] [CrossRef]
- Barbosa, T.M.; Bragada, J.A.; Reis, V.M.; Marinho, D.A.; Carvalho, C.; Silva, A.J. Energetics and biomechanics as determining factors of swimming performance: Updating the state of the art. J. Sci. Med. Sport 2010, 13, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Dopsaj, M.; Zuoziene, I.J.; Milić, R.; Cherepov, E.; Erlikh, V.; Masiulis, N.; di Nino, A.; Vodičar, J. Body Composition in International Sprint Swimmers: Are There Any Relations with Performance? Int. J. Environ. Res. Public Health 2020, 17, 9464. [Google Scholar] [CrossRef]
- Kwok, W.Y.; So, B.C.L.; Tse, D.H.T.; Ng, S.S.M. A systematic review and meta-analysis: Biomechanical evaluation of the effectiveness of strength and conditioning training programs on front crawl swimming performance. J. Sports Sci. Med. 2021, 20, 564–585. [Google Scholar] [CrossRef] [PubMed]
- Burns, J. The impact of intellectual disabilities on elite sports performance. Int. J. Sport Exerc. Psychol. 2015, 8, 251–267. [Google Scholar] [CrossRef]
- Sakalidis, K.E.; Burns, J.; Van Biesen, D.; Dreegia, W.; Hettinga, F.J. The impact of cognitive functions and intellectual impairment on pacing and performance in sports. Psychol. Sport Exerc. 2021, 52, 101840. [Google Scholar] [CrossRef]
- Van Biesen, D.; Mactavish, J.; McCulloch, K.; Lenaerts, L.; Vanlandewijck, Y.C. Cognitive profile of young well-trained athletes with intellectual disabilities. Res. Dev. Disabil. 2016, 53–54, 377–390. [Google Scholar] [CrossRef]
- Nikolaidis, P.T.; Zisimatos, D. Relationship of body mass index with 1600 m running, 50 m swimming, and pull-ups performance in army cadets. Saudi J. Sports Med. 2014, 14, 144–150. [Google Scholar] [CrossRef]
- Pitetti, K.H.; Yarmer, D.A.; Fernhall, B. Cardiovascular fitness and body composition of youth with and without mental retardation. Adapt. Phys. Act. Q. 2001, 18, 127–141. [Google Scholar] [CrossRef]
- Liu, T.; Kelly, J.; Davis, L.; Zamora, K. Nutrition, BMI and Motor Competence in Children with Autism Spectrum Disorder. Medicina 2019, 55, 135. [Google Scholar] [CrossRef] [PubMed]
- Sulton, K.; Jajat, J. Relationship between gross motor skills and body mass index of children with intellectual disability. In Proceedings of the 3rd International Conference on Sport Science, Health, and Physical Education (ICSSHPE 25-26/09/2018), Bandung, Indonesia, 25–26 September 2018; Advances in Health Sciences Research. Atlantis Press: Dordrecht, The Netherlands, 2019; Volume 11, pp. 210–213. [Google Scholar]
- Lemmer, J.T.; Hurlbut, D.E.; Martel, G.F.; Tracy, B.L.; Ivey, F.M.; Metter, E.J.; Fozard, J.L.; Fleg, J.L.; Hurley, B.F. Age and gender responses to strength training and detraining. Med. Sci. Sports Exerc. 2000, 32, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Garrido, N.D.; Silva, A.J.; Fernandes, R.J.; Barbosa, T.M.; Costa, A.M.; Marinho, D.A.; Marques, M.C. High level swimming performance and its relation to non-specific parameters: A cross-sectional study on maximum handgrip isometric strength. Percept. Mot. Skills 2012, 114, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Zampagni, M.L.; Casino, D.; Visani, A.; Martelli, S.; Benelli, P.; Marcacci, M.; De Vito, G. Influence of age and hand grip strength on freestyle performances in master swimmers. In Proceedings of the ISBS-Conference Proceedings Archive, Salzburg, Austria, 14–18 July 2006. [Google Scholar]
| Severity of Disability | Male | Female |
|---|---|---|
| Mild | 5 | 0 |
| Moderate | 9 | 3 |
| Severe | 2 | 2 |
| June | September | p | d | Change | |||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | ||||
| Age (years) | 28.1 | 8.7 | 28.4 | 8.7 | |||
| Height (m) | 1.74 | 0.1 | 1.74 | 0.1 | |||
| Weight (kg) | 80.2 | 16.1 | 81.7 | 15.9 * | 0.002 | 2.13 | ↑ 1.5 kg or 1.9% |
| ΒΜΙ (kg/m2) | 26.8 | 5.5 | 27.2 | 5.5 * | 0.004 | 0.74 | ↑ 0.4 kg/m2 or 1.8% |
| June | September | p | d | |||
|---|---|---|---|---|---|---|
| M | SD | M | SD | |||
| T25 (s) | 33.1 | 17.1 | 35.6 | 18.8 * | 0.002 | 3.48 |
| V20 (m·s−1) | 0.78 | 0.23 | 0.73 | 0.23 * | 0.003 | 0.08 |
| SC20 (cycles) | 16.3 | 6.1 | 17.7 | 7.2 * | 0.003 | 2.16 |
| SL20 (m) | 1.39 | 0.48 | 1.27 | 0.42 * | <0.001 | 0.13 |
| SR20 (cycles·min−1) | 35.4 | 10.4 | 35.4 | 9.2 | 0.491 | 4.06 |
| SC25 (cycles) | 19.3 | 6.1 | 20.7 | 7.2 * | 0.003 | 2.16 |
| SWOLF | 52.4 | 22.0 | 56.3 | 25.2 * | <0.001 | 4.84 |
| HGS (Ν) | 215 | 71 | 237 | 70 | 0.070 | 65.50 |
| June | September | |||
|---|---|---|---|---|
| Pearson’s r | p | Pearson’s r | p | |
| WEIGHT—T25 | 0.479 | 0.028 | 0.489 | 0.024 |
| BMI—T25 | 0.731 | 0.000 | 0.703 | 0.000 |
| BMI—SWOLF | 0.684 | 0.001 | 0.680 | 0.001 |
| HGS—T25 | −0.458 | 0.037 | −0.470 | 0.032 |
| SWOLF—T25 | 0.981 | 0,000 | 0.987 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyriakidou, G.; Tsalis, G.; Evaggelinou, C. Impact of a Three-Month Training Break on Swimming Performance in Athletes with Intellectual Disability. Sports 2024, 12, 330. https://doi.org/10.3390/sports12120330
Kyriakidou G, Tsalis G, Evaggelinou C. Impact of a Three-Month Training Break on Swimming Performance in Athletes with Intellectual Disability. Sports. 2024; 12(12):330. https://doi.org/10.3390/sports12120330
Chicago/Turabian StyleKyriakidou, Glykeria, George Tsalis, and Christina Evaggelinou. 2024. "Impact of a Three-Month Training Break on Swimming Performance in Athletes with Intellectual Disability" Sports 12, no. 12: 330. https://doi.org/10.3390/sports12120330
APA StyleKyriakidou, G., Tsalis, G., & Evaggelinou, C. (2024). Impact of a Three-Month Training Break on Swimming Performance in Athletes with Intellectual Disability. Sports, 12(12), 330. https://doi.org/10.3390/sports12120330







