Carbohydrate Supplementation Does Not Improve 10 km Swimming Intermittent Training
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Experimental Design
2.3. Continuous Glucose Monitoring
2.4. Statistical Analysis
3. Results
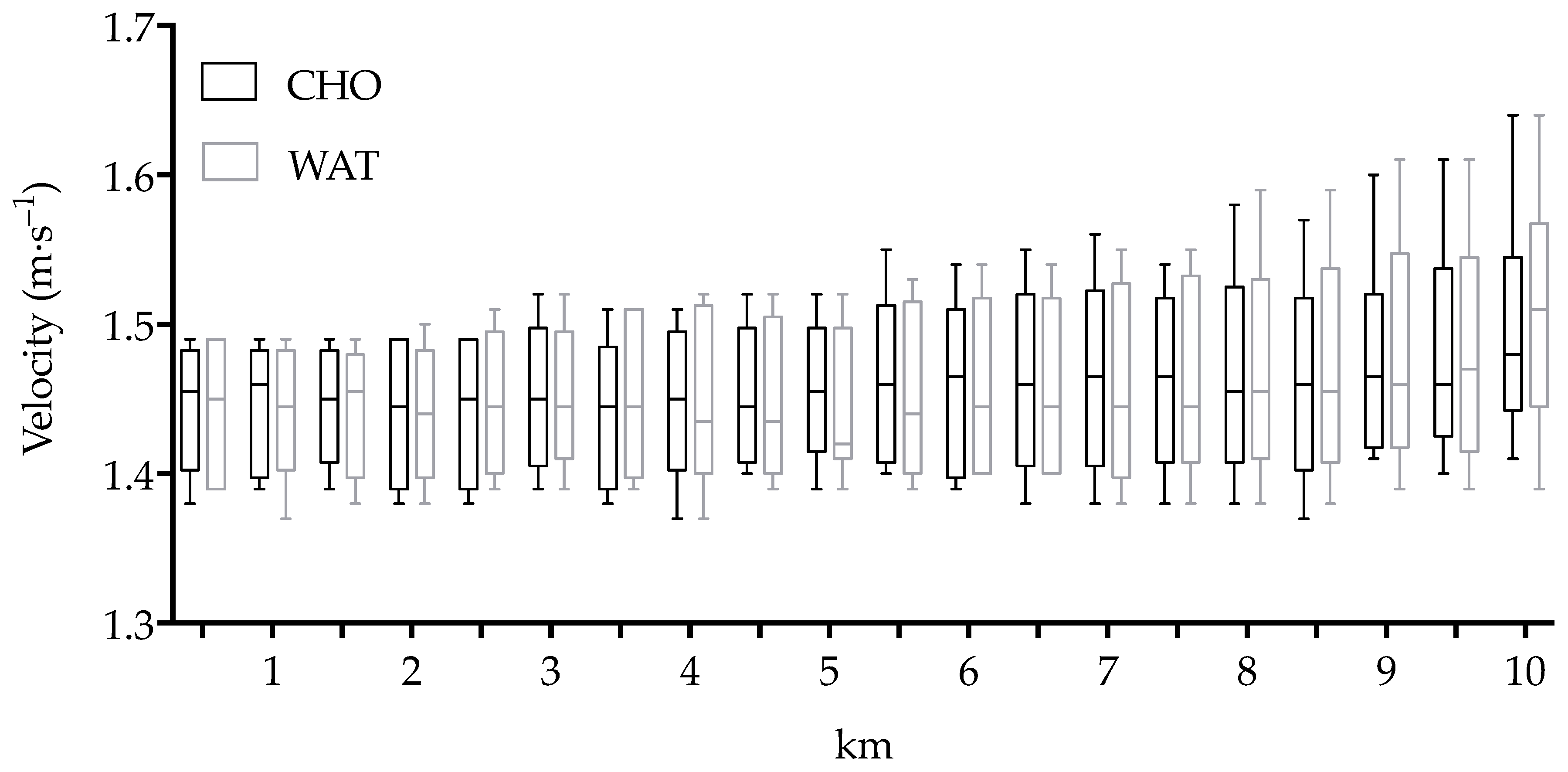
3.1. Performance
3.2. Rating of Perceived Exertion
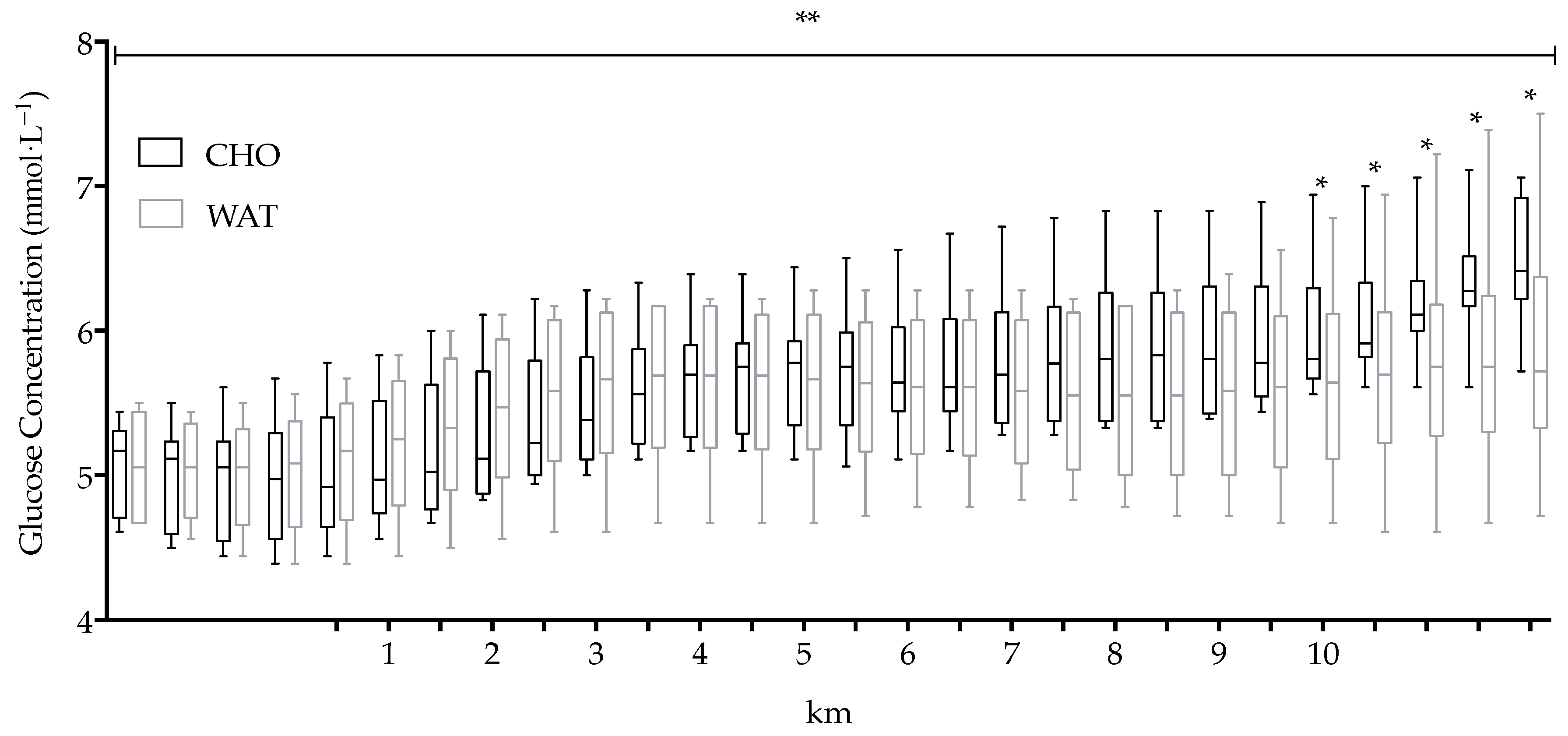
3.3. Glucose Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baldassarre, R.; Bonifazi, M.; Meeusen, R.; Piacentini, M.F. The Road to Rio: A Brief Report of Training-Load Distribution of Open-Water Swimmers During the Olympic Season. Int. J. Sports Physiol. Perform. 2018, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mujika, I.; Padilla, S.; Pyne, D. Swimming Performance Changes during the Final 3 Weeks of Training Leading to the Sydney 2000 Olympic Games. Int. J. Sports Med. 2002, 23, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Aspenes, S.T.; Karlsen, T. Exercise-training intervention studies in competitive swimming. Sport Med. 2012, 42, 527–543. [Google Scholar] [CrossRef] [PubMed]
- IOC—International Olympic Committee Olympic Swimming. Available online: https://www.olympic.org/swimming (accessed on 16 January 2018).
- Baldassarre, R.; Bonifazi, M.; Zamparo, P.; Piacentini, M.F. Characteristics and Challenges of Open-Water Swimming Performance: A Review. Int. J. Sports Physiol. Perform. 2017, 12, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.; Koivisto, A.; Gerrard, D.; Burke, L.M. Nutrition Considerations for Open-Water Swimming. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Dascombe, B.J.; Karunaratna, M.; Cartoon, J.; Fergie, B.; Goodman, C. Nutritional supplementation habits and perceptions of elite athletes within a state-based sporting institute. J. Sci. Med. Sport 2010. [Google Scholar] [CrossRef] [PubMed]
- Burgess, M.L.; Robertson, R.J.; Davis, J.M.; Norris, J.M. RPE, blood glucose, and carbohydrate oxidation during exercise: Effects of glucose feedings. Med. Sci. Sport Exerc. 1991, 23, 353–359. [Google Scholar] [CrossRef]
- Nybo, L. CNS fatigue and prolonged exercise: Effect of glucose supplementation. Med. Sci. Sports Exerc. 2003, 35, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Currell, K.; Jeukendrup, A.E. Superior endurance performance with ingestion of multiple transportable carbohydrates. Med. Sci. Sport Exerc. 2008, 40, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Coyle, E.F.; Coggan, A.R.; Hemmert, M.K.; Ivy, J.L. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. J. Appl. Physiol. 1986, 61, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Foskett, A.; Williams, C.; Boobis, L.; Tsintzas, K. Carbohydrate availability and muscle energy metabolism during intermittent running. Med. Sci. Sports Exerc. 2008, 40, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Karelis, A.D.; Smith, J.W.; Passe, D.H.; Péronnet, F. Carbohydrate administration and exercise performance: What are the potential mechanisms involved? Sport Med. 2010, 40, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Cermak, N.M.; Van Loon, L.J.C. The use of carbohydrates during exercise as an ergogenic aid. Sport Med. 2013, 43, 1139–1155. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, D.S.; Houltham, S.; Musa-Veloso, K.; Brown, F.; Paulionis, L.; Bailey, D. Fructose—Glucose Composite Carbohydrates and Endurance Performance: Critical Review and Future Perspectives. Sport Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Stellingwerff, T.; Cox, G.R. Systematic review: Carbohydrate supplementation on exercise performance or capacity of varying durations. Appl. Physiol. Nutr. Metab. 2014, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Vandenbogaerde, T.J.; Hopkins, W.G. Effects of acute carbohydrate supplementation on endurance performance: A meta-analysis. Sport Med. 2011, 41, 773–792. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Leckey, J.J. Carbohydrate Dependence during Prolonged, Intense Endurance Exercise. Sport Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Costill, D.L.; Hinrichs, D.; Fink, W.J.; Hoopes, D. Muscle glycogen depletion during swimming interval training. J. Swim. Res. 1988, 4, 15–18. [Google Scholar]
- Shaw, G.; Boyd, K.T.; Burke, L.M.; Koivisto, A. Nutrition for Swimming. Int. J. Sport Nutr. Exerc. Metab. 2014, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Utter, A.C.; Kang, J.; Nieman, D.C.; Dumke, C.L.; Mcanulty, S.R.; Vinci, D.M.; Mcanulty, L.S. Carbohydrate supplementation and perceived exertion during prolonged running. Med. Sci. Sports Exerc. 2014, 36, 1036–1041. [Google Scholar] [CrossRef]
- Utter, A.C.; Kang, J.; Niemann, D.C.; Williams, F.; Robertson, R.J.; Henson, D.A.; Davis, J.M.; Butterworth, D.E. Effect of carbohydrate ingestion and hormonal respones on ratings of perceived exertion during prolonged cycling and running. Eur. J. Appl. Physiol. 1999, 80, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Utter, A.C.; Kang, J.; Robertson, R.J.; Nieman, D.C.; Chaloupka, E.C.; Suminski, R.R.; Piccinni, C.R. Effect of carbohydrate ingestion on ratings of perceived exertion during a marathon. Med. Sci. Sports Exerc. 2002, 34, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Kumstát, M.; Rybářová, S.; Thomas, A.; Novotný, J. Case Study: Competition Nutrition Intakes during the Open Water Swimming Grand Prix Races in Elite Female Swimmer. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Mujika, I. Nutrition for Recovery in Aquatic Sports. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, R.; Bonifazi, M.; Piacentini, M.F. Pacing profile in the main international open-water swimming competitions. Eur. J. Sport Sci. 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.; Veiga, S. Effect of the Pacing Strategies on the Open Water 10km World Swimming Championships Performances. Int. J. Sports Physiol. Perform. 2017, 1–19. [Google Scholar] [CrossRef]
- Macaluso, F.; Di Felice, V.; Boscaino, G.; Bonsignore, G.; Stampone, T.; Farina, F.; Morici, G. Effects of three different water temperatures on dehydration in competitive swimmers. Sci. Sports 2010, 26, 265–271. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Weitgasser, R.; Gappmayer, B.; Pichler, M. Newer portable glucose meters—Analytical improvement compared with previous generation devices? Clin. Chem. 1999, 45, 1821–1825. [Google Scholar] [PubMed]
- Macdonald, A.L.; Philp, A.; Harrison, M.; Bone, A.J.; Watt, P.W. Monitoring exercise-induced changes in glycemic control in type 2 diabetes. Med. Sci. Sports Exerc. 2006, 38, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Green, J.M.; Sapp, A.L.; Pritchett, R.C.; Bishop, P.A. Pacing accuracy in collegiate and recreational runners. Eur. J. Appl. Physiol. 2010, 108, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.; MacLean, D.A.; Kiens, B.; Christensen, D. Effects of glucose, glucose plus branched-chain amino acids, or placebo on bike performance over 100 km. J. Appl. Physiol. 1996, 81, 2644–2650. [Google Scholar] [CrossRef] [PubMed]
- McConell, G.K.; Canny, B.J.; Daddo, M.C.; Nance, M.J.; Snow, R.J. Effect of carbohydrate ingestion on glucose kinetics and muscle metabolism during intense endurance exercise. J. Appl. Physiol. 2000. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, H.M.; Beek, P.J. Biomechanics of Competitive Front Crawl Swimming. Sports Med. Int. J. Appl. Med. Sci. Sport Exerc. 1992, 13, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Helge, J.W. Arm and leg substrate utilization and muscle adaptation after prolonged low-intensity training. Acta Physiol. 2010, 199, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, J.H.; PIronnet, F.; Lavoie, C.; Massicotte, D. Fuel selection during prolonged arm and leg exercise with 13c-glucose ingestion. Med. Sci. Sports Exerc. 2009, 41, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- Hetlelid, K.J.; Plews, D.J.; Herold, E.; Laursen, P.B.; Seiler, S. Rethinking the role of fat oxidation: Substrate utilisation during high-intensity interval training in well-trained and recreationally trained runners. Br. Med. J. Open Sport Exerc. Med. 2015, e000047. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.J.; Jeukendrup, A.E.; Saris, W.H.; Wagenmakers, A.J. Effect of training status on fuel selection during submaximal exercise with glucose ingestion. J. Appl. Physiol. 1999, 87, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.J.; Greenhaff, P.L.; Constantin-Teodosiu, D.; Saris, W.H.; Wagenmakers, A.J. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 2001, 536, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Utter, A.C.; Kang, J.; Nieman, D.C.; Dumke, C.L.; Mcanulty, S.R.; Mcanulty, L.S. Carbohydrate attenuates perceived exertion during intermittent exercise and recovery. Med. Sci. Sports Exerc. 2007, 39, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Marcora, S.M.; Staiano, W. The limit to exercise tolerance in humans: Mind over muscle? Eur. J. Appl. Physiol. 2010, 109, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Clark, V.R.; Hopkins, W.G.; Hawley, J.A.; Burke, L.M. Placebo effect of carbohydrate feedings during a 40-km cycling time trial. Med. Sci. Sports Exerc. 2000, 32, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Hulston, C.J.; Jeukendrup, A.E. No placebo effect from carbohydrate intake during prolonged exercise. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Shimizu, T.; Ota, M.; Hirata, R.; Sato, K.; Tamura, Y.; Imanishi, A.; Watanabe, M.; Sakuraba, K. Different training status may alter the continuous blood glucose kinetics in self-paced endurance running. Exp. Ther. Med. 2015, 10, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Sengoku, Y.; Nakamura, K.; Ogata, H.; Nabekura, Y.; Nagasaka, S.; Tokuyama, K. Continuous glucose monitoring during a 100-km race: A case study in an elite ultramarathon runner. Int. J. Sports Physiol. Perform. 2015, 10, 124–127. [Google Scholar] [CrossRef] [PubMed]

| CHO | WAT | 500max | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Average | 1st | 19th | 20th | Average | 1st | 19th | 20th | ||
| Velocity (m·s−1) | 1.46 ± 0.05 | 1.44 ± 0.04 | 1.48 ± 0.07 | 1.50 ± 0.07 | 1.46 ± 0.06 | 1.44 ± 0.04 | 1.48 ± 0.08 | 1.51 ± 0.08 | 1.60 ± 0.06 |
| %-500max (%) | 87.14 ± 3.33 | 86.28 ± 2.54 | 88.64 ± 4.21 | 89.39 ± 4.32 | 87.16 ± 3.61 | 86.20 ± 2.60 | 88.63 ± 4.72 | 90.20 ± 4.67 | |
| Males | |||||||||
| Velocity (m·s−1) | 1.49 ± 0.05 | 1.47 ± 0.03 | 1.51 ± 0.08 | 1.52 ± 0.08 | 1.50 ± 0.05 | 1.47 ± 0.03 | 1.53 ± 0.06 | 1.56 ± 0.05 | 1.64 ± 0.04 |
| %-500max (%) | 89.01 ± 2.82 | 87.80 ± 1.56 | 90.43 ± 4.51 | 90.76 ± 4.78 | 89.34 ± 3.02 | 87.83 ± 1.83 | 91.60 ± 3.61 | 93.20± 3.15 | |
| Females | |||||||||
| Velocity (m·s−1) | 1.41 ± 0.03 | 1.41 ± 0.03 | 1.44 ± 0.03 | 1.46 ± 0.05 | 1.40 ± 0.02 | 1.40 ± 0.02 | 1.41 ± 0.01 | 1.43 ± 0.03 | 1.55 ± 0.03 |
| %-500max (%) | 84.35 ± 1.68 | 84.01 ± 1.95 | 85.94 ± 1.77 | 87.35 ± 2.94 | 83.89 ± 1.06 | 83.76 ± 1.24 | 84.18 ± 0.88 | 85.70 ± 1.94 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldassarre, R.; Sacchetti, M.; Patrizio, F.; Nicolò, A.; Scotto di Palumbo, A.; Bonifazi, M.; Piacentini, M.F. Carbohydrate Supplementation Does Not Improve 10 km Swimming Intermittent Training. Sports 2018, 6, 147. https://doi.org/10.3390/sports6040147
Baldassarre R, Sacchetti M, Patrizio F, Nicolò A, Scotto di Palumbo A, Bonifazi M, Piacentini MF. Carbohydrate Supplementation Does Not Improve 10 km Swimming Intermittent Training. Sports. 2018; 6(4):147. https://doi.org/10.3390/sports6040147
Chicago/Turabian StyleBaldassarre, Roberto, Massimo Sacchetti, Federica Patrizio, Andrea Nicolò, Alessandro Scotto di Palumbo, Marco Bonifazi, and Maria Francesca Piacentini. 2018. "Carbohydrate Supplementation Does Not Improve 10 km Swimming Intermittent Training" Sports 6, no. 4: 147. https://doi.org/10.3390/sports6040147
APA StyleBaldassarre, R., Sacchetti, M., Patrizio, F., Nicolò, A., Scotto di Palumbo, A., Bonifazi, M., & Piacentini, M. F. (2018). Carbohydrate Supplementation Does Not Improve 10 km Swimming Intermittent Training. Sports, 6(4), 147. https://doi.org/10.3390/sports6040147









