Effect of Metabolic Stress to High-Load Exercise on Muscle Damage, Inflammatory and Hormonal Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
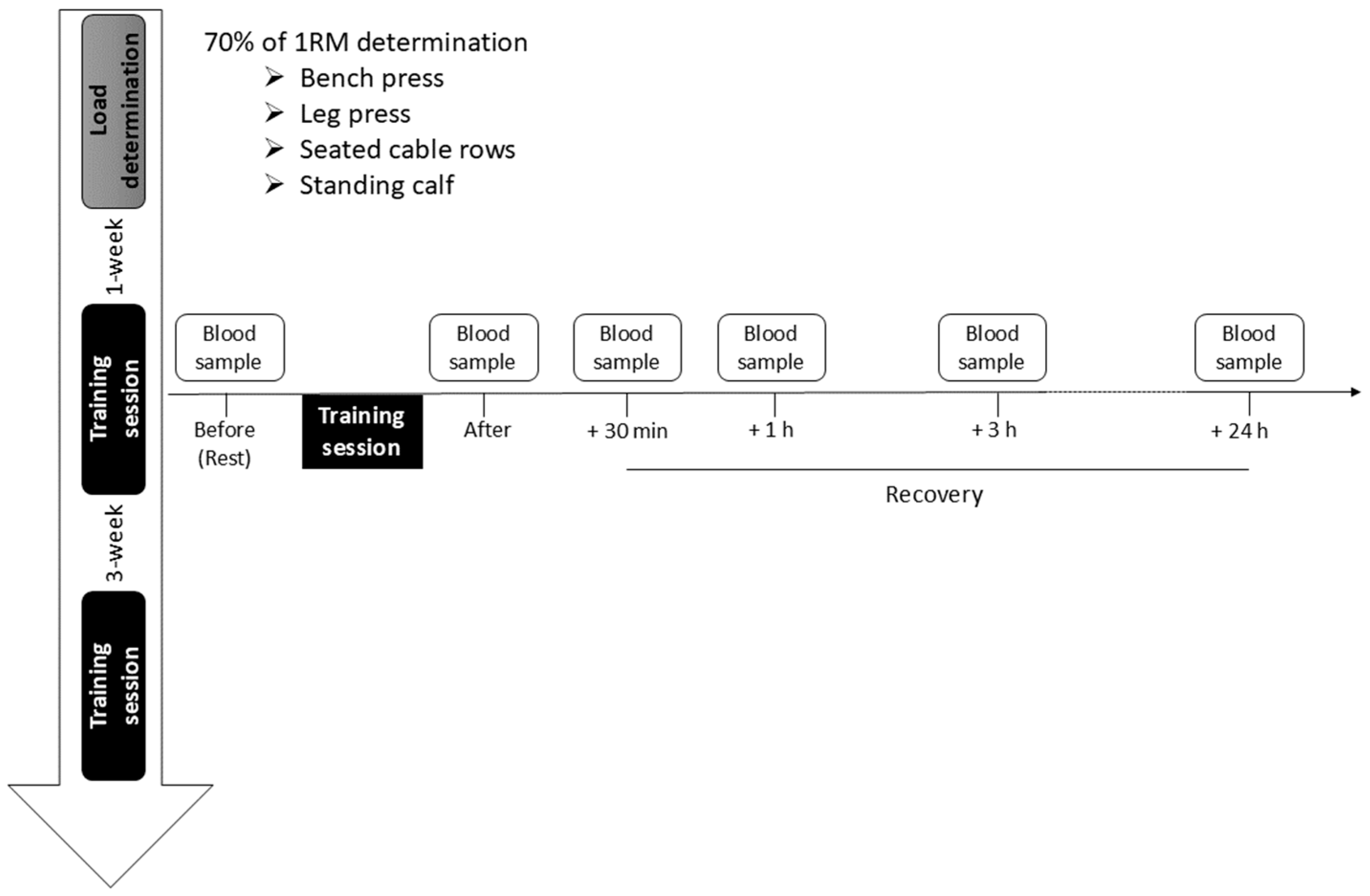
2.2. Experimental Protocol and Determination of the Training Load
2.3. Blood Collection
2.4. Biochemical Analyses
2.5. Statistical Analysis
3. Results
3.1. Workload During Training Session
3.2. Marker of Metabolic Response
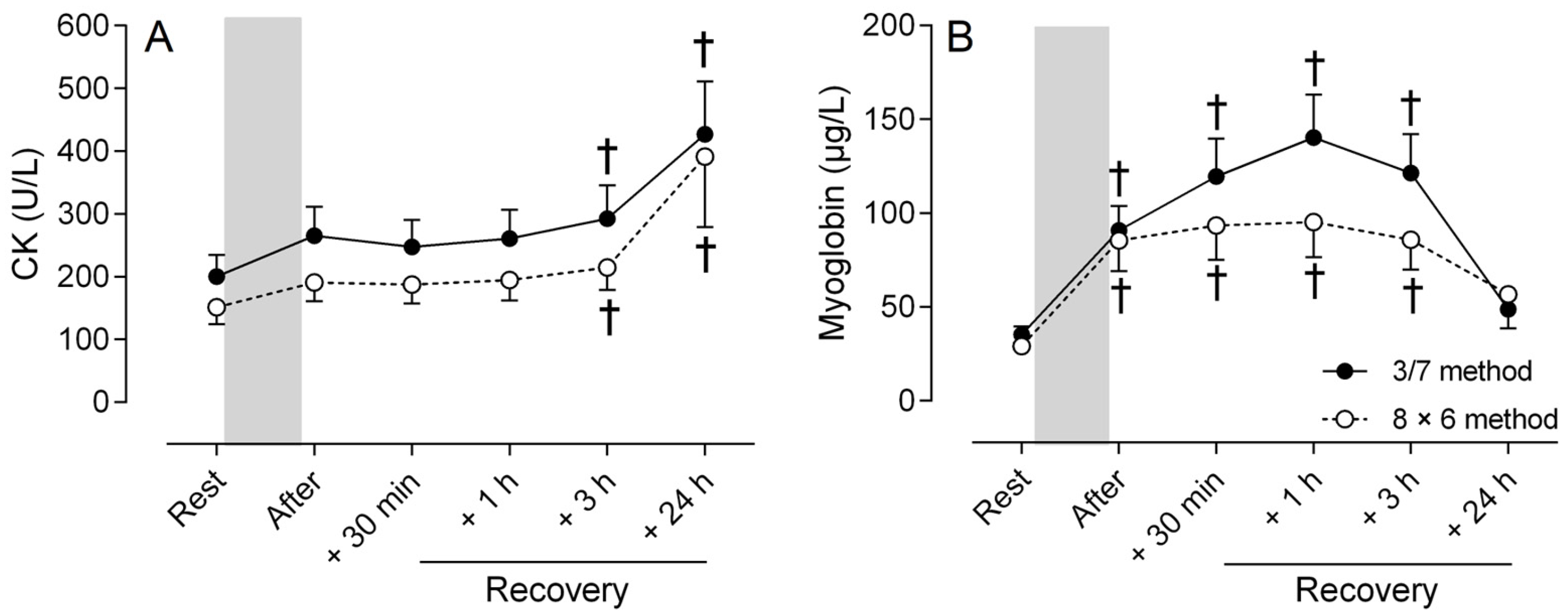
3.3. Markers of Muscle Damage
3.4. Markers of Inflammatory Response
3.5. Markers of Hormonal Responses
3.6. Association Between Biomarkers
4. Discussion
4.1. Workload Volume
4.2. Metabolic Response
4.3. Muscle Damage
4.4. Inflammatory Response
4.5. Hormonal Response
4.6. Potential Influence of Lactate in Muscle Adaptative Responses to Strength Training
4.7. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CK | creatine kinase |
| MB | myoglobin |
| IL-6 | interleukine-6 |
| GH | growth hormone |
| IGF-1 | insulin-like growth factor-1 |
| 1 RM | one repetition maximal |
| H+ | hydrogen ions |
| Pi | inorganic phosphate |
| 3RM | 3-repetitions maximum |
| PCr | phosphocreatine |
| MHC | myosin heavy chain |
| GPR81 | G protein-coupled receptor 81 |
| ERK1/2 | extracellular signal-regulated kinase-1/2 |
| Kla | lysine lactylation |
References
- Schoenfeld, B.J. Potential mechanisms for a role of metabolic stress in hypertrophic adaptations to resistance training. Sports Med. 2013, 43, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, E.T.; Villanueva, M.; West, D.D.W.; Phillips, S.M. Are acute post-resistance exercise increases in testosterone, growth hormone, and IGF-1 necessary to stimulate skeletal muscle anabolism and hypertrophy? Med. Sci. Sports Exerc. 2013, 45, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Duchateau, J.; Stragier, S.; Baudry, S.; Carpentier, A. Strength Training: In Search of Optimal Strategies to Maximize Neuromuscular Performance. Exerc. Sport Sci. Rev. 2021, 49, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Lawson, D.; Vann, C.; Schoenfeld, B.J.; Haun, C. Beyond Mechanical Tension: A Review of Resistance Exercise-Induced Lactate Responses & Muscle Hypertrophy. J. Funct. Morphol. Kinesiol. 2022, 7, 81. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005, 35, 339–361. [Google Scholar] [CrossRef]
- Vingren, J.L.; Kraemer, W.J.; Ratamess, N.A.; Anderson, J.M.; Volek, J.S.; Maresh, C.M. Testosterone physiology in resistance exercise and training: The up-stream regulatory elements. Sports Med. 2010, 40, 1037–1053. [Google Scholar] [CrossRef]
- Takarada, Y.; Sato, Y.; Ishii, N. Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur. J. Appl. Physiol. 2002, 86, 308–314. [Google Scholar] [CrossRef]
- Manini, T.M.; Clark, B.C. Blood flow restricted exercise and skeletal muscle health. Exerc. Sport Sci. Rev. 2009, 37, 78–85. [Google Scholar] [CrossRef]
- Lixandrão, M.E.; Ugrinowitsch, C.; Berton, R.; Vechin, F.C.; Conceiçao, M.S.; Damas, F.; Libardi, C.A.; Roschel, H. Magnitude of muscle strength and mass adaptations between high-load resistance training versus low-load resistance training associated with blood-flow restriction: A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 361–378. [Google Scholar] [CrossRef]
- Grønfeldt, B.M.; Lindberg Nielsen, J.; Mieritz, R.M.; Lund, H.; Aagaard, P. Effect of blood-flow restricted vs heavy-load strength training on muscle strength: Systematic review and meta-analysis. Scand. J. Med. Sci. Sports 2020, 30, 837–848. [Google Scholar] [CrossRef]
- McMahon, S.; Jenkins, D. Factors affecting the rate of phosphocreatine resynthesis following intense exercise. Sports Med. 2002, 32, 761–784. [Google Scholar] [CrossRef] [PubMed]
- Wernbom, M.; Aagaard, P. Muscle fibre activation and fatigue with low-load blood flow restricted resistance exercise-An integrative physiology review. Acta Physiol. 2020, 228, e13302. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, M.; Takeda, M. Lactate as a signaling molecule that regulates exercise-induced adaptations. Biology 2016, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, M.; Radak, Z.; Takeda, M. Lactate metabolism and satellite cell fate. Front. Physiol. 2020, 11, 610983. [Google Scholar] [CrossRef]
- Markus, I.; Constantini, K.; Hoffman, J.R.; Bartolomei, S.; Gepner, Y. Exercise-induced muscle damage: Mechanism, assessment and nutritional factors to accelerate recovery. Eur. J. Appl. Physiol. 2021, 121, 969–992. [Google Scholar] [CrossRef]
- Peake, J.; Nosaka, K.; Suzuki, K. Characterization of inflammatory responses to eccentric exercise in humans. Exerc. Immunol. Rev. 2005, 11, 64–85. [Google Scholar]
- Rossi, F.E.; Gerosa-Neto, J.; Zanchi, N.E.; Cholewa, J.M.; Lira, F.S. Impact of short and moderate rest intervals on the acute immunometabolic response to exhaustive strength exercise: Part I. J. Strength Cond. Res. 2016, 30, 1563–1569. [Google Scholar] [CrossRef]
- Rowbottom, D.G.; Green, K.J. Acute exercise effects on the immune system. Med. Sci. Sports Exerc. 2000, 32, S396–S405. [Google Scholar] [CrossRef]
- Smith, L.L. Tissue trauma: The underlying cause of overtraining syndrome? J. Strength Cond. Res. 2004, 18, 185–193. [Google Scholar] [CrossRef]
- Tidball, J.G. Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 2017, 17, 165–178. [Google Scholar] [CrossRef]
- Hojman, P.; Brolin, C.; Nørgaard-Christensen, N.; Dethlefsen, C.; Lauenborg, B.; Olsen, C.K.; Abom, M.M.; Krag, T.; Gehl, J.; Pedersen, B.K. IL-6 release from muscles during exercise is stimulated by lactate-dependent protease activity. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E940–E947. [Google Scholar] [CrossRef] [PubMed]
- Britto, F.A.; Gnimassou, O.; De Groote, E.; Balan, E.; Warnier, G.; Everard, A.; Cani, P.D.; Deldicque, L. Acute environmental hypoxia potentiates satellite cell-dependent myogenesis in response to resistance exercise through the inflammation pathway in human. FASEB J. 2020, 34, 1885–1900. [Google Scholar] [CrossRef]
- Benavente, C.; León, J.; Feriche, B.; Schoenfeld, B.J.; Bonitch-Gongora, J.; Almeida, F.; Pérez-Regalado, S.; Padial, P. Hormonal and inflammatory responses to hypertrophy-oriented resistance training at acute moderate altitude. Int. J. Environ. Res. Public Health 2021, 18, 4233. [Google Scholar] [CrossRef]
- Pearson, S.J.; Hussain, S.R. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2015, 45, 187–200. [Google Scholar] [CrossRef]
- Laurent, C.; Penzer, F.; Letroye, B.; Carpentier, A.; Baudry, S.; Duchateau, J. Effect of a strength training method characterized by an incremental number of repetitions across sets and a very short rest interval. Sci. Sports 2016, 31, e115–e121. [Google Scholar] [CrossRef]
- Stragier, S.; Baudry, S.; Carpentier, A.; Duchateau, J. Efficacy of a new strength training design: The 3/7 method. Eur. J. Appl. Physiol. 2019, 119, 1093–1104. [Google Scholar] [CrossRef]
- Penzer, F.; Cabrol, A.; Baudry, S.; Duchateau, J. Comparison of muscle activity and tissue oxygenation during strength training protocols that differ by their organisation, rest interval between sets, and volume. Eur. J. Appl. Physiol. 2016, 116, 1795–1806. [Google Scholar] [CrossRef]
- Brechue, W.F.; Mayhew, J.L. Upper-body work capacity and 1RM prediction are unaltered by increasing muscular strength in college football players. J. Strength Cond. Res. 2009, 23, 2477–2486. [Google Scholar] [CrossRef]
- Cole, T.J.; Altman, D.G. Statistics Notes: Percentage differences, symmetry, and natural logarithms. BMJ 2017, 358, j3683. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Marchitelli, L.; Gordon, S.E.; Harman, E.; Dziados, J.E.; Mello, R.; Frykman, P.; McCurry, D.; Fleck, S.J. Hormonal and growth factor responses to heavy resistance exercise protocols. J. Appl. Physiol. 1990, 69, 1442–1450. [Google Scholar] [CrossRef]
- Goto, K.; Ishii, N.; Kizuka, T.; Takamatsu, K. The impact of metabolic stress on hormonal responses and muscular adaptations. Med. Sci. Sports Exerc. 2005, 37, 955–963. [Google Scholar] [PubMed]
- Ohno, Y.; Nakatani, M.; Matsui, Y.; Suda, Y.; Ito, T.; Ando, K.; Yokoyama, S.; Goto, K. Effect of oral lactate administration on skeletal muscle mass in mice under different loading conditions. In Vivo 2025, 39, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Dankel, S.J.; Mattocks, K.T.; Jessee, M.B.; Buckner, S.L.; Mouser, J.G.; Loenneke, J.P. Do metabolites that are produced during resistance exercise enhance muscle hypertrophy? Eur. J. Appl. Physiol. 2017, 117, 2125–2135. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Nosaka, K.; Braun, B. Muscle function after exercise-induced muscle damage and rapid adaptation. Med. Sci. Sports Exerc. 1992, 24, 512–520. [Google Scholar] [CrossRef]
- Nosaka, K.; Newton, M. Concentric or eccentric training effect on eccentric exercise-induced muscle damage. Med. Sci. Sports Exerc. 2002, 34, 63–69. [Google Scholar] [CrossRef]
- Vieillevoye, S.; Poortmans, J.R.; Carpentier, A. Effects of essential amino acids supplementation on muscle damage following a heavy-load eccentric training session. Sci. Sports 2019, 35, e125–e134. [Google Scholar] [CrossRef]
- Lippi, G.; Schena, F.; Salvagno, G.L.; Montagnana, M.; Gelati, M.; Tarperi, C.; Banfi, G.; Guidi, G.C. Acute variation of biochemical markers of muscle damage following a 21-km, half-marathon run. Scand. J. Clin. Lab. Investig. 2008, 68, 667–672. [Google Scholar] [CrossRef]
- Spiering, B.A.; Kraemer, W.J.; Anderson, J.M.; Amstrong, L.E.; Nindl, B.C.; Volek, J.S.; Maresh, C.M. Resistance exercise biology: Manipulation of resistance exercise programme variables determines the responses of cellular and molecular signalling pathways. Sports Med. 2008, 38, 527–540. [Google Scholar] [CrossRef]
- Egner, I.M.; Bruusgaard, J.C.; Gundersen, K. Satellite cell depletion prevents fiber hypertrophy in skeletal muscle. Development 2016, 143, 2898–2906. [Google Scholar] [CrossRef]
- Koch, A.J. Immune response to resistance exercise. Am. J. Lifestyle Med. 2010, 4, 244–252. [Google Scholar] [CrossRef]
- Starkie, R.; Ostrowski, S.R.; Jauffred, S.; Febbraio, M.; Pedersen, B.K. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003, 17, 884–886. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.P. Interleukin-6 in acute exercise and training: What is the biological relevance? Exerc. Immunol. Rev. 2006, 12, 6–33. [Google Scholar] [PubMed]
- Febbraio, M.A.; Pedersen, B.K. Contraction-induced myokine production and release: Is skeletal muscle an endocrine organ? Exerc. Sport Sci. Rev. 2005, 33, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Bottaro, M.; Martins, B.; Gentil, P.; Wagner, D. Effects of rest duration between sets of resistance training on acute hormonal responses in trained women. J. Sci. Med. Sport 2009, 12, 73–78. [Google Scholar] [CrossRef]
- Pierce, J.R.; Clark, B.C.; Ploutz-Snyder, L.L.; Kanaley, J.A. Growth hormone and muscle function responses to skeletal muscle ischemia. J. Appl. Physiol. 2006, 101, 1588–1595. [Google Scholar] [CrossRef]
- Sotiropoulos, A.; Ohanna, M.; Kedzia, C.; Menon, R.K.; Kopchick, J.J.; Kelly, P.A.; Pende, M. Growth hormone promotes skeletal muscle cell fusion independent of insulin-like growth factor 1 up-regulation. Proc. Natl. Acad. Sci. USA 2006, 103, 7315–7320. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Dunn-Lewis, C.; Comstock, B.A.; Thomas, G.A.; Clark, J.E.; Nindl, B.C. Growth hormone, exercise, and athletic performance: A continued evolution of complexity. Curr. Sports Med. Rep. 2010, 9, 242–252. [Google Scholar] [CrossRef]
- Gregory, S.M.; Spiering, B.A.; Alemany, J.A.; Tuckow, A.P.; Rarick, K.R.; Staab, J.S.; Hatfield, D.L.; Kraemer, W.J.; Maresh, C.M.; Nindl, B.C. Exercise-induced insulin-like growth factor I system concentrations after training in women. Med. Sci. Sports Exerc. 2013, 45, 420–428. [Google Scholar] [CrossRef]
- Torres, R.; Koutakis, P.; Forsse, J. The effects of different exercise intensities and modalities on cortisol production in healthy individuals: A Review. J. Exerc. Nutr. 2021, 4, 19. [Google Scholar] [CrossRef]
- Hackney, A.C.; Walz, E.A. Hormonal adaptation and the stress of exercise training: The role of glucocorticoids. Trends Sport Sci. 2013, 20, 165–171. [Google Scholar] [PubMed]
- Debono, M.; Ghobadi, C.; Rostami-Hodjegan, A.; Huatan, H.; Campbell, M.J.; Newell-Price, J.; Darzy, K.; Merke, D.P.; Arlt, W.; Ross, R.J. Modified-release hydrocortisone to provide circadian cortisol profiles. J. Clin. Endocrinol. Metab. 2009, 94, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Shibasaki, A.; Naka, A.; Saito, H.; Iida, K. Lactate promotes myoblast differentiation and myotube hypertrophy via a pathway involving MyoD in vitro and enhances muscle regeneration in vivo. Int. J. Mol. Sci. 2018, 19, 3649. [Google Scholar] [CrossRef]
- Ohno, Y.; Nakatani, M.; Ito, T.; Matsui, Y.; Ando, K.; Suda, Y.; Ohashi, K.; Yokoyama, S.; Goto, K. Activation of lactate receptor positively regulates skeletal muscle mass in mice. Physiol. Res. 2023, 72, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.L.; Baeza-Raja, B.; Perdiguero, E.; Jardí, M.; Muñoz-Cánoves, P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008, 7, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Wang, Q.; Li, X.; Guo, Y. Ubiquitous protein lactylation in health and diseases. Cell. Mol. Biol. Lett. 2024, 29, 23. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Zhao, Y.; Du, J.; Wang, S.; Yang, X.; Li, W.; Song, J.; Zhang, S.; Zhang, Z.; Tan, Y.; et al. Exercise improves cognitive dysfunction and neuroinflammation in mice through histone H3 lactylation in microglia. Immun. Ageing 2023, 20, 63. [Google Scholar] [CrossRef]
- Baumert, P.; Lake, M.; Stewart, C.E.; Drust, B.; Erskine, R.M. Genetic variation and exercise-induced muscle damage: Implications for athletic performance, injury and ageing. Eur. J. Appl. Physiol. 2016, 116, 1595–1625. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stragier, S.; Duchateau, J.; Cotton, F.; Smet, J.; Wolff, F.; Tresnie, J.; Carpentier, A. Effect of Metabolic Stress to High-Load Exercise on Muscle Damage, Inflammatory and Hormonal Responses. Sports 2025, 13, 111. https://doi.org/10.3390/sports13040111
Stragier S, Duchateau J, Cotton F, Smet J, Wolff F, Tresnie J, Carpentier A. Effect of Metabolic Stress to High-Load Exercise on Muscle Damage, Inflammatory and Hormonal Responses. Sports. 2025; 13(4):111. https://doi.org/10.3390/sports13040111
Chicago/Turabian StyleStragier, Séverine, Jacques Duchateau, Frédéric Cotton, Julie Smet, Fleur Wolff, Jérémy Tresnie, and Alain Carpentier. 2025. "Effect of Metabolic Stress to High-Load Exercise on Muscle Damage, Inflammatory and Hormonal Responses" Sports 13, no. 4: 111. https://doi.org/10.3390/sports13040111
APA StyleStragier, S., Duchateau, J., Cotton, F., Smet, J., Wolff, F., Tresnie, J., & Carpentier, A. (2025). Effect of Metabolic Stress to High-Load Exercise on Muscle Damage, Inflammatory and Hormonal Responses. Sports, 13(4), 111. https://doi.org/10.3390/sports13040111






