Effect of Different Reduced Training Frequencies after 12 Weeks of Concurrent Resistance and Aerobic Training on Muscle Strength and Morphology
Abstract
:1. Introduction
2. Materials and Methods
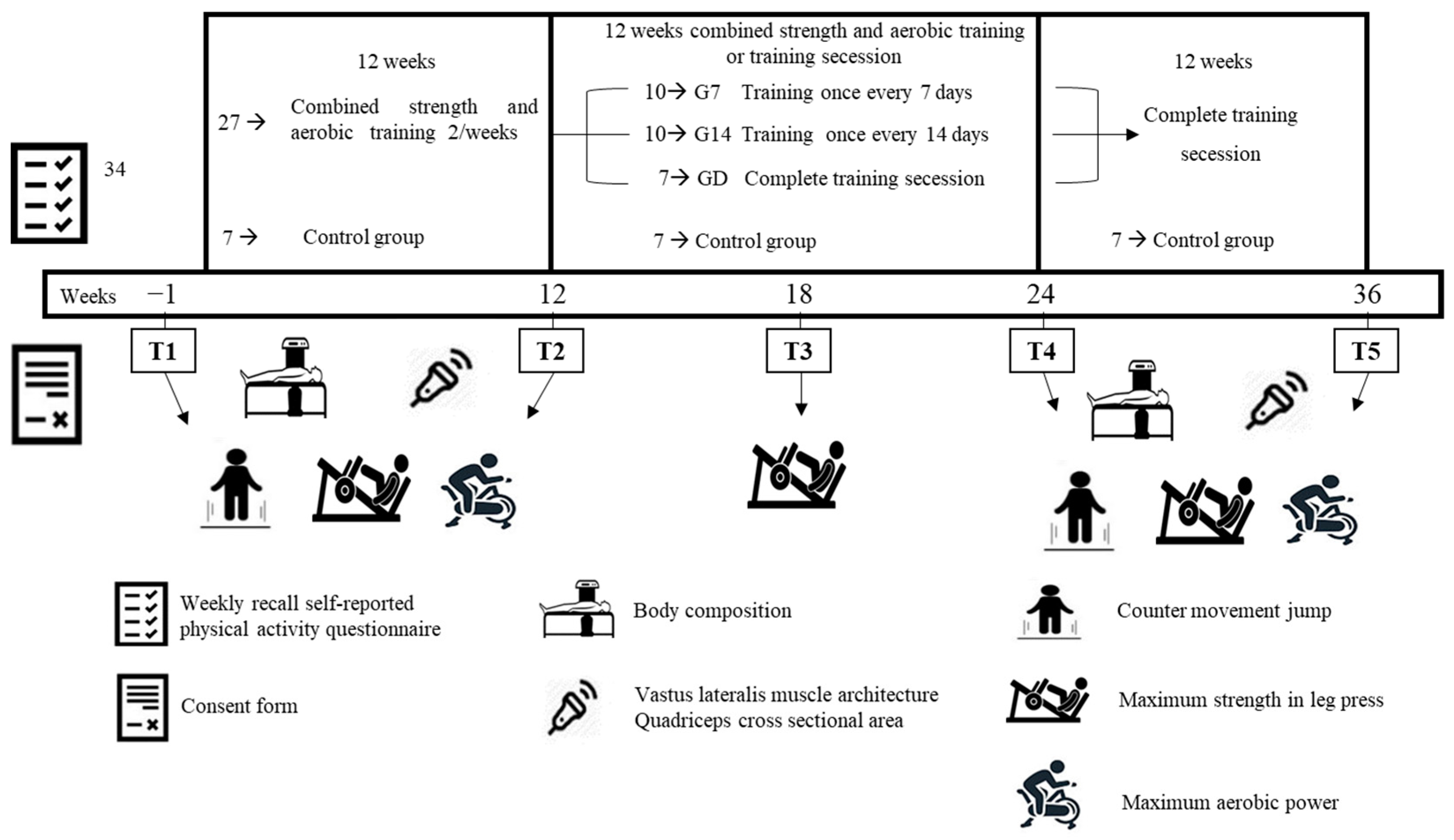
2.1. Experimental Approach
2.2. Participants
2.3. Procedures
2.3.1. Concurrent Resistance and Aerobic Training
2.3.2. Reduced Training and Complete Cessation of Exercise
2.3.3. Evaluation of Body Composition
2.3.4. Performance Evaluations
2.3.5. Ultrasonography
2.4. Statistical Analyses
3. Results
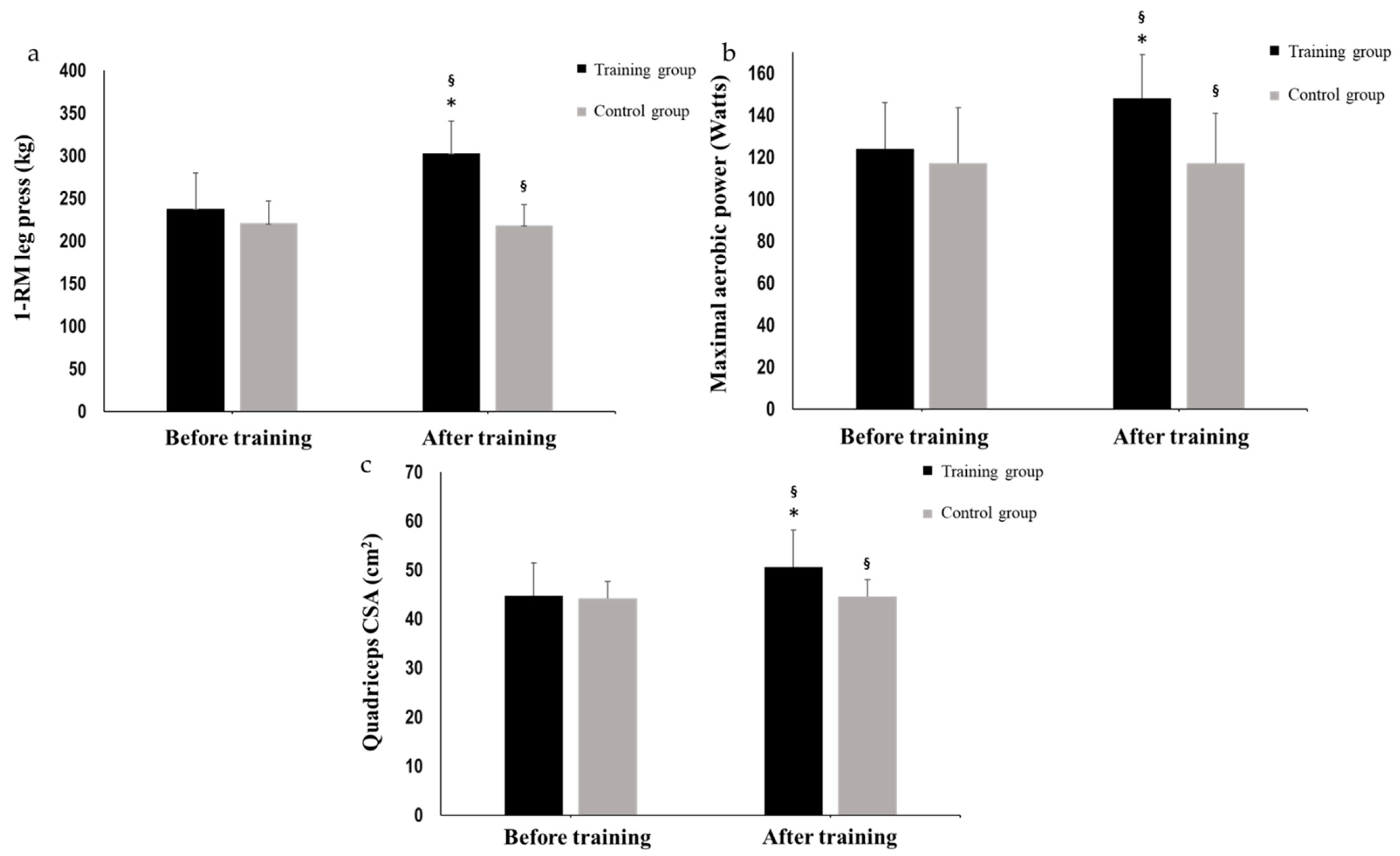
3.1. Systematic Concurrent Training (T1 to T2)
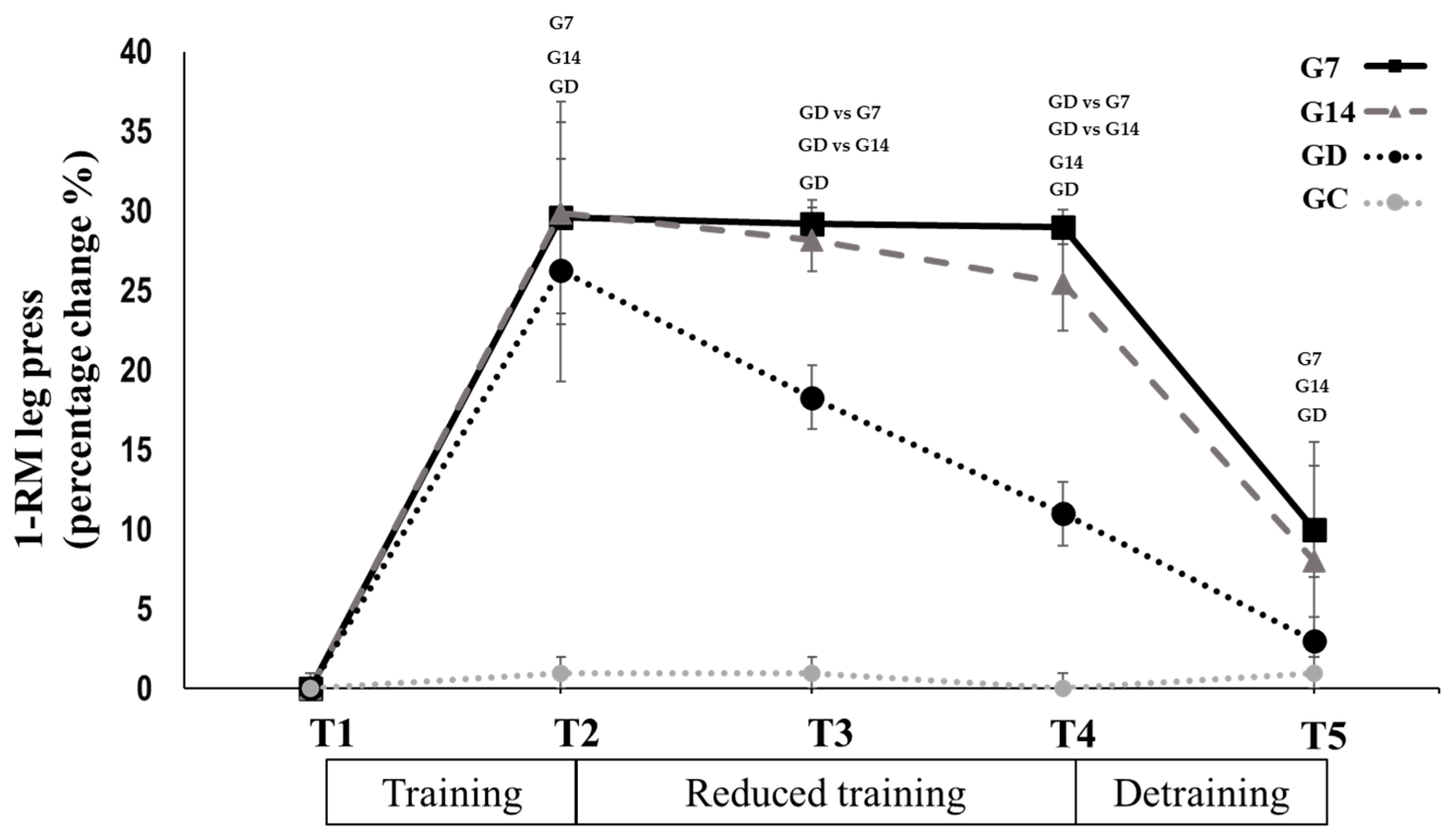
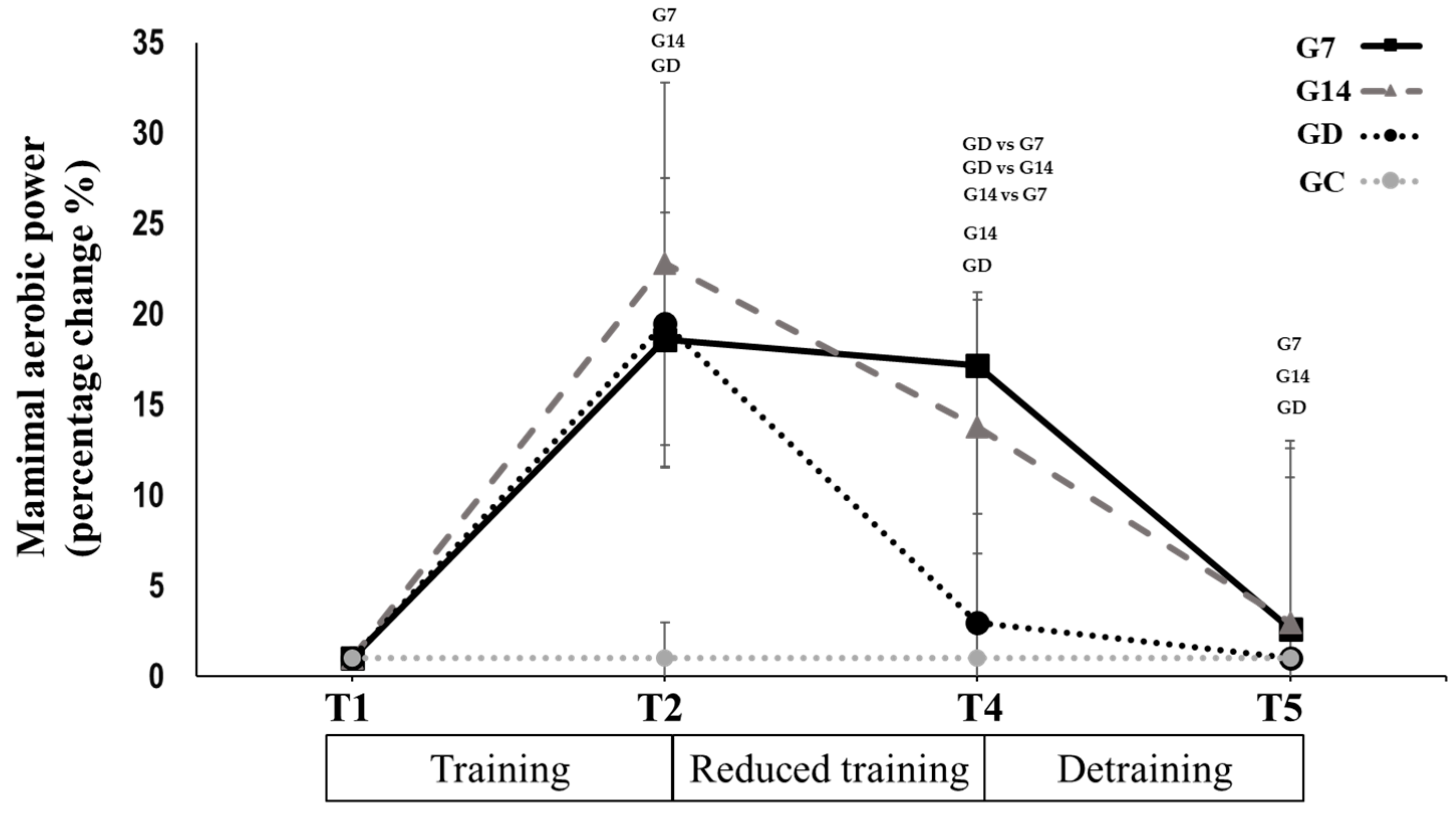
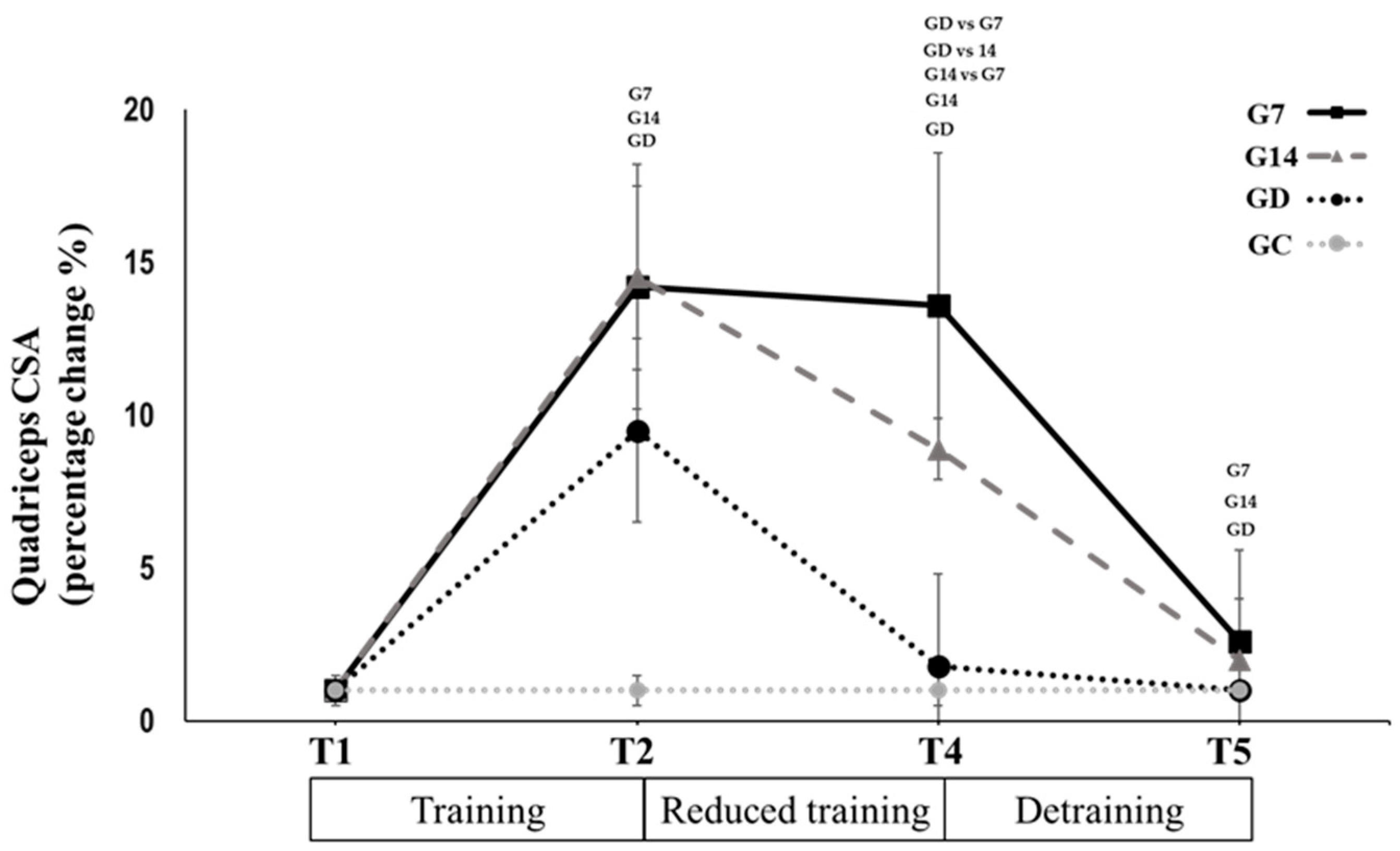
3.2. Reduced Training Frequency and Detraining (T2 to T5)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Kenney, L.W.; Wilmore, J.H.; Costill, D.L. Physiology of Sport and Exercise. In Physiology of Sport and Exercise, 6th ed.; Human Kenetics: West Yorkshire, UK, 2015. [Google Scholar]
- Tsitkanou, S.; Spengos, K.; Stasinaki, N.A.; Zaras, N.; Bogdanis, G.; Papadimas, G.; Terzis, G. Effects of high-intensity interval cycling performed after resistance training on muscle strength and hypertrophy. Scand. J. Med. Sci. Sports 2017, 27, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Haykowsky, M.; McGavock, J.; Vonder Muhll, I.; Koller, M.; Mandic, S.; Welsh, R.; Taylor, D. Effect of exercise training on peak aerobic power, left ventricular morphology, and muscle strength in healthy older women. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Billinger, S.A.; Arena, R.; Bernhardt, J.; Eng, J.J.; Franklin, B.A.; Johnson, C.M.; Mackay-Lyons, M.; Macko, R.F.; Mead, G.E.; Roth, E.J.; et al. Physical activity and exercise recommendations for stroke survivors: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 2532–2553. [Google Scholar] [CrossRef] [PubMed]
- Sigal, R.J.; Kenny, G.P.; Wasserman, D.H.; Castaneda-Sceppa, C.; White, R.D. Physical activity/exercise and type 2 diabetes: A consensus statement from the American Diabetes Association. Diabetes Care 2006, 29, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Middelkamp, J.; van Rooijen, M.; Wolfhagen, P.; Steenbergen, B. The effects of a self-efficacy intervention on exercise behavior of fitness club members in 52 weeks and long-term relationships of transtheoretical model constructs. J. Sports Sci. Med. 2017, 16, 163–171. [Google Scholar] [PubMed]
- International Health, Racquet & Sports club Association. The 2020 IHRSA Global Report; IHRSA: Boston, MA, USA, 2020; Available online: https://www.ihrsa.org/publications/the-2020-ihrsa-global-report/ (accessed on 20 November 2020).
- Vikne, H.; Strøm, V.; Pripp, A.H.; Gjøvaag, T. Human skeletal muscle fiber type percentage and area after reduced muscle use: A systematic review and meta-analysis. Scand. J. Med. Sci. Sports 2020, 30, 1298–1317. [Google Scholar] [CrossRef] [PubMed]
- Leitão, L.; Pereira, A.; Mazini, M.; Venturini, G.; Campos, Y.; Vieira, J.; Novaes, J.; Vianna, J.; da Silva, S.; Louro, H. Effects of three months of detraining on the health profile of older women after a multicomponent exercise program. Int. J. Environ. Res. Public Health 2019, 16, 3881. [Google Scholar] [CrossRef]
- Nikseresht, M.; Hafezi Ahmadi, M.R.; Hedayati, M. Detraining-induced alterations in adipokines and cardiometabolic risk factors after nonlinear periodized resistance and aerobic interval training in obese men. Appl. Physiol. Nutr. Metab. 2016, 41, 1018–1025. [Google Scholar] [CrossRef]
- Nolan, P.B.; Keeling, S.M.; Robitaille, C.A.; Buchanan, C.A.; Dalleck, L.C. The Effect of Detraining after a Period of Training on Cardiometabolic Health in Previously Sedentary Individuals. Int. J. Environ. Res. Public Health 2018, 15, 2303. [Google Scholar] [CrossRef]
- Tofas, T.; Draganidis, D.; Deli, C.K.; Georgakouli, K.; Fatouros, I.G.; Jamurtas, A.Z. Exercise-Induced Regulation of Redox Status in Cardiovascular Diseases: The Role of Exercise Training and Detraining. Antioxidants 2020, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Serra-Rexach, J.A.; Bustamante-Ara, N.; Hierro Villarán, M.; González Gil, P.; Sanz Ibáñez, M.J.; Blanco Sanz, N.; Ortega Santamaría, V.; Gutiérrez Sanz, N.; Marín Prada, A.B.; Gallardo, C.; et al. Short-term, light- to moderate-intensity exercise training improves leg muscle strength in the oldest old: A randomized controlled trial. J. Am. Geriatr. Soc. 2011, 59, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Lovell, D.I.; Cuneo, R.; Gass, G.C. The effect of strength training and short-term detraining on maximum force and the rate of force development of older men. Eur. J. Appl. Physiol. 2010, 109, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; Ibañez, J.; González-Badillo, J.J.; Ratamess, N.A.; Kraemer, W.J.; Häkkinen, K.; Bonnabau, H.; Granados, C.; French, D.N.; Gorostiaga, E.M. Detraining and tapering effects on hormonal responses and strength performance. J. Strength Cond. Res. 2007, 21, 768–775. [Google Scholar] [CrossRef]
- Graves, J.E.; Pollock, M.L.; Leggett, S.H.; Braith, R.W.; Carpenter, D.M.; Bishop, L.E. Effect of reduced training frequency on muscular strength. Int. J. Sports Med. 1988, 9, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Sallinen, J.; Fogelholm, M.; Volek, J.S.; Kraemer, W.J.; Alen, M.; Häkkinen, K. Effects of strength training and reduced training on functional performance and metabolic health indicators in middle-aged men. Int. J. Sports Med. 2007, 28, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Trappe, S.; Williamson, D.; Godard, M. Maintenance of whole muscle strength and size following resistance training in older men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, B138–B143. [Google Scholar] [CrossRef] [PubMed]
- Bickel, C.S.; Cross, J.M.; Bamman, M.M. Exercise dosing to retain resistance training adaptations in young and older adults. Med. Sci. Sports Exerc. 2011, 43, 1177–1187. [Google Scholar] [CrossRef]
- Mujika, I.; Padilla, S. Muscular characteristics of detraining in humans. Med. Sci. Sports Exerc. 2001, 33, 1297–1303. [Google Scholar] [CrossRef]
- Hickson, R.C.; Rosenkoetter, M.A. Reduced training frequencies and maintenance of increased aerobic power. Med. Sci. Sports Exerc. 1981, 13, 13–16. [Google Scholar] [CrossRef]
- Tavares, L.D.; de Souza, E.O.; Ugrinowitsch, C.; Laurentino, G.C.; Roschel, H.; Aihara, A.Y.; Cardoso, F.N.; Tricoli, V. Effects of different strength training frequencies during reduced training period on strength and muscle cross-sectional area. Eur. J. Sport Sci. 2017, 17, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Methenitis, S.A. Brief Review on Concurrent Training: From Laboratory to the Field. Sports 2018, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Zaras, N.; Stasinaki, A.N.; Spiliopoulou, P.; Mpampoulis, T.; Hadjicharalambous, M.; Terzis, G. Effect of Inter-Repetition Rest vs. Traditional Strength Training on Lower Body Strength, Rate of Force Development, and Muscle Architecture. Appl. Sci. 2021, 11, 45. [Google Scholar] [CrossRef]
- Methenitis, S.; Mpampoulis, T.; Spiliopoulou, P.; Papadimas, G.; Papadopoulos, C.; Chalari, E.; Evangelidou, E.; Stasinaki, A.N.; Nomikos, T.; Terzis, G. Muscle fiber composition, jumping performance, and rate of force development adaptations induced by different power training volumes in females. Appl. Physiol. Nutr. Metab. 2020, 45, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Spiliopoulou, P.; Zaras, N.; Methenitis, S.; Papadimas, G.; Papadopoulos, C.; Bogdanis, G.C.; Terzis, G. Effect of Concurrent Power Training and High-Intensity Interval Cycling on Muscle Morphology and Performance. J. Strength Cond. Res. 2021, 35, 2464–2471. [Google Scholar] [CrossRef]
- Borg, G.A.V. Borg’s Perceived Exertion and Pain Scales; Human Kinetics Press: Champaign, IL, USA, 1998. [Google Scholar]
- A.C.S.M. Laboratory Submaximal Exercise Testing: YMCA CycleνErgometer Test. In ACSM’s Health-Related Physical Fitness Assessment Manual, 2nd ed.; Wolters Kluwer: Philadelphia, PA, USA, 2008; pp. 112–116. [Google Scholar]
- Noorkoiv, M.; Nosaka, K.; Blazevich, A.J. Assessment of quadriceps muscle cross-sectional area by ultrasound extended-field-of-view imaging. Eur. J. Appl. Physiol. 2010, 109, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Blazevich, A.J. Effects of physical training and detraining, immobilisation, growth and aging on human fascicle geometry. Sports Med. 2006, 36, 1003–1017. [Google Scholar] [CrossRef]
- Stasinaki, A.N.; Zaras, N.; Methenitis, S.; Bogdanis, G.; Terzis, G. Rate of Force Development and Muscle Architecture after Fast and Slow Velocity Eccentric Training. Sports 2019, 7, 41. [Google Scholar] [CrossRef]
- Fatouros, I.G.; Kambas, A.; Katrabasas, I.; Nikolaidis, K.; Chatzinikolaou, A.; Leontsini, D.; Taxildaris, K. Strength training and detraining effects on muscular strength, anaerobic power, and mobility of inactive older men are intensity dependent. Br. J. Sports Med. 2005, 39, 776–780. [Google Scholar] [CrossRef]
- Sakugawa, R.L.; Moura, B.M.; Orssatto, L.B.D.R.; Bezerra, E.S.; Cadore, E.L.; Diefenthaeler, F. Effects of resistance training, detraining, and retraining on strength and functional capacity in elderly. Aging Clin. Exp. Res. 2019, 31, 31–39. [Google Scholar] [CrossRef]
- Andersen, L.L.; Andersen, J.L.; Magnusson, S.P.; Aagaard, P. Neuromuscular adaptations to detraining following resistance training in previously untrained subjects. Eur. J. Appl. Physiol. 2005, 93, 511–518. [Google Scholar] [CrossRef]
- Häkkinen, K.; Newton, R.U.; Walker, S.; Häkkinen, A.; Krapi, S.; Rekola, R.; Koponen, P.; Kraemer, W.J.; Haff, G.G.; Blazevich, A.J.; et al. Effects of Upper Body Eccentric versus Concentric Strength Training and Detraining on Maximal Force, Muscle Activation, Hypertrophy and Serum Hormones in Women. J. Sports Sci. Med. 2022, 21, 200–213. [Google Scholar] [CrossRef]
- Taaffe, D.R.; Marcus, R. Dynamic muscle strength alterations to detraining and retraining in elderly men. Clin. Physiol. 1997, 17, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Hickson, R.C.; Kanakis, C., Jr.; Davis, J.R.; Moore, A.M.; Rich, S. Reduced training duration effects on aerobic power, endurance, and cardiac growth. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1982, 53, 225–229. [Google Scholar] [CrossRef]
- Hickson, R.C.; Foster, C.; Pollock, M.L.; Galassi, T.M.; Rich, S. Reduced training intensities and loss of aerobic power, endurance, and cardiac growth. J. Appl. Physiol. 1985, 58, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, T.K.; Inoue, Y.; Hirata, M.; Shamsuddin, A.K.; Kondo, N. Enhanced heat loss responses induced by short-term endurance training in exercising women. Exp. Physiol. 2009, 94, 90–102. [Google Scholar] [CrossRef]
- Baechle, T.R.; Earle, R.W. Essentials of Strength Training and Conditioning; Human Kinetics: Champaign, IL, USA, 2008. [Google Scholar]
- Morton, R.W.; Oikawa, S.Y.; Wavell, C.G.; Mazara, N.; McGlory, C.; Quadrilatero, J.; Baechler, B.L.; Baker, S.K.; Phillips, S.M. Neither load nor systemic hormones determine resistance training-mediated hypertrophy or strength gains in resistance-trained young men. J. Appl. Physiol. 2016, 121, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, K.; Alen, M.; Kraemer, W.J.; Gorostiaga, E.; Izquierdo, M.; Rusko, H.; Mikkola, J.; Häkkinen, A.; Valkeinen, H.; Kaarakainen, E.; et al. Neuromuscular adaptations during concurrent strength and endurance training versus strength training. Eur. J. Appl. Physiol. 2003, 89, 42–52. [Google Scholar] [CrossRef]
- Cadore, E.L.; Izquierdo, M.; Alberton, C.L.; Pinto, R.S.; Conceição, M.; Cunha, G.; Radaelli, R.; Bottaro, M.; Trindade, G.T.; Kruel, L.F. Strength prior to endurance intra-session exercise sequence optimizes neuromuscular and cardiovascular gains in elderly men. Exp. Gerontol. 2012, 47, 164–169. [Google Scholar] [CrossRef]
- Cantrell, G.S.; Schilling, B.K.; Paquette, M.R.; Murlasits, Z. Maximal strength, power, and aerobic endurance adaptations to concurrent strength and sprint interval training. Eur. J. Appl. Physiol. 2014, 114, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, J.J.; Bartlett, J.D.; Hanson, E.D.; Stepto, N.K.; Bishop, D.J. Endurance Training Intensity Does Not Mediate Interference to Maximal Lower-Body Strength Gain during Short-Term Concurrent Training. Front. Physiol. 2016, 7, 487. [Google Scholar] [CrossRef] [PubMed]
- Terzis, G.; Spengos, K.; Methenitis, S.; Aagaard, P.; Karandreas, N.; Bogdanis, G. Early phase interference between low-intensity running and power training in moderately trained females. Eur. J. Appl. Physiol. 2016, 116, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.W.; Howatson, G.; Russell, M.; French, D.N. Performance and Endocrine Responses to Differing Ratios of Concurrent Strength and Endurance Training. J. Strength Cond. Res. 2016, 30, 693–702. [Google Scholar] [CrossRef]
- Lee, M.J.; Ballantyne, J.K.; Chagolla, J.; Hopkins, W.G.; Fyfe, J.J.; Phillips, S.M.; Bishop, D.J.; Bartlett, J.D. Order of same-day concurrent training influences some indices of power development, but not strength, lean mass, or aerobic fitness in healthy, moderately-active men after 9 weeks of training. PLoS ONE 2020, 15, e0233134. [Google Scholar] [CrossRef]
| Training Group (N = 27) | Control Group (Ν = 7) | ||||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Fat mass (kg) | 17.6 ± 4.2 | 17.9 ± 4.1 | 17.8 ± 2.8 | 18 ± 1.8 | |
| Total LBM (kg) | 40.7 ± 3.8 | 40.9 ± 4.2 | 38.4 ± 2.2 | 37.7 ± 2.4 | |
| LBM of lower extremities (kg) | 13.8 ± 1.7 | 14.5 ± 1.8 *,# | 13.3 ± 1.5 | 12.7 ± 1.4 # | |
| Bone mass density | 1.208 ± 0.07 | 1.212 ± 0.09 | 1.219 ± 0.08 | 1.220 ± 0.06 | |
| VL fascicle length (cm) | 6.6 ± 0.5 | 6.7 ± 0.7 | 6.5 ± 0.4 | 6.5 ± 0.4 | |
| VL fascicle angle (°) | 16.1 ± 2.4 | 18.6 ± 2.2 *,# | 16.1 ± 4.0 | 16.9 ± 4.1 # | |
| VL thickness (cm) | 1.8 ± 0.2 | 2.0 ± 0.2 *,# | 1.8 ± 1.0 | 1.8 ± 1.1 # | |
| CSA Quad (cm2) | 44.7 ± 6.7 | 50.5 ± 7.6 *,# | 44.2 ± 3.4 | 44.5 ± 3.5 # | |
| CSA VL (cm2) | 14.2 ± 2.1 | 16.3 ± 2.1 *,# | 14.1 ± 2.0 | 14.3 ± 2.1 # | |
| CSA RF (cm2) | 4.7 ± 1.1 | 5.5 ± 1.2 *,# | 3.9 ± 0.6 | 3.8 ± 0.6 # | |
| CSA VI (cm2) | 15.9 ± 2.9 | 17.7 ± 3.2 *,# | 16.3 ± 2.1 | 16.6 ± 2.1 # | |
| CSA VM (cm2) | 9.5 ± 1.9 | 10.8 ± 1.8 *,# | 9.6 ± 1.6 | 9.6 ± 1.8 # | |
| CMJ power (W) | 1669 ± 453 | 1681 ± 443 | 1186 ± 300 | 1138 ± 325 | |
| CMJ height (cm) | 19.4 ± 3.7 | 19.6 ± 3.8 | 17.3 ± 1.8 | 16.7 ± 1.7 | |
| Maximal aerobic power (W) | 124.5 ± 22.4 | 148.0 ± 21.2 *,# | 117.2 ± 12.2 | 117.4 ± 12.7 # | |
| Heart rate | 100 W | 153.1 ± 15.3 | 137.2 ± 14.1 *,# | 151.5 ± 8.2 | 155.4 ± 9.3 # |
| 125 W | 166.5 ± 11.3 | 148.2 ± 10.4 *,# | 169.9 ± 11.2 | 173.5 ± 7.7 # | |
| 1-RM leg press (Kg) | 237.9 ± 41.9 | 302.9 ± 37.7 *,# | 220.7 ± 26.3 | 218.5 ± 24.2 # | |
| 1-RM leg press (Kg) | G7 (N = 10) | |||
| T2 | T3 | T4 | T5 | |
| 314 ± 41.4 | 312.5 ± 41.1 | 310.5 ± 40 | 257 ± 43.1 *,‡ | |
| G14 (N = 10) | ||||
| 308 ± 35.7 | 303 ± 30.9 | 293 ± 27.4 *,± | 246 ± 38 *,‡ | |
| GD (N = 7) | ||||
| 280.4 ± 26.4 | 254.2 ± 23.5 *,G7,G14 | 237.1 ± 26.1 *,±,G7,G14 | 215 ± 26.3 *,‡ | |
| G7 (N = 10) | G14 (N = 10) | GD (N = 7) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T2 | T4 | T5 | T2 | T4 | T5 | T2 | T4 | T5 | ||
| Fat mass (kg) | 15.5 ± 2.7 | 14.8 ± 2.9 | 15.6 ± 2.8 | 19.5 ± 4.4 | 19.8 ± 5.1 | 20.5 ± 4.7 | 20.1 ± 3.5 | 19.8 ± 4.4 | 20.7 ± 4.7 | |
| LBM (Kg) | 40.9 ± 3.9 | 40.9 ± 3.9 | 40.1 ± 3.8 | 41.6 ± 4.3 | 41.5 ± 4 | 41.3 ± 4.6 | 39.5 ± 4.8 | 39.3 ± 4.6 | 38.5 ± 4.5 | |
| LBM LE (Kg) | 14.4 ± 1.9 | 14.3 ± 1.9 | 13.9 ± 1.9 *,± | 14.9 ± 1.9 | 14.6 ± 1.7 * | 14.3 ± 1.6 *,± | 14.1 ± 1.6 | 13.5 ± 1.4 * | 13.5 ± 1.5 * | |
| BMD | 1.227 ± 0.1 | 1.230 ± 0.1 | 1.228 ± 0.1 | 1.224 ± 0.1 | 1.226 ± 0.1 | 1.222 ± 0.1 | 1.174 ± 0.7 | 1.175 ± 0.7 | 1.173 ± 0.7 | |
| VL FL (cm) | 6.4 ± 0.9 | 6.6 ± 0.9 | 6.4 ± 0.7 | 6.7 ± 0.6 | 6.8 ± 0.4 | 6.8 ± 0.5 | 6.9 ± 0.3 | 6.8 ± 0.4 | 6.8 ± 0.4 | |
| VL FA (°) | 18.4 ± 1.9 | 18.1 ± 1.7 | 15.7 ± 2.4 *,± | 19.1 ± 2.8 | 17.7 ± 2.7 *,G7 | 16.3 ± 3.2 *,± | 18.4 ± 2.0 | 16.7 ± 1.0 *,G7 | 16.6 ± 1.1 * | |
| VL T (cm) | 2.0 ± 0.2 | 2.0 ± 0.2 | 1.8 ± 0.2 *,± | 2.0 ± 0.2 | 1.9 ± 0.2 *,G7 | 1.8 ± 0.2 *,± | 2.0 ± 0.1 | 1.8 ± 0.1 *,G7 | 1.8 ± 0.1 | |
| QCSA (cm2) | 49.9 ± 8.8 | 49.6 ± 9.2 | 43.8 ± 7.8 *,± | 53.8 ± 6.6 | 50.7 ± 6.6 *,G7 | 47.1 ± 5.7 *,± | 46.6 ± 5.5 | 43.3 ± 6.5 *,G7,G14 | 42.8 ± 6.4 * | |
| CSA VL (cm2) | 16 ± 2.8 | 16 ± 2.9 | 14.1 ± 2.1 *,± | 17.7 ± 2.5 | 16.7 ± 2.4 *,G7 | 15.4 ± 2 *,± | 14.8 ± 1.5 | 13.7 ± 1.6 *,G7 | 13.6 ± 1.5 * | |
| CSA RF (cm2) | 5.3 ± 1.3 | 5.2 ± 1.4 | 4.4 ± 0.9 *,± | 5.7 ± 0.9 | 5.2 ± 0.9 *,G7 | 4.8 ± 0.9 *,± | 5.4 ± 1.7 | 4.9 ± 1.6 *,G7 | 4.9 ± 1.6 * | |
| CSA VI (cm2) | 17.2 ± 3.7 | 17.2 ± 3.7 | 15.5 ± 3.1 *,± | 19.3 ± 2.8 | 18.3 ± 2.2 *,G7 | 17 ± 2.5 *,± | 16.1 ± 2.1 | 15.4 ± 2.5 *,G7 | 15.3 ± 2.5 * | |
| CSA VM (cm2) | 11.1 ± 1.9 | 11 ± 1.9 | 9.7 ± 2.4 *,± | 10.9 ± 2.1 | 10.1 ± 2 *,G7 | 9.7 ± 1.3 * | 10.1 ± 0.7 | 8.8 ± 1.4 * G7 G14 | 8.7 ± 1.4 * | |
| CMJ power (W) | 1892 ± 585 | 1863 ± 461 | 1768 ± 509 | 1675 ± 274 | 1654 ± 294 | 1600 ± 217 | 1388 ± 222 | 1358 ± 232 | 1337 ± 289 | |
| CMJ height (cm) | 22.5 ± 4.1 | 22.9 ± 3.9 | 22.5 ± 4.2 | 18.0 ± 1.8 | 17.8 ± 2.4 | 18.0 ± 1.9 | 17.7 ± 3.1 | 17.9 ± 2.3 | 16.9 ± 1.6 | |
| Maximal AP (W) | 147.5 ± 24.8 | 145.2 ± 22.9 | 125.3 ± 33.3 *,± | 157.5 ± 16.8 | 142.5 ± 12.1 *,G7 | 127.5 ± 18.0 *,± | 135.7 ± 15.0 | 114.2 ± 19.6 *,G7,G14 | 107.5 ± 26.7 * | |
| HR | 100 W | 141.2 ± 13.2 | 146.1 ± 13.8 | 152.5 ± 10.1 *,± | 128.9 ± 16.0 | 142.7 ± 8.2 * | 148.1 ± 14.3 * | 145.1 ± 9.1 | 159.8 ± 9.2 * | 162.8 ± 10.3 * |
| 125 W | 160.2 ± 13.3 | 162.5 ± 12.2 | 167.5 ± 7.5 *,± | 148.4 ± 14.1 | 160.2 ± 8.2 *,G7 | 164.7 ± 6.8 * | 156.3 ± 8.0 | 167.0 ± 8.6 *,G7 | 167.2 ± 4.0 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mpampoulis, T.; Stasinaki, A.N.; Methenitis, S.; Zaras, N.; Bogdanis, G.C.; Terzis, G. Effect of Different Reduced Training Frequencies after 12 Weeks of Concurrent Resistance and Aerobic Training on Muscle Strength and Morphology. Sports 2024, 12, 198. https://doi.org/10.3390/sports12070198
Mpampoulis T, Stasinaki AN, Methenitis S, Zaras N, Bogdanis GC, Terzis G. Effect of Different Reduced Training Frequencies after 12 Weeks of Concurrent Resistance and Aerobic Training on Muscle Strength and Morphology. Sports. 2024; 12(7):198. https://doi.org/10.3390/sports12070198
Chicago/Turabian StyleMpampoulis, Thomas, Angeliki N. Stasinaki, Spyridon Methenitis, Nikolaos Zaras, Gregory C. Bogdanis, and Gerasimos Terzis. 2024. "Effect of Different Reduced Training Frequencies after 12 Weeks of Concurrent Resistance and Aerobic Training on Muscle Strength and Morphology" Sports 12, no. 7: 198. https://doi.org/10.3390/sports12070198









