The Impact of Modified Tabata Training on Segmental Fat Accumulation, Muscle Mass, Muscle Thickness, and Physical and Cardiorespiratory Fitness in Overweight and Obese Participants: A Randomized Control Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design: Randomized and Non-Blinded
2.2. Screening of Participants
2.3. Sample Size
2.4. Tabata Training
2.5. Study End Points
2.5.1. Body Composition and Vital Signs
2.5.2. Muscle Thickness
2.5.3. Physical Fitness
2.5.4. Cardiorespiratory Fitness
2.6. Statistical Analysis
3. Results
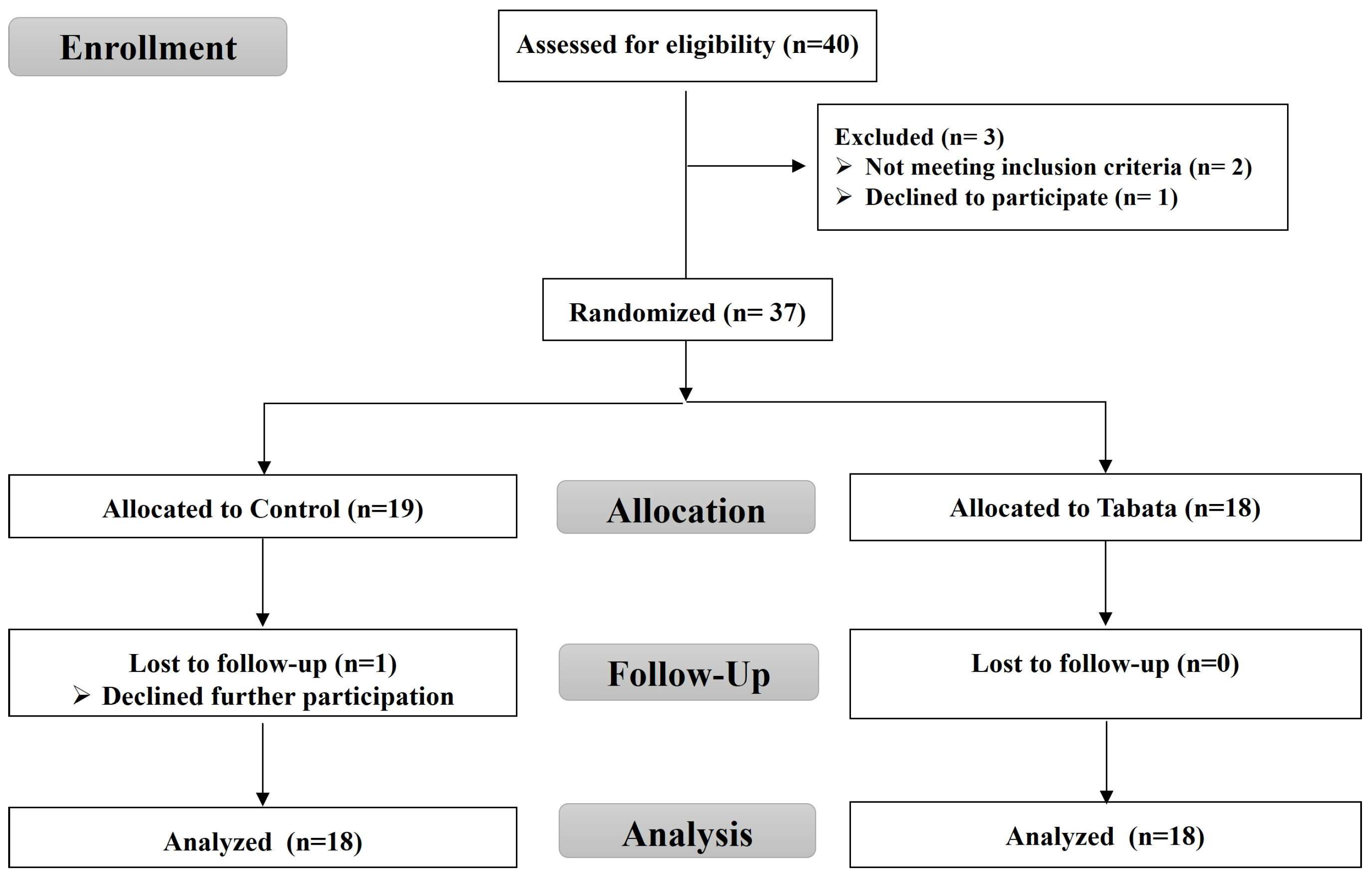
3.1. Participant Characteristics and Feasibility
3.2. Impact on Body Composition
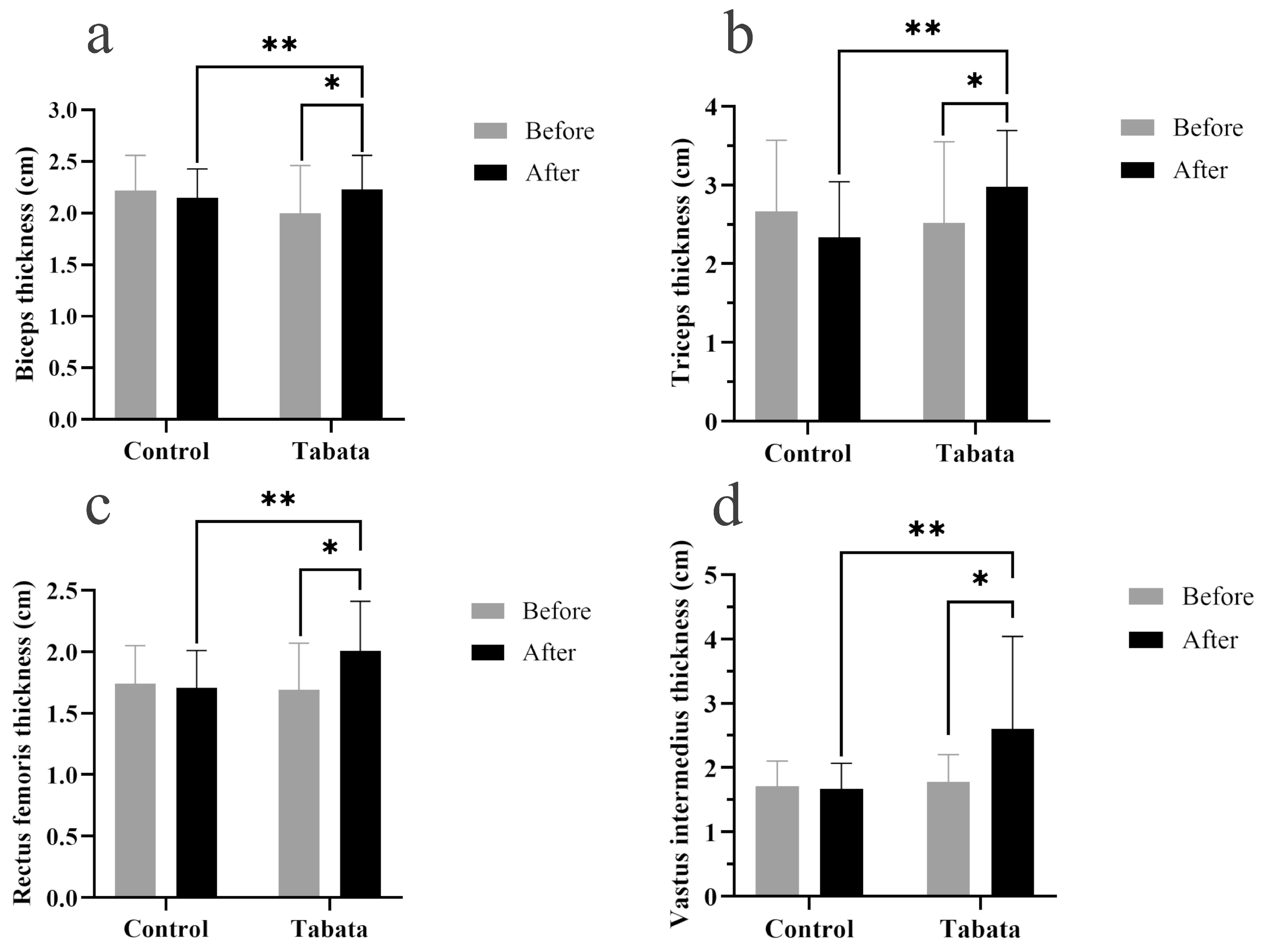
3.3. Impact on Muscle Thickness
3.4. Impact on Physical Fitness
3.5. Impact on Cardiorespiratory Fitness
4. Discussion
4.1. Modified Tabata Training Improves Body Proportions
4.2. Modified Tabata Training Improves Muscle Thickness
4.3. Modified Tabata Training Improves Muscle Strength and Endurance
4.4. Modified Tabata Training Improves Cardiorespiratory Fitness
4.5. Limitations of Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BB | Biceps muscle |
| BF | Body fat |
| BMI | Body mass index |
| BMR | Basal metabolic rate |
| BW | Body weight |
| DBP | Diastolic blood pressure |
| ES | Effect size |
| FFM | Fat-free mass |
| FMs | Fat mass |
| FM | Femur |
| HM | Humerus |
| HIIT | High-intensity intermittent training |
| HR | Heart rate |
| HRmax | Maximal heart rate |
| MAP | Mean arterial pressure |
| MM | Muscle mass |
| NEAT | Non-exercise activity thermogenesis |
| REP | Rating of perceived exertion |
| RER | Respiratory exchange ratio |
| RF | Rectus femoris muscle |
| RR | Respiratory rate |
| SBP | Systolic blood pressure |
| SpO2 | Partial oxygen saturation |
| TC | Triceps muscle |
| VCO2 | Carbon dioxide production |
| VI | Vastus intermedius muscle |
| VO2 | Oxygen uptake |
| VO2max | Maximal oxygen consumption |
| VT | Ventilatory threshold |
| WHO | World Health Organization |
| WHR | Waist–hip ratio |
References
- Murray, C.J.L.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Okunogbe, A.; Nugent, R.; Spencer, G.; Powis, J.; Ralston, J.; Wilding, J. Economic impacts of overweight and obesity: Current and future estimates for 161 countries. BMJ Glob. Health 2022, 7, e009773. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Sundararajan, E.A.; Driss, M.; Boulila, W.; Shapi’i, A. A systematic literature review on obesity: Understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput. Biol. Med. 2021, 136, 104754. [Google Scholar] [CrossRef]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Gerdes, M.W.; Martinez, S.G. Identification of Risk Factors Associated with Obesity and Overweight—A Machine Learning Overview. Sensors 2020, 20, 2734. [Google Scholar] [CrossRef]
- Jensen, M.; Ryan, D.; Donato, K.; Apovian, C.; Ard, J.; Comuzzie, A.; Hu, F.; Hubbard, V.; Jakicic, J.; Kushner, R.; et al. Expert Panel Report: Guidelines (2013) for the management of overweight and obesity in adults. Obesity 2014, 22 (Suppl. S2), S41–S410. [Google Scholar] [CrossRef]
- American College of Sports Medicine; Liguori, G.; Feito, Y.; Fountaine, C.; Roy, B.A. ACSM’s Guidelines for Exercise Testing and Prescription, 11th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2022. [Google Scholar]
- Tschakert, G.; Hofmann, P. High-Intensity Intermittent Exercise: Methodological and Physiological Aspects. Int. J. Sports Physiol. Perform. 2013, 8, 600–610. [Google Scholar] [CrossRef]
- Boutcher, S.H. High-intensity intermittent exercise and fat loss. J. Obes. 2011, 2011, 868305. [Google Scholar] [CrossRef]
- Cooper, S.B.; Dring, K.J.; Nevill, M.E. High-Intensity Intermittent Exercise: Effect on Young People’s Cardiometabolic Health and Cognition. Curr. Sports. Med. Rep. 2016, 15, 245–251. [Google Scholar] [CrossRef]
- Cassidy, S.; Thoma, C.; Hallsworth, K.; Parikh, J.; Hollingsworth, K.G.; Taylor, R.; Jakovljevic, D.G.; Trenell, M.I. High intensity intermittent exercise improves cardiac structure and function and reduces liver fat in patients with type 2 diabetes: A randomised controlled trial. Diabetologia 2016, 59, 56–66. [Google Scholar] [CrossRef]
- Murawska-Cialowicz, E.; Wolanski, P.; Zuwala-Jagiello, J.; Feito, Y.; Petr, M.; Kokstejn, J.; Stastny, P.; Goliński, D. Effect of HIIT with Tabata Protocol on Serum Irisin, Physical Performance, and Body Composition in Men. Int. J. Environ. Res. Public Health 2020, 17, 3589. [Google Scholar] [CrossRef] [PubMed]
- Tabata, I. Tabata training: One of the most energetically effective high-intensity intermittent training methods. J. Physiol. Sci. 2019, 69, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Tabata, I.; Nishimura, K.; Kouzaki, M.; Hirai, Y.; Ogita, F.; Miyachi, M.; Yamamoto, K. Effects of moderate-intensity endurance and high-intensity intermittent training on anaerobic capacity and VO2max. Med. Sci. Sports Exerc. 1996, 28, 1327–1330. [Google Scholar] [CrossRef] [PubMed]
- Tabata, I.; Irisawa, K.; Kouzaki, M.; Nishimura, K.; Ogita, F.; Miyachi, M. Metabolic profile of high intensity intermittent exercises. Med. Sci. Sports Exerc. 1997, 29, 390–395. [Google Scholar] [CrossRef]
- Viana, R.B.; de Lira, C.A.B.; Naves, J.P.A.; Coswig, V.S.; Del Vecchio, F.B.; Gentil, P. Tabata protocol: A review of its application, variations and outcomes. Clin. Physiol. Funct. Imaging 2019, 39, 1–8. [Google Scholar] [CrossRef]
- Miyamoto-Mikami, E.; Tsuji, K.; Horii, N.; Hasegawa, N.; Fujie, S.; Homma, T.; Uchida, M.; Hamaoka, T.; Kanehisa, H.; Tabata, I.; et al. Gene expression profile of muscle adaptation to high-intensity intermittent exercise training in young men. Sci. Rep. 2018, 8, 16811. [Google Scholar] [CrossRef]
- Chuensiri, N.; Suksom, D.; Tanaka, H. Effects of High-Intensity Intermittent Training on Vascular Function in Obese Preadolescent Boys. Child. Obes. 2018, 14, 41–49. [Google Scholar] [CrossRef]
- Logan, G.R.; Harris, N.; Duncan, S.; Plank, L.D.; Merien, F.; Schofield, G. Low-Active Male Adolescents: A Dose Response to High-Intensity Interval Training. Med. Sci. Sports Exerc. 2016, 48, 481–490. [Google Scholar] [CrossRef]
- Ekkekakis, P.; Hall, E.E.; Petruzzello, S.J. The relationship between exercise intensity and affective responses demystified: To crack the 40-year-old nut, replace the 40-year-old nutcracker! Ann. Behav. Med. 2008, 35, 136–149. [Google Scholar] [CrossRef]
- World Health Organization; Regional Office for the Western Pacific. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; Health Communications Australia: Sydney, NSW, Australia, 2000. [Google Scholar]
- Jalayondeja, C.; Jalayondeja, W.; Vachalathiti, R.; Bovonsunthonchai, S.; Sakulsriprasert, P.; Kaewkhuntee, W.; Bunprajun, T.; Upiriyasakul, R. Cross-Cultural Adaptation of the Compendium of Physical Activity: Thai Translation and Content Validity. J. Med. Assoc. Thail. 2015, 98 (Suppl. S5), S53–S59. [Google Scholar]
- Baecke, J.A.; Burema, J.; Frijters, J.E. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am. J. Clin. Nutr. 1982, 36, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Triyulianti, S.P.A.; Utami, R. The Effect of High Intensity Interval Training on Maximal Oxygen Uptake (VO2max) in Overweight Adolescents. Int. J. Aging Health Mov. 2023, 5, 21–28. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Perceived exertion as an indicator of somatic stress. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar] [CrossRef]
- Czartoryski, P.; Garcia, J.; Manimaleth, R.; Napolitano, P.; Weber, H.W.C.; Alvarez-Beaton, A.; Nieto, A.C.; Patel, A.; Peacock, C.; Banks, J.; et al. Body Composition Assessment: A Comparison of the DXA, InBody 270, and Omron. J. Exerc. Nutr. 2020, 3, 1–6. [Google Scholar]
- Hentzen, J.; van Wijk, L.; Buis, C.; Viddeleer, A.; Bock, G.; van der Schans, C.; Dam, G.; Kruijff, S.; Klaase, J. Impact and risk factors for clinically relevant surgery-related muscle loss in patients after major abdominal cancer surgery: Study protocol for a prospective observational cohort study (MUSCLE POWER). Int. J. Clin. Trials 2019, 6, 138. [Google Scholar] [CrossRef]
- Spineti, J.; Figueiredo, T.; Miranda, H.; de Salles, B.; Oliveira, L.; Simão, R. The effects of exercise order and periodized resistance training on maximum strength and muscle thickness. Int. Sport. J. 2014, 15, 374–390. [Google Scholar]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]
- Bruce, R.A. Exercise testing of patients with coronary heart disease. Principles and normal standards for evaluation. Ann. Clin. Res. 1971, 3, 323–332. [Google Scholar]
- Sanca-Valeriano, S.; Espinola-Sánchez, M.; Caballero-Alvarado, J.; Canelo-Aybar, C. Effect of high-intensity interval training compared to moderate-intensity continuous training on body composition and insulin sensitivity in overweight and obese adults: A systematic review and meta-analysis. Heliyon 2023, 9, e20402. [Google Scholar] [CrossRef]
- D’Amuri, A.; Sanz, J.M.; Capatti, E.; Di Vece, F.; Vaccari, F.; Lazzer, S.; Zuliani, G.; Dalla Nora, E.; Passaro, A. Effectiveness of high-intensity interval training for weight loss in adults with obesity: A randomised controlled non-inferiority trial. BMJ Open Sport Exerc. Med. 2021, 7, e001021. [Google Scholar] [CrossRef]
- Airin, S.; Linoby, A.; Mohamad Zaki, M.S.; Baki, H.; Sariman, H.; Esham, B.; Mohd Azam, M.Z.; Mohamed, M.N. The Effects of High-Intensity Interval Training and Continuous Training on Weight Loss and Body Composition in Overweight Females. In Proceedings of the International Colloquium on Sports Science, Exercise, Engineering and Technology 2014 (ICoSSEET 2014), Singapore, 7–9 April 2014; pp. 401–409. [Google Scholar]
- Türk, Y.; Theel, W.; Kasteleyn, M.J.; Franssen, F.M.E.; Hiemstra, P.S.; Rudolphus, A.; Taube, C.; Braunstahl, G.J. High intensity training in obesity: A Meta-analysis. Obes. Sci. Pract. 2017, 3, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Wewege, M.; van den Berg, R.; Ward, R.E.; Keech, A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: A systematic review and meta-analysis. Obes. Rev. 2017, 18, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Ardavani, A.; Aziz, H.; Smith, K.; Atherton, P.J.; Phillips, B.E.; Idris, I. The Effects of Very Low Energy Diets and Low Energy Diets with Exercise Training on Skeletal Muscle Mass: A Narrative Review. Adv. Ther. 2021, 38, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Astorino, T.A.; Schubert, M.M.; Palumbo, E.; Stirling, D.; McMillan, D.W. Effect of two doses of interval training on maximal fat oxidation in sedentary women. Med. Sci. Sports Exerc. 2013, 45, 1878–1886. [Google Scholar] [CrossRef]
- Tjønna, A.E.; Stølen, T.O.; Bye, A.; Volden, M.; Slørdahl, S.A.; Ødegård, R.; Skogvoll, E.; Wisløff, U. Aerobic interval training reduces cardiovascular risk factors more than a multitreatment approach in overweight adolescents. Clin. Sci. 2009, 116, 317–326. [Google Scholar] [CrossRef]
- King, N.A.; Caudwell, P.; Hopkins, M.; Byrne, N.M.; Colley, R.; Hills, A.P.; Stubbs, J.R.; Blundell, J.E. Metabolic and behavioral compensatory responses to exercise interventions: Barriers to weight loss. Obesity 2007, 15, 1373–1383. [Google Scholar] [CrossRef]
- Melanson, E.L.; Keadle, S.K.; Donnelly, J.E.; Braun, B.; King, N.A. Resistance to exercise-induced weight loss: Compensatory behavioral adaptations. Med. Sci. Sports Exerc. 2013, 45, 1600. [Google Scholar] [CrossRef]
- Blue, M.N.; Smith-Ryan, A.E.; Trexler, E.T.; Hirsch, K.R. The effects of high intensity interval training on muscle size and quality in overweight and obese adults. J. Sci. Med. Sport 2018, 21, 207–212. [Google Scholar] [CrossRef]
- Abe, T.; DeHoyos, D.V.; Pollock, M.L.; Garzarella, L. Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. Eur. J. Appl. Physiol. 2000, 81, 174–180. [Google Scholar] [CrossRef]
- Wideman, L.; Weltman, J.; Hartman, M.; Johannes, D.; Weltman, A. Growth Hormone Release During Acute and Chronic Aerobic and Resistance Exercise. Sports Med. 2002, 32, 987–1004. [Google Scholar] [CrossRef]
- Francois, M.E.; Little, J.P. Effectiveness and safety of high-intensity interval training in patients with type 2 diabetes. Diabetes Spectr. 2015, 28, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, W.; Xirouchaki, C.E.; El-Gilany, A.-H. The Comparative Effects of High-Intensity Interval Training and Traditional Resistance Training on Hormonal Responses in Young Women: A 10-Week Intervention Study. Sports 2025, 13, 67. [Google Scholar] [CrossRef]
- Zaroni, R.S.; Brigatto, F.A.; Schoenfeld, B.J.; Braz, T.V.; Benvenutti, J.C.; Germano, M.D.; Marchetti, P.H.; Aoki, M.S.; Lopes, C.R. High Resistance-Training Frequency Enhances Muscle Thickness in Resistance-Trained Men. J. Strength Cond. Res. 2019, 33 (Suppl. S1), S140–S151. [Google Scholar] [CrossRef] [PubMed]
- McRae, G.; Payne, A.; Zelt, J.G.; Scribbans, T.D.; Jung, M.E.; Little, J.P.; Gurd, B.J. Extremely low volume, whole-body aerobic-resistance training improves aerobic fitness and muscular endurance in females. Appl. Physiol. Nutr. Metab. 2012, 37, 1124–1131. [Google Scholar] [CrossRef]
- Caparrós-Manosalva, C.; Garrido-Muñoz, N.; Alvear-Constanzo, B.; Sanzana-Laurié, S.; Artigas-Arias, M.; Alegría-Molina, A.; Vidal-Seguel, N.; Espinoza-Araneda, J.; Huard, N.; Pagnussat, A.S.; et al. Effects of high-intensity interval training on lean mass, strength, and power of the lower limbs in healthy old and young people. Front. Physiol. 2023, 14, 1223069. [Google Scholar] [CrossRef]
- Laursen, P.B.; Jenkins, D.G. The scientific basis for high-intensity interval training: Optimising training programmes and maximising performance in highly trained endurance athletes. Sports Med. 2002, 32, 53–73. [Google Scholar] [CrossRef]
- Menz, V.; Marterer, N.; Amin, S.B.; Faulhaber, M.; Hansen, A.B.; Lawley, J.S. Functional vs. Running low-volume high-intensity interval training: Effects on vo2max and muscular endurance. J. Sports Sci. Med. 2019, 18, 497. [Google Scholar]
- Gibala, M.J.; McGee, S.L. Metabolic adaptations to short-term high-intensity interval training: A little pain for a lot of gain? Exerc. Sport Sci. Rev. 2008, 36, 58–63. [Google Scholar] [CrossRef]
- Rohmansyah, N.A.; Ka Praja, R.; Phanpheng, Y.; Hiruntrakul, A. High-Intensity Interval Training Versus Moderate-Intensity Continuous Training for Improving Physical Health in Elderly Women. Inquiry 2023, 60, 1–13. [Google Scholar] [CrossRef]
- Tjønna, A.E.; Lee, S.J.; Rognmo, Ø.; Stølen, T.O.; Bye, A.; Haram, P.M.; Loennechen, J.P.; Al-Share, Q.Y.; Skogvoll, E.; Slørdahl, S.A.; et al. Aerobic Interval Training Versus Continuous Moderate Exercise as a Treatment for the Metabolic Syndrome. Circulation 2008, 118, 346–354. [Google Scholar] [CrossRef]
- Bo, B.; Guo, A.; Kaila, S.J.; Hao, Z.; Zhang, H.; Wei, J.; Yao, Y. Elucidating the primary mechanisms of high-intensity interval training for improved cardiac fitness in obesity. Front. Physiol. 2023, 14, 1170324. [Google Scholar] [CrossRef] [PubMed]
- Sharp, R.L.; Costill, D.L.; Fink, W.J.; King, D.S. Effects of eight weeks of bicycle ergometer sprint training on human muscle buffer capacity. Int. J. Sports Med. 1986, 7, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Torma, F.; Gombos, Z.; Jokai, M.; Takeda, M.; Mimura, T.; Radak, Z. High intensity interval training and molecular adaptive response of skeletal muscle. Sports Med. Health Sci. 2019, 1, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Gist, N.H.; Freese, E.C.; Cureton, K.J. Comparison of responses to two high-intensity intermittent exercise protocols. J. Strength Cond. Res. 2014, 28, 3033–3040. [Google Scholar] [CrossRef]
- Murawska-Ciałowicz, E.; de Assis, G.G.; Clemente, F.M.; Feito, Y.; Stastny, P.; Zuwała-Jagiełło, J.; Bibrowicz, B.; Wolański, P. Effect of four different forms of high intensity training on BDNF response to Wingate and Graded Exercise Test. Sci. Rep. 2021, 11, 8599. [Google Scholar] [CrossRef]




| Control Group (n = 18) | Tabata Group (n = 18) | |
|---|---|---|
| Number | 18 | 18 |
| Sex (n, male:female) | 5:13 | 5:13 |
| Age (years) | 21.61 ± 2.06 | 20.72 ± 1.32 |
| WHO BMI classification (n, overweight:obese) | 2:16 | 5:13 |
| Physical activity score | ||
| - Pre-test | 7.01 ± 1.07 | 7.04 ± 0.99 |
| - Post-test | 6.96 ± 0.79 | 7.31 ± 1.22 |
| Physical activity level | ||
| - Sedentary (n, %) | 3 (17%) | 3 (17%) |
| - Active (n, %) | 11 (61%) | 12 (66%) |
| - Athletic (n, %) | 4 (22%) | 3 (17%) |
| Resting HR (/min) | 77.22 ± 9.35 | 77.00 ± 9.83 |
| Resting RR (/min) | 18.33 ± 3.29 | 16.11 ± 2.03 |
| Resting SBP (mmHg) | 118.65 ± 16.16 | 118.31 ± 11.77 |
| Resting DBP (mmHg) | 74.41 ± 12.54 | 73.94 ± 8.54 |
| Resting MAP (mmHg) | 89.15 ± 13.04 | 88.73 ± 9.00 |
| Resting SpO2 (%) | 97.78 ± 1.21 | 97.50 ± 1.29 |
| Control Group (n = 18) | Tabata Group (n = 18) | p-Value of Change Between Groups | |||||
|---|---|---|---|---|---|---|---|
| Pre-Test | Post-Test | Mean Change | Pre-Test | Post-Test | Mean Change | ||
| BW (kg) | 74.25 ± 19.70 | 73.85 ± 17.80 | −0.20 ± 4.77 | 73.85 ± 28.05 | 74.70 ± 25.80 | 1.45 ± 2.78 | 0.281 |
| Height (cm) | 163.00 ± 9.06 | 163.11 ± 8.86 | 0.00 ± 0.00 | 163.00 ± 9.06 | 163.16 ± 8.77 | −0.05 ± 0.23 | 0.956 |
| BMI (kg/m2) | 27.10 ± 5.15 | 28.00 ± 4.72 | −0.05 ± 1.73 | 27.00 ± 7.47 | 27.75 ± 7.98 | 0.40 ± 1.13 | 0.269 |
| MM (kg) | 23.35 ± 7.80 | 23.55 ± 7.78 | −0.30 ± 1.15 | 23.30 ± 9.85 | 23.60 ± 10.55 | 0.20 ± 1.38 | 0.290 |
| FMs (kg) | 31.00 ± 11.13 | 30.00 ± 9.52 | 0.25 ± 2.68 | 29.20 ± 15.20 | 30.85 ± 14.48 | 0.75 ± 3.97 | 0.529 |
| BF (%) | 40.20 ± 10.25 | 39.60 ± 10.73 | 0.80 ± 2.22 | 41.10 ± 11.90 | 39.90 ± 15.02 | −0.15 ± 3.90 | 0.927 |
| FFM (kg) | 42.65 ± 13.02 | 42.80 ± 12.93 | −0.70 ± 1.88 | 42.75 ± 16.63 | 43.15 ± 17.00 | 0.45 ± 2.48 | 0.361 |
| BMR (kcal) | 1291.50 ± 281.25 | 1295.00 ± 280.25 | −16.00 ± 41.25 | 1293.50 ± 359.75 | 1302.00 ± 368.00 | 9.5 ± 53.75 | 0.361 |
| Water (L) | 31.20 ± 9.55 | 31.30 ± 9.67 | −0.45 ± 1.33 | 31.30 ± 12.08 | 31.60 ± 12.50 | 0.25 ± 1.83 | 0.384 |
| Protein (kg) | 8.45 ± 2.68 | 8.50 ± 2.55 | −0.10 ± 0.35 | 8.40 ± 3.20 | 8.50 ± 3.60 | 0.10 ± 0.33 | 0.300 |
| Mineral (kg) | 3.11 ± 0.87 | 3.01 ± 0.80 | −0.05 ± 0.14 | 2.98 ± 1.11 | 3.00 ± 1.10 | 0.10 ± 0.23 | 0.293 |
| WHR | 0.92 ± 0.06 | 0.94 ± 0.06 * | 0.02 ± 0.03 | 0.91 ± 0.06 | 0.89 ± 0.06 ** | −0.01 ± 0.03 | 0.043 |
| Control Group (n = 18) | Tabata Group (n = 18) | p-Value of Change Between Groups | |||||
|---|---|---|---|---|---|---|---|
| Pre-Test | Post-Test | Mean Change | Pre-Test | Post-Test | Mean Change | ||
| Right arm (kg) | 2.38 ± 0.66 | 2.40 ± 0.65 | 0.01 ± 0.10 | 2.44 ± 0.70 | 2.45 ± 0.71 | 0.01 ± 0.10 | 0.937 |
| Right arm (%) | 93.38 ± 9.00 | 94.08 ± 8.17 | 0.70 ± 4.36 | 96.60 ± 12.35 | 96.80 ± 13.30 | 0.20 ± 3.51 | 0.707 |
| Left arm (kg) | 2.35 ± 0.64 | 2.35 ± 0.63 | −0.00 ± 0.11 | 2.38 ± 0.66 | 2.43 ± 0.69 | 0.04 ± 0.10 | 0.198 |
| Left arm (%) | 92.20 ± 8.17 | 92.11 ± 7.05 | −0.09 ± 4.76 | 94.78 ± 12.71 | 95.98 ± 13.50 | 1.19 ± 3.51 | 0.363 |
| Right leg (kg) | 7.20 ± 1.50 | 7.04 ± 1.49 * | −0.16 ± 0.24 | 7.41 ± 1.88 | 7.43 ± 1.88 ** | 0.02 ± 0.24 | 0.031 |
| Right leg (%) | 93.71 ± 6.55 | 91.56 ± 6.11 * | −1.72 ± 2.58 | 96.18 ± 8.03 | 96.22 ± 8.44 ** | 0.06 ± 2.45 | 0.026 |
| Left leg (kg) | 7.13 ± 1.48 | 6.84 ± 1.78 | −0.29 ± 0.84 | 7.38 ± 1.83 | 7.42 ± 1.84 | 0.04 ± 0.25 | 0.117 |
| Left leg (%) | 92.78 ± 6.02 | 91.06 ± 6.26 * | −1.72 ± 2.58 | 95.87 ± 7.59 | 95.97 ± 8.23 ** | 0.10 ± 2.60 | 0.043 |
| Abdomen (kg) | 20.75 ± 4.10 | 20.79 ± 4.06 | 0.04 ± 0.61 | 20.95 ± 4.30 | 21.17 ± 4.37 | 0.22 ± 0.52 | 0.358 |
| Abdomen (%) | 94.56 ± 5.16 | 94.80 ± 4.49 | 0.23 ± 2.80 | 95.99 ± 7.25 | 96.50 ± 7.84 | 0.50 ± 2.11 | 0.750 |
| Control Group (n = 18) | Tabata Group (n = 18) | p-Value of Change Between Groups | |||||
|---|---|---|---|---|---|---|---|
| Pre-Test | Post-Test | Mean Change | Pre-Test | Post-Test | Mean Change | ||
| Right arm (kg) | 2.69 ± 1.38 | 2.63 ± 1.32 | 0.33 ± 0.21 | 2.58 ± 1.50 | 2.71 ± 1.56 | 0.09 ± 0.25 | 0.445 |
| Right arm (%) | 321.72 ± 161.12 | 324.39 ± 153.33 | 2.67 ± 25.11 | 308.11 ± 170.64 | 322.76 ± 43.70 | 11.03 ± 31.57 | 0.385 |
| Left arm (kg) | 2.62 ± 1.37 | 2.66 ± 1.32 | 0.04 ± 0.22 | 2.63 ± 1.50 | 2.67 ± 1.54 | 0.07 ± 0.25 | 0.675 |
| Left arm (%) | 324.87 ± 161.29 | 329.37 ± 154.56 | 4.50 ± 25.21 | 313.80 ± 173.20 | 319.14 ± 183.72 | 8.95 ± 31.53 | 0.643 |
| Right leg (kg) | 4.85 ± 1.93 | 4.72 ± 1.59 | −0.12 ± 0.56 | 4.75 ± 1.74 | 4.87 ± 1.94 | 0.12 ± 0.41 | 0.138 |
| Right leg (%) | 226.24 ± 82.10 | 220.52 ± 69.46 | −5.71 ± 23.97 | 217.58 ± 80.25 | 222.93 ± 89.87 | 5.29 ± 18.89 | 0.134 |
| Left leg (kg) | 4.82 ± 1.89 | 4.68 ± 1.58 | −0.13 ± 0.52 | 4.72 ± 1.71 | 4.85 ± 1.90 | 0.12 ± 0.39 | 0.100 |
| Left leg (%) | 225.03 ± 80.70 | 219.66 ± 68.57 | −5.36 ± 23.46 | 216.13 ± 78.25 | 221.43 ± 88.22 | 5.35 ± 18.93 | 0.142 |
| Abdomen (kg) | 15.20 ± 3.84 | 15.58 ± 3.88 * | 0.37 ± 0.82 | 15.05 ± 4.83 | 15.26 ± 1.18 | 0.21 ± 1.11 | 0.625 |
| Abdomen (%) | 316.30 ± 88.06 | 324.00 ± 87.19 * | 7.70 ± 16.94 | 305.77 ± 102.02 | 309.73 ± 108.02 | 3.96 ± 23.14 | 0.583 |
| Control Group (n = 18) | Tabata Group (n = 18) | p-Value of Change Between Groups | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre-Test | Post-Test | Mean Change | Pre-Test | Post-Test | Mean Change | |||
| Rest | ||||||||
| HR (beats/min) | 94.44 ± 10.31 | 92.00 ± 11.90 | −2.44 ± 7.51 | 91.72 ± 10.39 | 90.16 ± 10.40 | −1.55 ± 8.84 | 0.747 | |
| BF (breaths/min) | 20.16 ± 3.85 | 19.50 ± 3.83 | −0.66 ± 2.97 | 21.05 ± 4.24 | 21.66 ± 5.20 | 0.61 ± 2.70 | 0.186 | |
| RER | 0.80 ± 0.05 | 0.84 ± 0.03 * | 0.04 ± 0.06 | 0.81 ± 0.04 | 0.86 ± 0.04 * | 0.04 ± 0.06 | 0.740 | |
| VO2 (L/min) | 0.33 ± 0.07 | 0.29 ± 0.07 * | −0.04 ± 0.04 | 0.36 ± 0.07 | 0.34 ± 0.08 ** | −0.02 ± 0.06 | 0.265 | |
| VO2 (ml/min/kg) | 4.44 ± 0.92 | 3.94 ± 0.80 * | −0.50 ± 0.71 | 4.66 ± 0.77 | 4.44 ± 0.86 | −0.22 ± 1.00 | 0.344 | |
| Exercise | ||||||||
| HR (/min) | 177.22 ± 14.28 | 181.44 ± 7.90 | 4.22 ± 9.72 | 186.00 ± 8.40 | 191.00 ± 9.57 | 5.00 ± 8.68 | 0.802 | |
| HR (% age-predicted maximum HR) | 89.33 ± 7.15 | 91.46 ± 4.03 | 2.13 ± 4.89 | 93.34 ± 4.45 | 95.86 ± 5.14 | 2.51 ± 4.34 | 0.806 | |
| VT1 (mL/min/kg) | 17.22 ± 4.60 | 16.06 ± 3.19 | −1.17 ± 3.29 | 19.89 ± 3.20 | 18.06 ± 3.62 | −1.83 ± 3.91 | 0.584 | |
| VT2 (mL/min/kg) | 25.56 ± 6.54 | 22.17 ± 4.72 * | −3.39 ± 4.79 | 27.78 ± 4.62 | 26.72 ± 5.69 ** | −1.06 ± 4.39 | 0.137 | |
| VO2peak (L/min) | 2.18 ± 0.48 | 2.14 ± 0.49 | −0.04 ± 0.24 | 2.42 ± 0.62 | 2.43 ± 0.63 ** | 0.01 ± 0.28 | 0.323 | |
| BF (/min) | 45.94 ± 9.25 | 54.44 ± 10.19 * | 8.50 ± 9.30 | 48.11 ± 8.87 | 62.22 ± 12.75 * | 14.11 ± 12.96 | 0.145 | |
| RER | 1.06 ± 0.06 | 1.29 ± 0.05 * | 0.23 ± 0.08 | 1.07 ± 0.05 | 1.29 ± 1.28 * | 0.21 ± 0.08 | 0.625 | |
| SV (mL) | 76.38 ± 17.04 | 74.20 ± 15.99 | −2.17 ± 9.41 | 79.86 ± 18.31 | 78.62 ± 18.99 | −1.24 ± 15.98 | 0.767 | |
| CO (L/min) | 13.47 ± 2.98 | 13.19 ± 3.02 | −0.28 ± 1.52 | 14.92 ± 3.85 | 14.50 ± 3.49 | −0.42 ± 2.83 | 0.481 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padkao, T.; Prasertsri, P. The Impact of Modified Tabata Training on Segmental Fat Accumulation, Muscle Mass, Muscle Thickness, and Physical and Cardiorespiratory Fitness in Overweight and Obese Participants: A Randomized Control Trial. Sports 2025, 13, 99. https://doi.org/10.3390/sports13040099
Padkao T, Prasertsri P. The Impact of Modified Tabata Training on Segmental Fat Accumulation, Muscle Mass, Muscle Thickness, and Physical and Cardiorespiratory Fitness in Overweight and Obese Participants: A Randomized Control Trial. Sports. 2025; 13(4):99. https://doi.org/10.3390/sports13040099
Chicago/Turabian StylePadkao, Tadsawiya, and Piyapong Prasertsri. 2025. "The Impact of Modified Tabata Training on Segmental Fat Accumulation, Muscle Mass, Muscle Thickness, and Physical and Cardiorespiratory Fitness in Overweight and Obese Participants: A Randomized Control Trial" Sports 13, no. 4: 99. https://doi.org/10.3390/sports13040099
APA StylePadkao, T., & Prasertsri, P. (2025). The Impact of Modified Tabata Training on Segmental Fat Accumulation, Muscle Mass, Muscle Thickness, and Physical and Cardiorespiratory Fitness in Overweight and Obese Participants: A Randomized Control Trial. Sports, 13(4), 99. https://doi.org/10.3390/sports13040099







