Are Blood Pressure and Cardiovascular Stress Greater in Isometric or in Dynamic Resistance Exercise?
Abstract
:1. Introduction
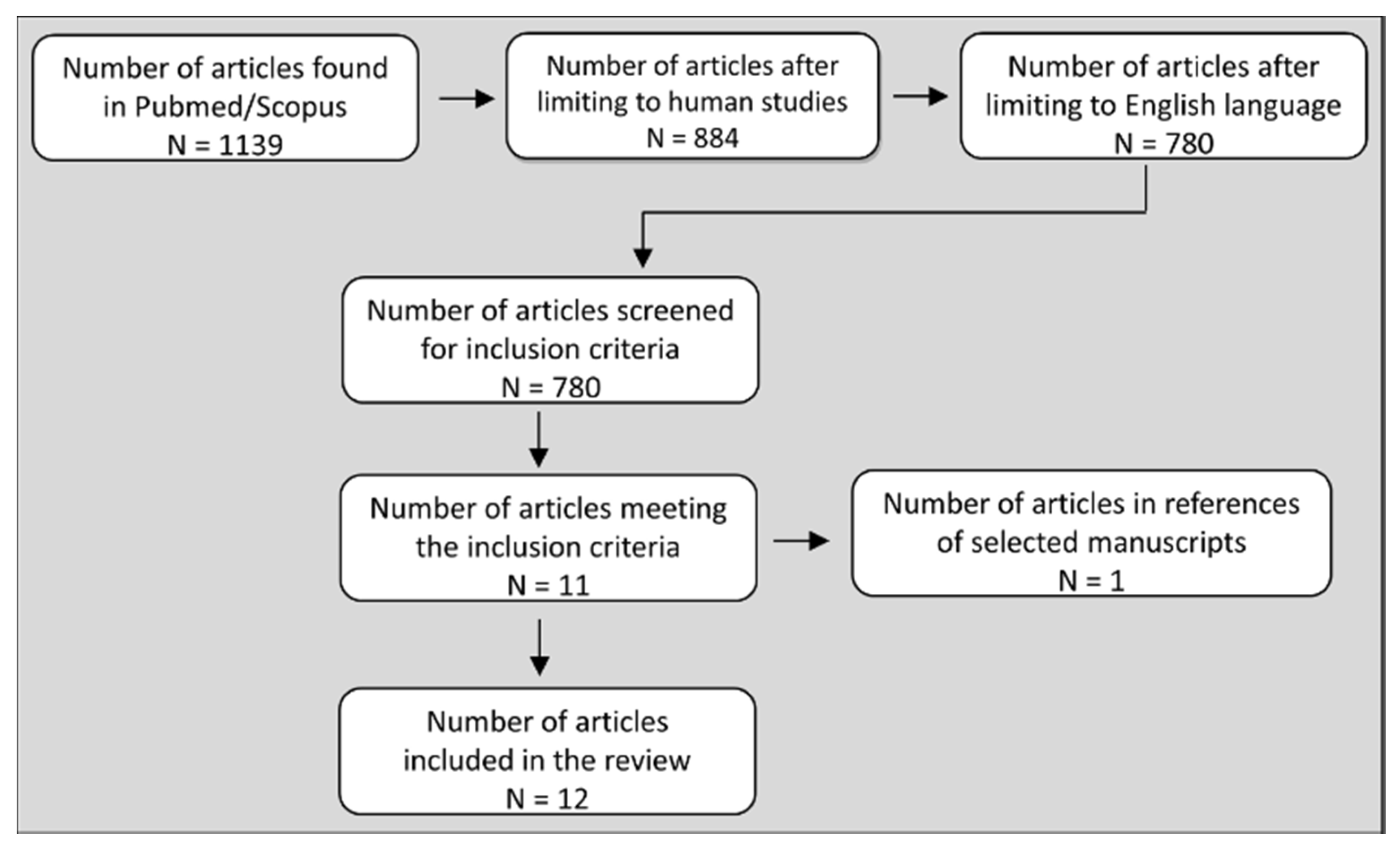
2. Methods
3. Studies that Compared BP and Hemodynamic Responses between Isometric and Dynamic RE
| Study | Participants | Muscle Mass | Workload (TTI) | Study Design | Results |
|---|---|---|---|---|---|
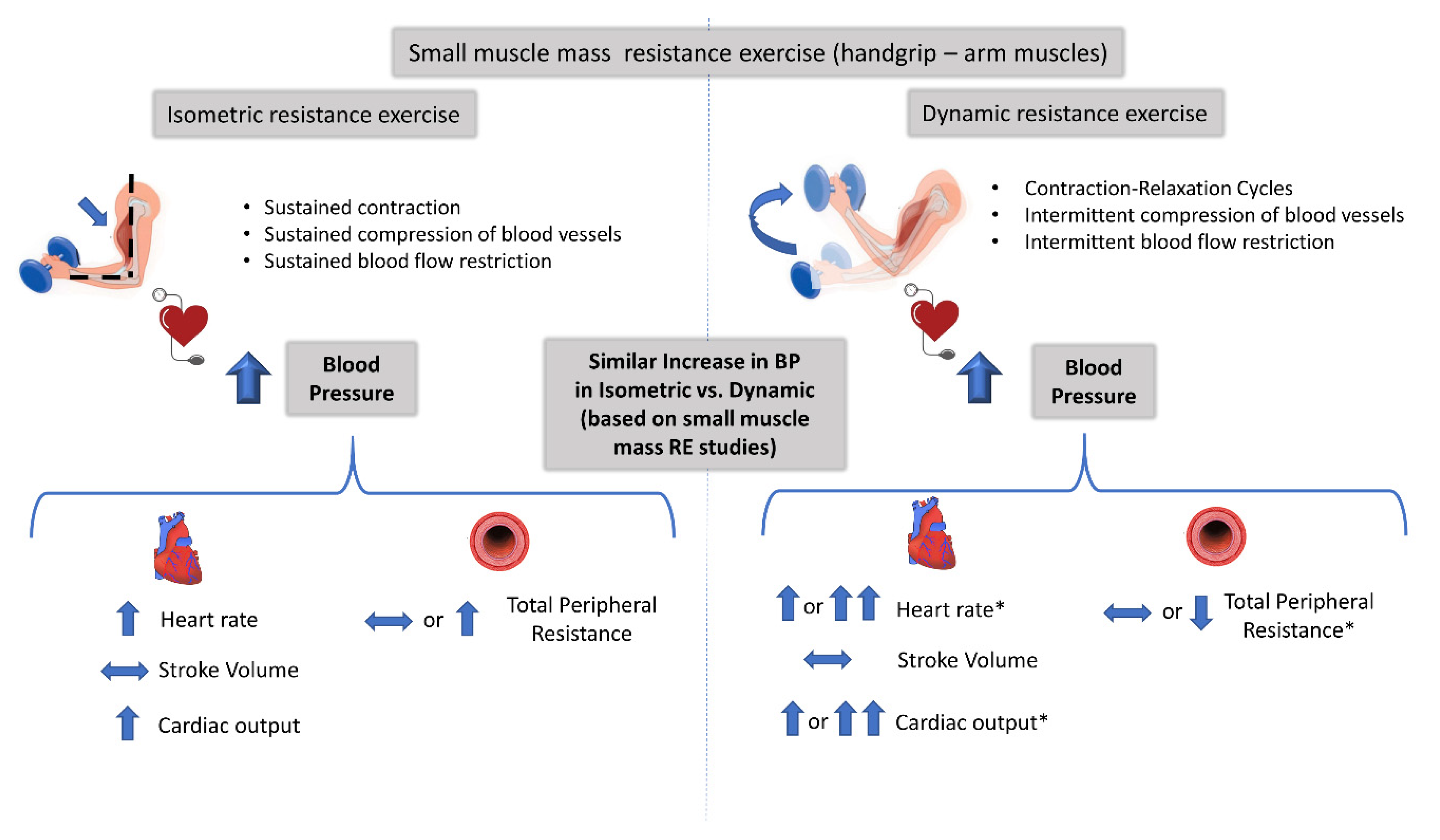
| Lewis [32] | 6 healthy males (26 ± 3 years) | Small | Not Measured | Handgrip (to Fatigue) Isometric: 25% MVC Dynamic: 20–40 reps/min 11 kg | ↑SBP, ↑DBP, ↑MAP: Isometric = Dynamic ↑HR, ↑CO: Isometric = Dynamic →SV: Isometric = Dynamic ↑TPR only in Isometric ↑VO2: Isometric = Dynamic |
| Lewis [34] | 6 healthy males (27 ± 7 years) | Small | Not Measured | Handgrip (to Fatigue) Isometric: 25% MVC Dynamic: 33-40 reps/min 11 kg | ↑SBP, ↑DBP ↑MAP: Isometric = Dynamic ↑HR, ↑CO: Isometric = Dynamic →SV, →TPR: Isometric = Dynamic |
| Haennel [37] | 5 healthy males (26 ± 3 years) | Small | Not Measured | Elbow Extension Isometric: 20 s maximal contraction Isokinetic: 20 s as fast as possible (3 speeds) | ↑MAP: Isometric = Isokinetic →SV: Isometric = Isokinetic ↑HR, ↑CO, ↑RPP: Isometric < Isokinetic ↓ SVR: Only in high speed Isokinetic > Isometric |
| Louhevaara [33] | 21 healthy males (33 ± 6 years) | Small | Not Measured | Handgrip (to Fatigue) Isometric: 50% MVC Dynamic: 50% MVC (50 reps/min) | ↑SBP, ↑DBP: Isometric =Dynamic ↑HR: Isometric = Dynamic ↑VO2, ↑VE: Isometric = Dynamic |
| Vedsted [41] | 8 healthy, 1 male and 7 females (45-69 years) | Small | Equivalent in isometric and dynamic protocols | Elbow Flexion/Extension Isometric: 1 min, 10% MVC (4 s contraction, 4 s rest) Isometric: 1 min, 20% MVC (4 s contraction, 4 s rest) Dynamic: 1 min, 10% MVC (2 s concentric, 2 s eccentric, 2 s rest) Dynamic: 1 min, 20% MVC (2 s concentric, 2 s eccentric, 2 s rest) | ↑SBP: Isometric = Dynamic ↑DBP: Isometric = Dynamic ↑Intramuscular pressure: Isometric > Dynamic ↓ Muscle Oxygenation: Isometric = Dynamic EMG and MMG: Isometric < Dynamic |
| Stebbins [40] | 10 healthy, 7 males and 3 females (20-51 years) | Small | Equivalent among 3 protocols | Handgrip Isometric: 90 s, 30% MVC Dynamic: 180 s, 30% MVC, 1 rep/s Dynamic: 90 s, 60% MVC, 1 rep/s | Similar tension with variable time (equal TTI) ↑SBP,↑DBP,↑MAP: Isometric = Dynamic ↑HR, ↑RPP: Isometric = Dynamic ↑CO only in Dynamic: Isometric = Dynamic →SV, →SVR: Isometric = Dynamic Blood Flow: Isometric < Dynamic RPE: Isometric = Dynamic Increased tension in Dynamic with similar time (equal TTI) ↑SBP,↑DBP,↑MAP: Isometric < Dynamic ↑HR, ↑CO, ↑RPP: Isometric < Dynamic →SV, →SVR: Isometric = Dynamic ↑Blood Flow: Isometric < Dynamic ↑RPE: Isometric < Dynamic |
| Edwards [42] | 14 healthy, 9 males and 5 females (23 ± 19 years) | Small | Equivalent in isometric and dynamic protocols | Handgrip Isometric: 90 s, 30% MVC Dynamic: 180 s, 30% MVC, 1 rep/s | ↑SBP,↑DBP: Isometric = Dynamic ↑HR: Isometric = Dynamic ↑cSBP,↑cDBP: Isometric = Dynamic ↑Augmentation index: Isometric = Dynamic ↑STI, ↑DTI: Isometric = Dynamic |
| Study | Participants | Muscle Mass | Workload (TTI) | Study Design | Results |
|---|---|---|---|---|---|
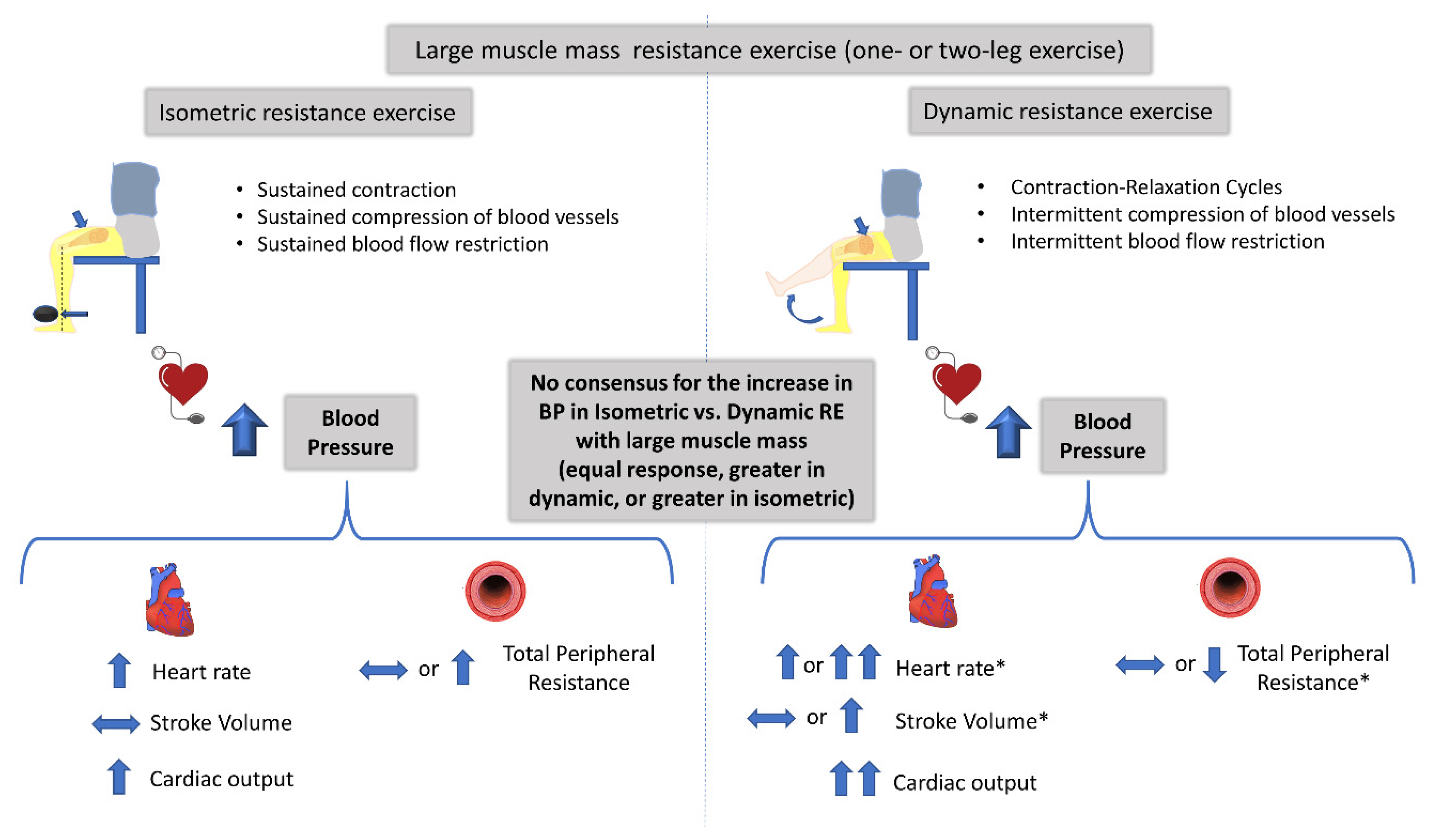
| Lewis [34] | 6 healthy males (27 ± 7 years) | Large | Not Measured | Two-Leg Knee Extension (to Fatigue) Isometric: 25% MVC, 90° Dynamic: 33-40 reps/min 35 kg | ↑SBP,↑MAP: Isometric = Dynamic ↑DBP: Isometric > Dynamic ↑HR: Isometric = Dynamic↑CO: Isometric < Dynamic ↑SV, ↓TPR: Only in Dynamic > Isometric ↑VO2: Isometric < Dynamic |
| Chapman [36] | 5 healthy females (21–22 years) | Large | Uncertain | Two-Leg Knee Extension Isometric: 1 min, 50% MVC 3 Dynamic: 1 min (50 reps), 50% MVC (Displacement: 5, 10, 15 cm) | ↑SBP: Isometric < Dynamic ↑DBP: Isometric > Dynamic ↑MAP*: Isometric = Dynamic ↑HR: Isometric < Dynamic |
| Haennel [37] | 5 healthy males (26 ± 3 years) | Intermediate | Not Measured | One-Leg Knee Extension Isometric: 20 s maximal contraction Isokinetic: 20 s as fast as possible (3 speeds) | ↑MAP: Isometric = Dynamic ↑HR: Isometric < Dynamic ↑CO: Isometric < Dynamic →SV: Isometric = Dynamic ↓ SVR: Only in Dynamic > Isometric ↑RPP: Isometric = Dynamic |
| Iellamo [38] | 10 healthy males (22–42 years) | Intermediate | Not Equivalent Isometric > Both Dynamic | One-Leg Knee Extension Isometric: 1 min, 40% MVC Isokinetic: 30 reps, 40% Peak Torque Isotonic: 30 reps, 40% MVC | ↑SBP: Isometric < Isokinetic = Isotonic ↑DBP: Isometric < Isokinetic = Isotonic ↑HR: Isometric < Isokinetic = Isotonic ↑VO2, ↑VE: Isometric < Isokinetic = Isotonic |
| Koba [43] | 9 healthy, 4 males and 5 females (27 ± 9 years) | Intermediate | Equivalent among 3 protocols | One-Leg Knee Extension Isometric: 2 min sustained, 20% MVC Isometric: 2 min sustained, 40% MVC Dynamic: 4 min, 40% MVC (1 s contraction and 1 s relaxation) | Similar tension-variable time (equal TTI) ↑MAP: Isometric > Dynamic ↑HR: Isometric = Dynamic ↑Blood Flow ↑VO2: Isometric < Dynamic Increased tension-similar time (equal TTI) ↑MAP: Isometric > Dynamic ↑HR: Isometric = Dynamic ↑Blood Flow ↑VO2: Isometric < Dynamic |
| Arimoto [39] | 7 healthy males (20 ± 1 years) | Intermediate Large | Not Measured | One- and Two-Leg Knee Extension Isometric: 6 min, 20% MVC, angle 90º Isometric: 3 min, 40% MVC, angle 90º Dynamic: 6 min, 20% MVC, range 90º Dynamic: 6 min, 40% MVC, range 90º | One-Leg Knee Extension (20 and 40% MVC) ↑SBP, ↑DBP, ↑RPP: Isometric > Dynamic ↑HR: Isometric ≥ Dynamic ↑VO2: Isometric < Dynamic Two-Leg Knee Extension (20 and 40% MVC) ↑SBP, ↑RPP: Isometric = Dynamic (at 20%) ↑SBP, ↑RPP: Isometric > Dynamic (at 40%) ↑DBP: Isometric > Dynamic ↑HR: Isometric ≥ Dynamic ↑VO2: Isometric < Dynamic |
| Yamauchi [44] | 18 healthy participants (19 ± 1 years) | Intermediate | Not Measured | One-Leg Knee Extension Isometric: 1 contraction, 100% F0 Dynamic: 1 contraction, 12% F0 Dynamic: 1 contraction, 22% F0 Dynamic: 1 contraction, 33% F0 Dynamic: 1 contraction, 46% F0 Dynamic: 1 contraction, 66% F0 | ↑MAP: Isometric > all Dynamic |
4. Discussion
4.1. Mechanisms Controlling the Increase in BP during Isometric and Dynamic Resistance Exercise
4.2. Safety of Isometric versus Dynamic Resistance Exercise
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Braith, R.W.; Beck, D.T. Resistance exercise: Training adaptations and developing a safe exercise prescription. Heart Fail. Rev. 2008, 13, 69–79. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Williams, M.A.; Haskell, W.L.; Ades, P.A.; Amsterdam, E.A.; Bittner, V.; Franklin, B.A.; Gulanick, M.; Laing, S.T.; Stewart, K.J. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: A scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation 2007, 116, 572–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornelissen, V.A.; Fagard, R.H.; Coeckelberghs, E.; Vanhees, L. Impact of resistance training on blood pressure and other cardiovascular risk factors: A meta-analysis of randomized, controlled trials. Hypertension 2011, 58, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Chrysant, S.G. Current evidence on the hemodynamic and blood pressure effects of isometric exercise in normotensive and hypertensive persons. J. Clin. Hypertens. 2010, 12, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Pescatello, L.S.; Franklin, B.A.; Fagard, R.; Farquhar, W.B.; Kelley, G.A.; Ray, C.A. American College of Sports Medicine position stand. Exercise and hypertension. Med. Sci. Sports Exerc. 2004, 36, 533–553. [Google Scholar] [CrossRef]
- Topp, R.; Woolley, S.; Hornyak, J., 3rd; Khuder, S.; Kahaleh, B. The effect of dynamic versus isometric resistance training on pain and functioning among adults with osteoarthritis of the knee. Arch. Phys. Med. Rehabil. 2002, 83, 1187–1195. [Google Scholar] [CrossRef]
- Pluim, B.M.; Zwinderman, A.H.; van der Laarse, A.; van der Wall, E.E. The athlete’s heart. A meta-analysis of cardiac structure and function. Circulation 2000, 101, 336–344. [Google Scholar] [CrossRef] [Green Version]
- Fagard, R.H. Athlete’s heart: A meta-analysis of the echocardiographic experience. Int. J. Sports Med. 1996, 17 (Suppl. 3), S140–S144. [Google Scholar] [CrossRef]
- Umpierre, D.; Stein, R. Hemodynamic and vascular effects of resistance training: Implications for cardiovascular disease. Arq. Bras. Cardiol. 2007, 89, 256–262. [Google Scholar] [CrossRef]
- Tanimoto, M.; Ishii, N. Effects of low-intensity resistance exercise with slow movement and tonic force generation on muscular function in young men. J. Appl. Physiol. 2006, 100, 1150–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, J.H.; Haskell, W.; Snell, P.; Van Camp, S.P. Task Force 8: Classification of sports. J. Am. Coll. Cardiol. 2005, 45, 1364–1367. [Google Scholar] [CrossRef] [Green Version]
- Sadamoto, T.; Bonde-Petersen, F.; Suzuki, Y. Skeletal muscle tension, flow, pressure, and EMG during sustained isometric contractions in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1983, 51, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Kagaya, A.; Ogita, F. Blood flow during muscle contraction and relaxation in rhythmic exercise at different intensities. Ann. Physiol. Anthropol. 1992, 11, 251–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radegran, G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J. Appl. Physiol. 1997, 83, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, J.D.; Tuxen, D.; Sale, D.G.; Moroz, J.R.; Sutton, J.R. Arterial blood pressure response to heavy resistance exercise. J. Appl. Physiol. 1985, 58, 785–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wernbom, M.; Augustsson, J.; Thomee, R. The influence of frequency, intensity, volume and mode of strength training on whole muscle cross-sectional area in humans. Sports Med. 2007, 37, 225–264. [Google Scholar] [CrossRef]
- Millar, P.J.; McGowan, C.L.; Cornelissen, V.A.; Araujo, C.G.; Swaine, I.L. Evidence for the role of isometric exercise training in reducing blood pressure: Potential mechanisms and future directions. Sports Med. 2014, 44, 345–356. [Google Scholar] [CrossRef]
- Carlson, D.J.; Dieberg, G.; Hess, N.C.; Millar, P.J.; Smart, N.A. Isometric exercise training for blood pressure management: A systematic review and meta-analysis. Mayo Clin. Proc. 2014, 89, 327–334. [Google Scholar] [CrossRef]
- Brook, R.D.; Appel, L.J.; Rubenfire, M.; Ogedegbe, G.; Bisognano, J.D.; Elliott, W.J.; Fuchs, F.D.; Hughes, J.W.; Lackland, D.T.; Staffileno, B.A.; et al. Beyond medications and diet: Alternative approaches to lowering blood pressure: A scientific statement from the american heart association. Hypertension 2013, 61, 1360–1383. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018, 138, e426–e483. [Google Scholar] [PubMed]
- Sharman, J.E.; Smart, N.A.; Coombes, J.S.; Stowasser, M. Exercise and sport science australia position stand update on exercise and hypertension. J. Hum. Hypertens. 2019, 33, 837–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compton, D.; Hill, P.M.; Sinclair, J.D. Weight-lifters’ blackout. Lancet 1973, 2, 1234–1237. [Google Scholar] [CrossRef]
- Goetting, M.G.; Swanson, S.E. Massive hemorrhage into intracranial neurinomas. Surg. Neurol. 1987, 27, 168–172. [Google Scholar] [CrossRef] [Green Version]
- Haykowsky, M.J.; Findlay, J.M.; Ignaszewski, A.P. Aneurysmal subarachnoid hemorrhage associated with weight training: Three case reports. Clin. J. Sport Med. 1996, 6, 52–55. [Google Scholar] [CrossRef]
- Pott, F.; Van Lieshout, J.J.; Ide, K.; Madsen, P.; Secher, N.H. Middle cerebral artery blood velocity during intense static exercise is dominated by a Valsalva maneuver. J. Appl. Physiol. 2003, 94, 1335–1344. [Google Scholar] [CrossRef] [Green Version]
- Vlak, M.H.; Rinkel, G.J.; Greebe, P.; van der Bom, J.G.; Algra, A. Trigger factors for rupture of intracranial aneurysms in relation to patient and aneurysm characteristics. J. Neurol. 2012, 259, 1298–1302. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, M.; Watanabe, K.; Saito, A.; Matsumura, K.; Ichikawa, M. Circumstances, activities, and events precipitating aneurysmal subarachnoid hemorrhage. J. Stroke Cerebrovasc. Dis. 2007, 16, 25–29. [Google Scholar] [CrossRef]
- Daniels, J.W.; Stebbins, C.L.; Longhurst, J.C. Hemodynamic responses to static and dynamic muscle contractions at equivalent workloads. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R1849–R1855. [Google Scholar] [CrossRef]
- Greer, M.; Dimick, S.; Burns, S. Heartrate and blood pressure response to several methods of strength training. Phys. Ther. 1984, 64, 179–183. [Google Scholar] [CrossRef]
- Kordi, R.; Mazaheri, R.; Rostami, M.; Mansournia, M.A. Hemodynamic changes after static and dynamic exercises and treadmill stress test; different patterns in patients with primary benign exertional headache? Acta Med. Iran. 2012, 50, 399–403. [Google Scholar] [PubMed]
- Lewis, S.F.; Taylor, W.F.; Bastian, B.C.; Graham, R.M.; Pettinger, W.A.; Blomqvist, C.G. Haemodynamic responses to static and dynamic handgrip before and after autonomic blockade. Clin. Sci. 1983, 64, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Louhevaara, V.; Smolander, J.; Aminoff, T.; Korhonen, O.; Shen, N. Cardiorespiratory responses to fatiguing dynamic and isometric hand-grip exercise. Eur. J. Appl. Physiol. 2000, 82, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.F.; Snell, P.G.; Taylor, W.F.; Hamra, M.; Graham, R.M.; Pettinger, W.A.; Blomqvist, C.G. Role of muscle mass and mode of contraction in circulatory responses to exercise. J. Appl. Physiol. 1985, 58, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason-Wehrens, B.; Mayer-Berger, W.; Meister, E.R.; Baum, K.; Hambrecht, R.; Gielen, S. Recommendations for resistance exercise in cardiac rehabilitation. Recommendations of the German Federation for Cardiovascular Prevention and Rehabilitation. Eur. J. Cardiovasc. Prev. Rehabil. 2004, 11, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.H.; Elliott, P.W. Cardiovascular effects of static and dynamic exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1988, 58, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Haennel, R.G.; Snydmiller, G.D.; Teo, K.K.; Greenwood, P.V.; Quinney, H.A.; Kappagoda, C.T. Changes in blood pressure and cardiac output during maximal isokinetic exercise. Arch. Phys. Med. Rehabil. 1992, 73, 150–155. [Google Scholar] [PubMed]
- Iellamo, F.; Legramante, J.M.; Raimondi, G.; Castrucci, F.; Damiani, C.; Foti, C.; Peruzzi, G.; Caruso, I. Effects of isokinetic, isotonic and isometric submaximal exercise on heartrate and blood pressure. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 75, 89–96. [Google Scholar] [CrossRef]
- Arimoto, M.; Kijima, A.; Muramatsu, S. Cardiorespiratory response to dynamic and static leg press exercise in humans. J. Physiol. Anthropol. Appl. Hum. Sci. 2005, 24, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Stebbins, C.L.; Walser, B.; Jafarzadeh, M. Cardiovascular responses to static and dynamic contraction during comparable workloads in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R568–R575. [Google Scholar] [CrossRef]
- Vedsted, P.; Blangsted, A.K.; Sogaard, K.; Orizio, C.; Sjogaard, G. Muscle tissue oxygenation, pressure, electrical, and mechanical responses during dynamic and static voluntary contractions. Eur. J. Appl. Physiol. 2006, 96, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.G.; Mastin, C.R.; Kenefick, R.W. Wave reflection and central aortic pressure are increased in response to static and dynamic muscle contraction at comparable workloads. J. Appl. Physiol. 2008, 104, 439–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koba, S.; Hayashi, N.; Miura, A.; Endo, M.; Fukuba, Y.; Yoshida, T. Pressor response to static and dynamic knee extensions at equivalent workload in humans. Jpn. J. Physiol. 2004, 54, 471–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamauchi, J.; Nakayama, S.; Ishii, N. Blood pressure response to force-velocity properties of the knee-hip extension movement. Eur. J. Appl. Physiol. 2008, 102, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.H.; Payne, F.C.; Saltin, B.; Schibye, B. The role of muscle mass in the cardiovascular response to static contractions. J. Physiol. 1980, 309, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Hart, E.C.; Charkoudian, N.; Wallin, B.G.; Curry, T.B.; Eisenach, J.H.; Joyner, M.J. Sex differences in sympathetic neural-hemodynamic balance: Implications for human blood pressure regulation. Hypertension 2009, 53, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Lovell, D.I.; Cuneo, R.; Gass, G.C. The blood pressure response of older men to maximum and sub-maximum strength testing. J. Sci. Med. Sport 2011, 14, 254–258. [Google Scholar] [CrossRef]
- Joyner, M.J.; Wallin, B.G.; Charkoudian, N. Sex differences and blood pressure regulation in humans. Exp. Physiol. 2016, 101, 349–355. [Google Scholar] [CrossRef]
- Blomqvist, C.G.; Lewis, S.F.; Taylor, W.F.; Graham, R.M. Similarity of the hemodynamic responses to static and dynamic exercise of small muscle groups. Circ. Res. 1981, 48, I87–I92. [Google Scholar]
- MacDougall, J.D.; McKelvie, R.S.; Moroz, D.E.; Sale, D.G.; McCartney, N.; Buick, F. Factors affecting blood pressure during heavy weight lifting and static contractions. J. Appl. Physiol. 1992, 73, 1590–1597. [Google Scholar] [CrossRef]
- Boushel, R. Muscle metaboreflex control of the circulation during exercise. Acta Physiol. 2010, 199, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.H.; Kaufman, M.P.; Iwamoto, G.A. The exercise pressor reflex: Its cardiovascular effects, afferent mechanisms, and central pathways. Annu. Rev. Physiol. 1983, 45, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.J.; Nauss, L.A.; Warner, M.A.; Warner, D.O. Sympathetic modulation of blood flow and O2 uptake in rhythmically contracting human forearm muscles. Am. J. Physiol. 1992, 263, H1078–H1083. [Google Scholar] [CrossRef]
- Victor, R.G.; Secher, N.H.; Lyson, T.; Mitchell, J.H. Central command increases muscle sympathetic nerve activity during intense intermittent isometric exercise in humans. Circ. Res. 1995, 76, 127–131. [Google Scholar] [CrossRef]
- Gallagher, K.M.; Fadel, P.J.; Smith, S.A.; Stromstad, M.; Ide, K.; Secher, N.H.; Raven, P.B. The interaction of central command and the exercise pressor reflex in mediating baroreflex resetting during exercise in humans. Exp. Physiol. 2006, 91, 79–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartwich, D.; Dear, W.E.; Waterfall, J.L.; Fisher, J.P. Effect of muscle metaboreflex activation on spontaneous cardiac baroreflex sensitivity during exercise in humans. J. Physiol. 2011, 589, 6157–6171. [Google Scholar] [CrossRef]
- Hureau, T.J.; Weavil, J.C.; Thurston, T.S.; Broxterman, R.M.; Nelson, A.D.; Bledsoe, A.D.; Jessop, J.E.; Richardson, R.S.; Wray, D.W.; Amann, M. Identifying the role of group III/IV muscle afferents in the carotid baroreflex control of mean arterial pressure and heartrate during exercise. J. Physiol. 2018, 596, 1373–1384. [Google Scholar] [CrossRef] [Green Version]
- Iellamo, F.; Legramante, J.M.; Raimondi, G.; Peruzzi, G. Baroreflex control of sinus node during dynamic exercise in humans: Effects of central command and muscle reflexes. Am. J. Physiol. 1997, 272, H1157–H1164. [Google Scholar] [CrossRef]
- Victor, R.G.; Pryor, S.L.; Secher, N.H.; Mitchell, J.H. Effects of partial neuromuscular blockade on sympathetic nerve responses to static exercise in humans. Circ. Res. 1989, 65, 468–476. [Google Scholar] [CrossRef] [Green Version]
- Zouhal, H.; Jacob, C.; Delamarche, P.; Gratas-Delamarche, A. Catecholamines and the effects of exercise, training and gender. Sports Med. 2008, 38, 401–423. [Google Scholar] [CrossRef]
- Mitchell, J.H. Cardiovascular control during exercise: Central and reflex neural mechanisms. Am. J. Cardiol. 1985, 55, 34D–41D. [Google Scholar] [CrossRef]
- Kjaer, M.; Secher, N.H. Neural influence on cardiovascular and endocrine responses to static exercise in humans. Sports Med. 1992, 13, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, R.S. Catecholamine responses to acute and chronic exercise. Med. Sci. Sports Exerc. 1991, 23, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, M.; Secher, N.H.; Galbo, H. Physical stress and catecholamine release. Baillieres Clin. Endocrinol. Metab. 1987, 1, 279–298. [Google Scholar] [CrossRef]
- Seals, D.R.; Victor, R.G. Regulation of muscle sympathetic nerve activity during exercise in humans. Exerc. Sport Sci. Rev. 1991, 19, 313–349. [Google Scholar] [CrossRef]
- Okamoto, T.; Masuhara, M.; Ikuta, K. Upper but not lower limb resistance training increases arterial stiffness in humans. Eur. J. Appl. Physiol. 2009, 107, 127–134. [Google Scholar] [CrossRef]
- Wiles, J.D.; Taylor, K.; Coleman, D.; Sharma, R.; O’Driscoll, J.M. The safety of isometric exercise: Rethinking the exercise prescription paradigm for those with stage 1 hypertension. Medicine 2018, 97, e0105. [Google Scholar] [CrossRef]
- McCartney, N. Acute responses to resistance training and safety. Med. Sci. Sports Exerc. 1999, 31, 31–37. [Google Scholar] [CrossRef]
- Bertagnoli, K.; Hanson, P.; Ward, A. Attenuation of exercise-induced ST depression during combined isometric and dynamic exercise in coronary artery disease. Am. J. Cardiol. 1990, 65, 314–317. [Google Scholar] [CrossRef]
- DeBusk, R.F.; Valdez, R.; Houston, N.; Haskell, W. Cardiovascular responses to dynamic and static effort soon after myocardial infarction. Application to occupational work assessment. Circulation 1978, 58, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Franklin, B.A.; Bonzheim, K.; Gordon, S.; Timmis, G.C. Resistance training in cardiac rehabilitation. J. Cardiopulm. Rehabil. 1991, 11, 99–107. [Google Scholar] [CrossRef]
- Chaney, R.H.; Arndt, S. Comparison of cardiovascular risk in maximal isometric and dynamic exercise. South. Med. J. 1983, 76, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Haissly, J.C.; Messin, R.; Degre, S.; Vandermoten, P.; Demaret, B.; Denolin, H. Comparative response to isometric (static) and dynamic exercise tests in coronary disease. Am. J. Cardiol. 1974, 33, 791–796. [Google Scholar] [CrossRef]
- Kerber, R.E.; Miller, R.A.; Najjar, S.M. Myocardial ischemic effects of isometric, dynamic and combined exercise in coronary artery disease. Chest 1975, 67, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Kelley, G.A.; Kelley, K.S. Isometric handgrip exercise and resting blood pressure: A meta-analysis of randomized controlled trials. J. Hypertens. 2010, 28, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Zebrowska, A.; Gasior, Z.; Jastrzebski, D. Cardiovascular effects of the valsalva maneuver during static arm exercise in elite power lifting athletes. Adv. Exp. Med. Biol. 2013, 755, 335–342. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kounoupis, A.; Papadopoulos, S.; Galanis, N.; Dipla, K.; Zafeiridis, A. Are Blood Pressure and Cardiovascular Stress Greater in Isometric or in Dynamic Resistance Exercise? Sports 2020, 8, 41. https://doi.org/10.3390/sports8040041
Kounoupis A, Papadopoulos S, Galanis N, Dipla K, Zafeiridis A. Are Blood Pressure and Cardiovascular Stress Greater in Isometric or in Dynamic Resistance Exercise? Sports. 2020; 8(4):41. https://doi.org/10.3390/sports8040041
Chicago/Turabian StyleKounoupis, Anastasios, Stavros Papadopoulos, Nikiforos Galanis, Konstantina Dipla, and Andreas Zafeiridis. 2020. "Are Blood Pressure and Cardiovascular Stress Greater in Isometric or in Dynamic Resistance Exercise?" Sports 8, no. 4: 41. https://doi.org/10.3390/sports8040041
APA StyleKounoupis, A., Papadopoulos, S., Galanis, N., Dipla, K., & Zafeiridis, A. (2020). Are Blood Pressure and Cardiovascular Stress Greater in Isometric or in Dynamic Resistance Exercise? Sports, 8(4), 41. https://doi.org/10.3390/sports8040041






