Acute Metabolic Changes with Lower Leg-Positioned Wearable Resistances during Submaximal Running in Endurance-Trained Runners
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
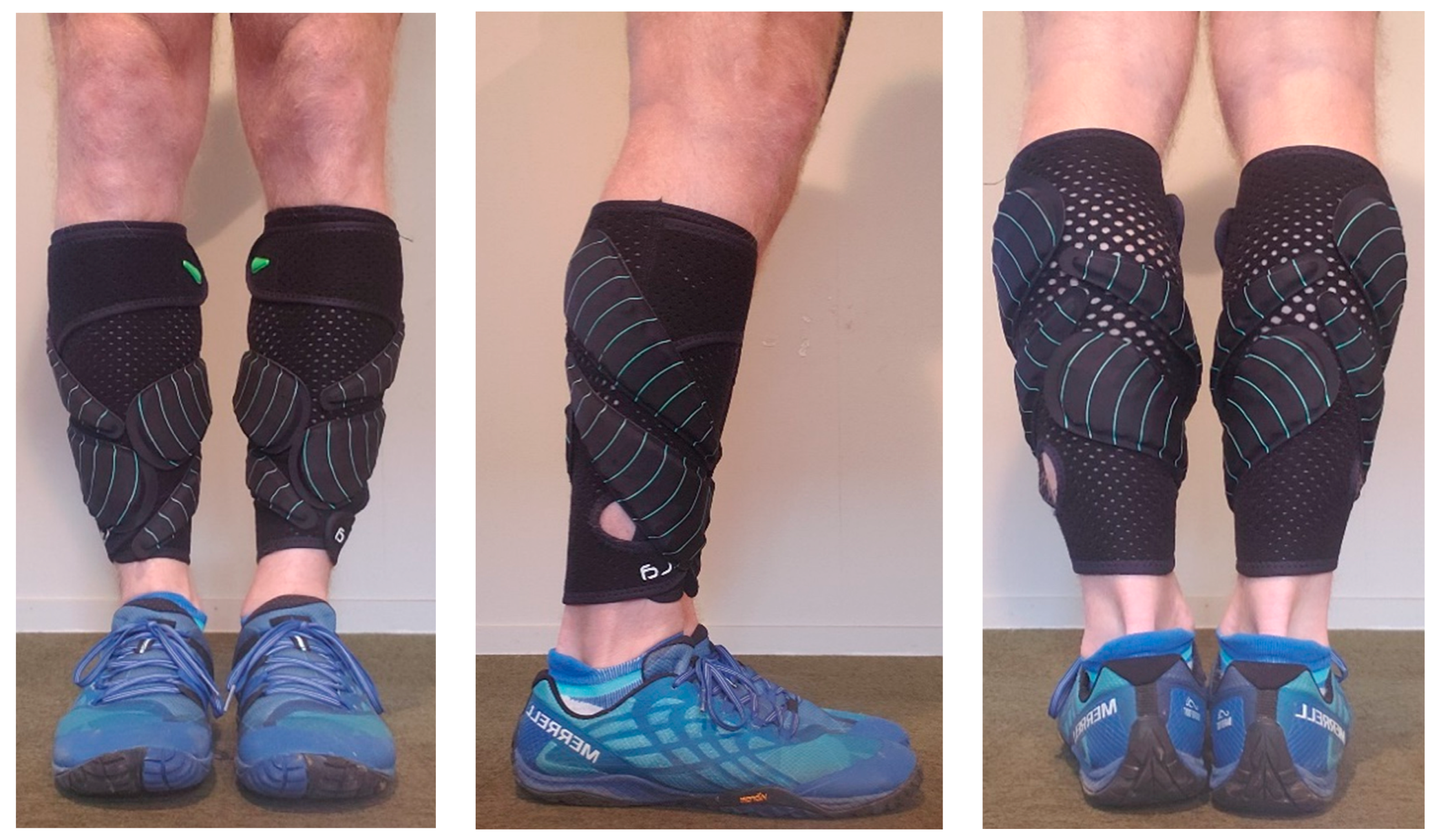
2.2. Procedure
2.3. Statistical Analysis
3. Results
Metabolic Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bramble, D.M.; Lierberman, D.E. Endurance running and the evolution of Homo. Nature 2004, 432, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D.; Myburgh, K.H.; Schall, R. Peak treadmill running velocity during the VO2 max test predicts running performance. J. Sports Sci. 1990, 8, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, R.M.; Sleivert, G.G. Indices of lactate threshold and their relationship with 10-km running velocity. Med. Sci. Sports Exerc. 2001, 33, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Tjelta, L.I.; Shalfawi, S.A. Physiological factors affecting performance in elite distance runners. Acta Kinesiol. Univ. Tartu. 2016, 22, 7–19. [Google Scholar] [CrossRef]
- Alcaraz, P.E.; Carlos-Vivas, J.; Oponjuru, B.O.; Martínez-Rodríguez, A. Correction to: The Effectiveness of Resisted Sled Training (RST) for Sprint Performance: A Systematic Review and Meta-analysis. Sports Med. 2018, 48, 2167–2168. [Google Scholar] [CrossRef] [Green Version]
- Macadam, P.; Cronin, J.B.; Simperingham, K.D. The effects of wearable resistance training on metabolic, kinematic and kinetic variables during walking, running, sprint running and jumping: A systematic review. Sports Med. 2017, 47, 887–906. [Google Scholar] [CrossRef]
- Macadam, P.; Cronin, J.; Uthoff, A.; Feser, E. The effects of different wearable resistance placements on sprint-running performance: A review and practical applications. Strength Cond. J. 2018, 41, 79–96. [Google Scholar] [CrossRef]
- Field, A.P.; Gill, N.; Macadam, P.; Plews, D. Acute metabolic changes with thigh-positioned wearable resistances during submaximal running in endurance-trained runners. Sports 2019, 7, 187. [Google Scholar] [CrossRef]
- Soule, R.G.; Goldman, R.F. Energy cost of loads carried on the head, hands, or feet. J. Appl. Physiol. 1969, 27, 687–690. [Google Scholar] [CrossRef]
- Jones, B.H.; Toner, M.M.; Daniels, W.L.; Knapik, J.J. The energy cost and heart-rate response of trained and untrained subjects walking and running in shoes and boots. Ergonomics 1984, 27, 895–902. [Google Scholar] [CrossRef]
- Martin, P.E. Mechanical and physiological responses to lower extremity loading during running. Med. Sci. Sports Exerc. 1985, 17, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Doust, J. A 1% treadmill grade most accurately reflects the energetic cost of outdoor running. J. Sports Sci. 1996, 14, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Seiler, K.S.; Kjerland, G.Ø. Quantifying training intensity distribution in elite endurance athletes: Is there evidence for an “optimal” distribution? Scand. J. Med. Sci. Sports 2006, 16, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.N.; Webster, C.; Lamberts, R.P.; Lambert, M.I. Effect of exercise intensity on post-exercise oxygen consumption and heart rate recovery. Eur. J. Appl. Physiol. 2014, 114, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Scharhag-Rosenberger, F.; Carlsohn, A.; Cassel, M.; Mayer, F.; Scharhag, J. How to test maximal oxygen uptake: A study on timing and testing procedure of a supramaximal verification test. Appl. Physiol. Nutr. Metab. 2011, 36, 153–160. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3. [Google Scholar] [CrossRef]
- Batterham, A.M.; Hopkins, W.G. Making meaningful inferences about magnitudes. Sportscience 2005, 9, 6–13. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Batterham, A. The vindication of magnitude-basaed inference. Sportscience 2018, 22, 19–29. [Google Scholar]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Wallace, L.K.; Slattery, K.M.; Coutts, A.J. A comparison of methods for quantifying training load: Relationships between modelled and actual training responses. Eur. J. Appl. Physiol. 2014, 114, 11–20. [Google Scholar] [CrossRef]
- Coggan, A. Normalized Power, Intensity Factor and Training Stress Score. Available online: https://www.trainingpeaks.com/blog/normalized-power-intensity-factor-training-stress/ (accessed on 2 May 2019).
- Claremont, A.D.; Hall, S.J. Effects of extremity loading upon energy expenditure and running mechanics. Med. Sci. Sports Exerc. 1988, 20, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Borresen, J.; Lambert, M.I. The quantification of training load, the training response and the effect on performance. Sports Med. 2009, 39, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Sainani, K.L. The problem with “magnitude-based inference”. Med. Sci. Sports Exerc. 2018, 50, 2166–2176. [Google Scholar] [CrossRef] [PubMed]





| Training Load (% BM) | V̇O2 (L) Mean (SD) | Effect Size (90% CI) | Rating |
|---|---|---|---|
| 0% | 3.22 (0.48) | - | - |
| 0.5% | 3.28 (0.53) | 0.09 (−0.02 to 0.19) | (4/96/0) very likely trivial increase |
| 1% | 3.36 (0.59) | 0.22 (0.9 to 0.34) | (60/40/0) possible small increase |
| 1.5% | 3.39 (0.56) | 0.28 (0.17 to 0.39) | 88/12/0) likely moderate increase |
| 2% | 3.39 (0.53) | 0.3 (0.19 to 0.40) | (94/6/0) likely moderate increase |
| 2.5% | 3.43 (0.59) | 0.34 (0.22 to 0.44) | (97/3/0) very likely large increase |
| 3% | 3.52 (0.54) | 0.51 (0.42 to 0.60) | (100/0/0) most likely very large increase |
| Training Load (% BM) | HR (bpm) Mean (SD) | Effect Size (90% CI) | Rating |
|---|---|---|---|
| 0% | 150.2 (10.2) | - | - |
| 0.5% | 151.6 (9.09) | 0.13 (−0.06 to 0.33) | (28/71/1) possible small increase |
| 1% | 153.5 (12.1) | 0.30 (0.8 to 0.52) | (78/22/0) likely moderate increase |
| 1.5% | 154.1 (10.6) | 0.35 (0.19 to 0.52) | (94/6/0) likely moderate increase |
| 2% | 155.5 (9.84) | 0.49 (0.32 to 0.67) | (100/0/0) most likely very large increase |
| 2.5% | 156.7 (9.17) | 0.60 (0.44 to 0.76) | (100/0/0) most likely very large increase |
| 3% | 156.8 (7.95) | 0.62 (0.4 to 0.84) | (100/0/0) most likely very large increase |
| Training Load (% BM) | La (mmol/L) Mean (SD) | Effect Size (90%CI) | Rating |
|---|---|---|---|
| 0% | 1.89 (0.60) | - | - |
| 0.5% | 2.29 (0.89) | 0.49 (0.3 to 0.95) | (86/13/1) likely moderate increase |
| 1% | 2.35 (0.72) | 0.63 (0.22 to 1.03) | (96/4/0) very likely large increase |
| 1.5% | 2.37 (1.11) | 0.45 (−0.03 to 0.93) | (82/16/2) likely moderate increase |
| 2% | 2.44 (0.95) | 0.65 (0.12 to 1.19) | (92/7/1) likely moderate increase |
| 2.5% | 2.61 (0.66) | 0.96 (0.52 to 1.39) | (100/0/0) most likely very large increase |
| 3% | 2.83 (1.22) | 1.05 (0.6 to 1.51) | (100/0/0) most likely very large increase |
| Training Load (% BM) | Rate of Perceived Exertion (RPE) Mean (SD) | Effect Size (90% CI) | Rating |
|---|---|---|---|
| 0% | 2.53 (0.88) | - | - |
| 0.5% | 3.03 (1.22) | 0.40 (0.07 to 0.72) | (85/15/0) likely moderate increase |
| 1% | 3.27 (1.07) | 0.63 (0.35 to 0.90) | (99/1/0) very likely large increase |
| 1.5% | 3.40 (1.02) | 0.73 (0.35 to 1.11) | (99/1/0) very likely large increase |
| 2% | 4.00 (1.28) | 1.11 (0.78 to 1.43) | (100/0/0) most likely very large increase |
| 2.5% | 4.30 (1.15) | 1.30 (1.05 to 1.55) | (100/0/0) most likely very large increase |
| 3% | 4.53 (1.59) | 1.38 (0.98 to 1.79) | (100/0/0) most likely very large increase |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Field, A.P.; Gill, N.; Uthoff, A.M.; Plews, D. Acute Metabolic Changes with Lower Leg-Positioned Wearable Resistances during Submaximal Running in Endurance-Trained Runners. Sports 2019, 7, 220. https://doi.org/10.3390/sports7100220
Field AP, Gill N, Uthoff AM, Plews D. Acute Metabolic Changes with Lower Leg-Positioned Wearable Resistances during Submaximal Running in Endurance-Trained Runners. Sports. 2019; 7(10):220. https://doi.org/10.3390/sports7100220
Chicago/Turabian StyleField, Allister P., Nicholas Gill, Aaron M. Uthoff, and Dan Plews. 2019. "Acute Metabolic Changes with Lower Leg-Positioned Wearable Resistances during Submaximal Running in Endurance-Trained Runners" Sports 7, no. 10: 220. https://doi.org/10.3390/sports7100220
APA StyleField, A. P., Gill, N., Uthoff, A. M., & Plews, D. (2019). Acute Metabolic Changes with Lower Leg-Positioned Wearable Resistances during Submaximal Running in Endurance-Trained Runners. Sports, 7(10), 220. https://doi.org/10.3390/sports7100220






