Low-Dose Ammonium Preconditioning Enhances Endurance in Submaximal Physical Exercises
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Participants
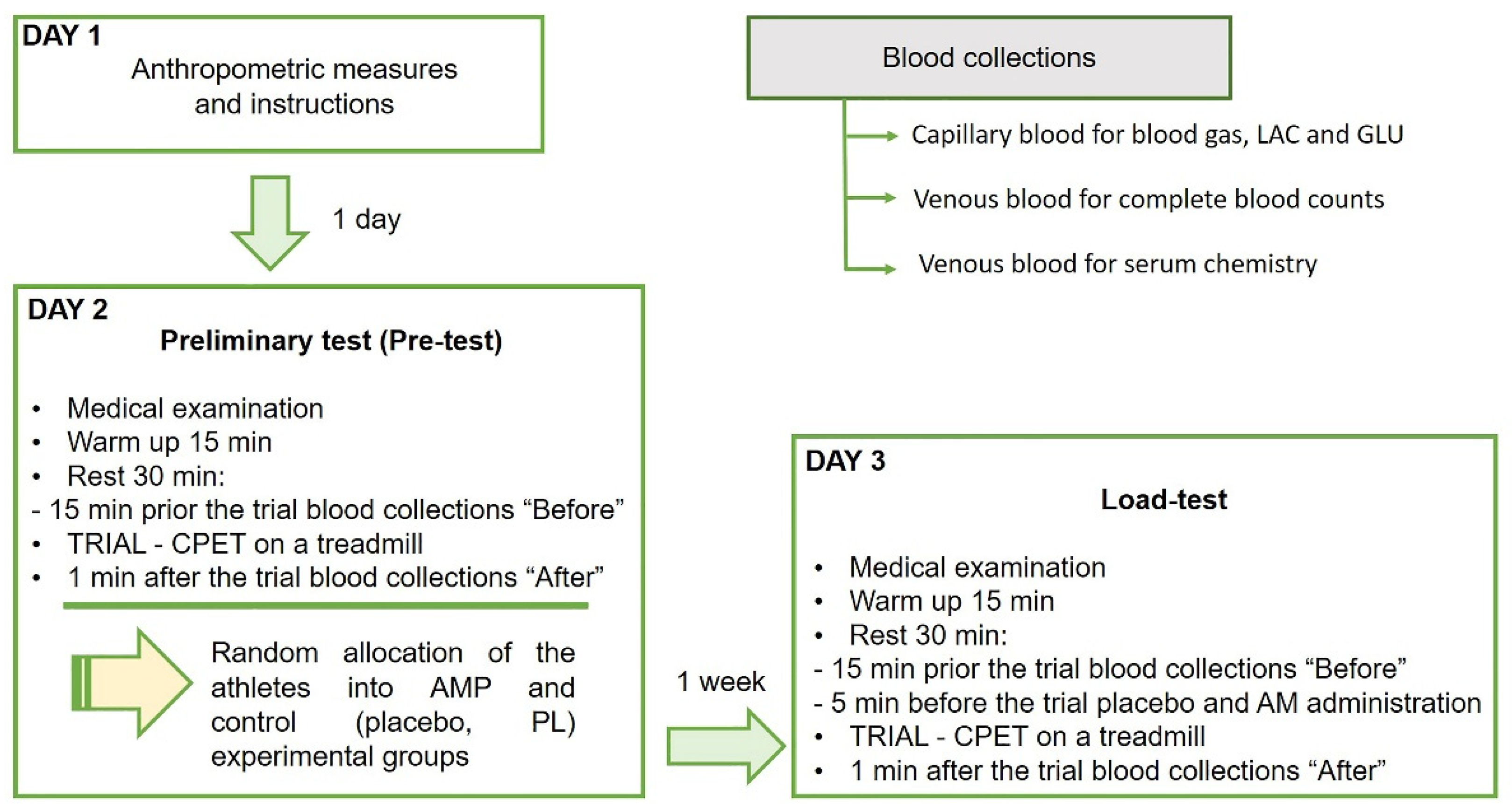
2.3. Experimental Design
2.3.1. Preliminary Test (Pre-Test)
2.3.2. Cessation Criteria for the Trial
2.3.3. Methodology of Group Formation
2.3.4. Testing Day
2.3.5. Ammonium and Placebo Preconditioning
2.4. Instrumentation and Measurements
2.4.1. Cardiopulmonary Endurance Test (CPET)
2.4.2. Blood Collections and Analyses
2.5. Data Calculations
2.6. Statistical Analysis
3. Results
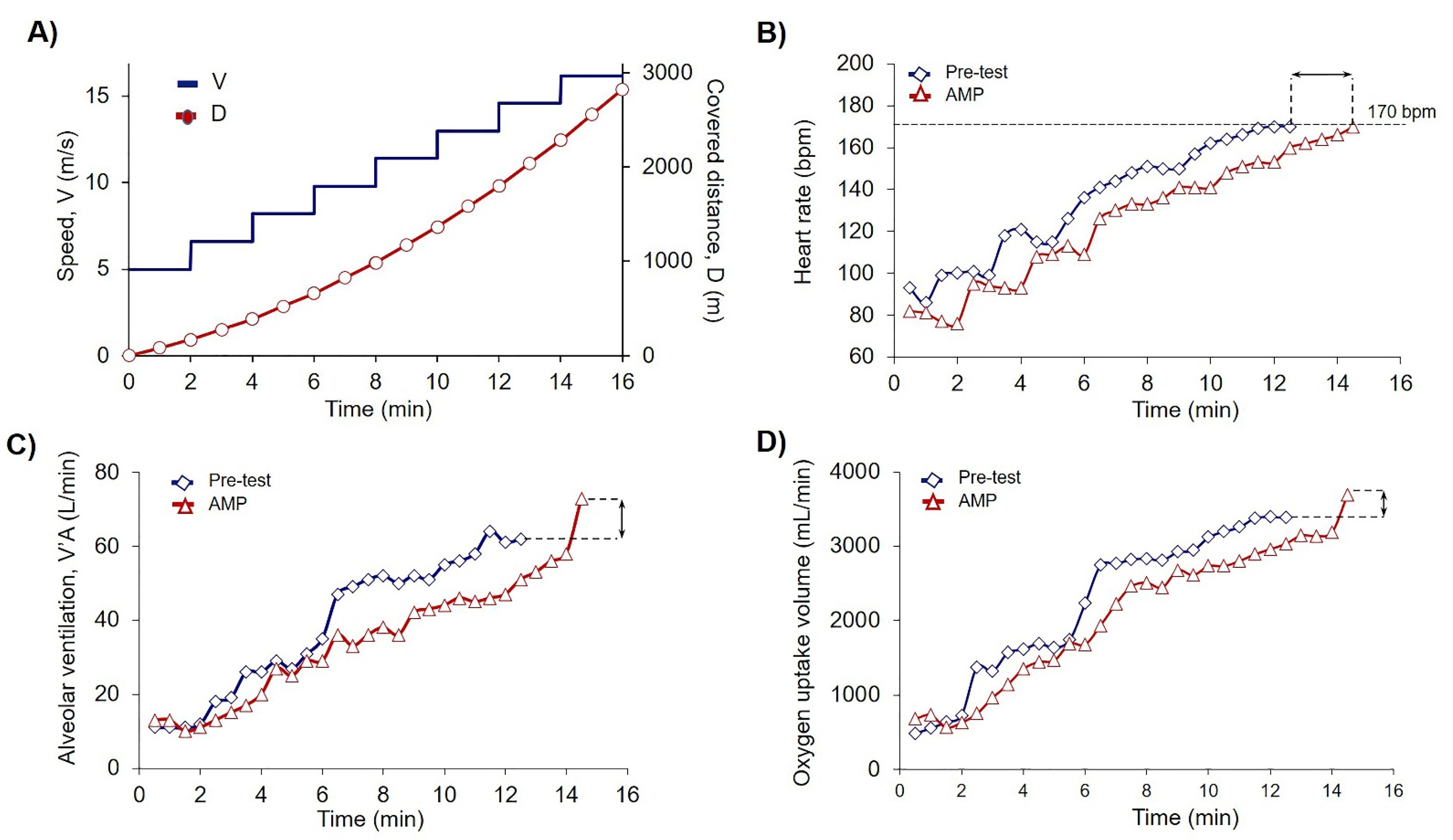
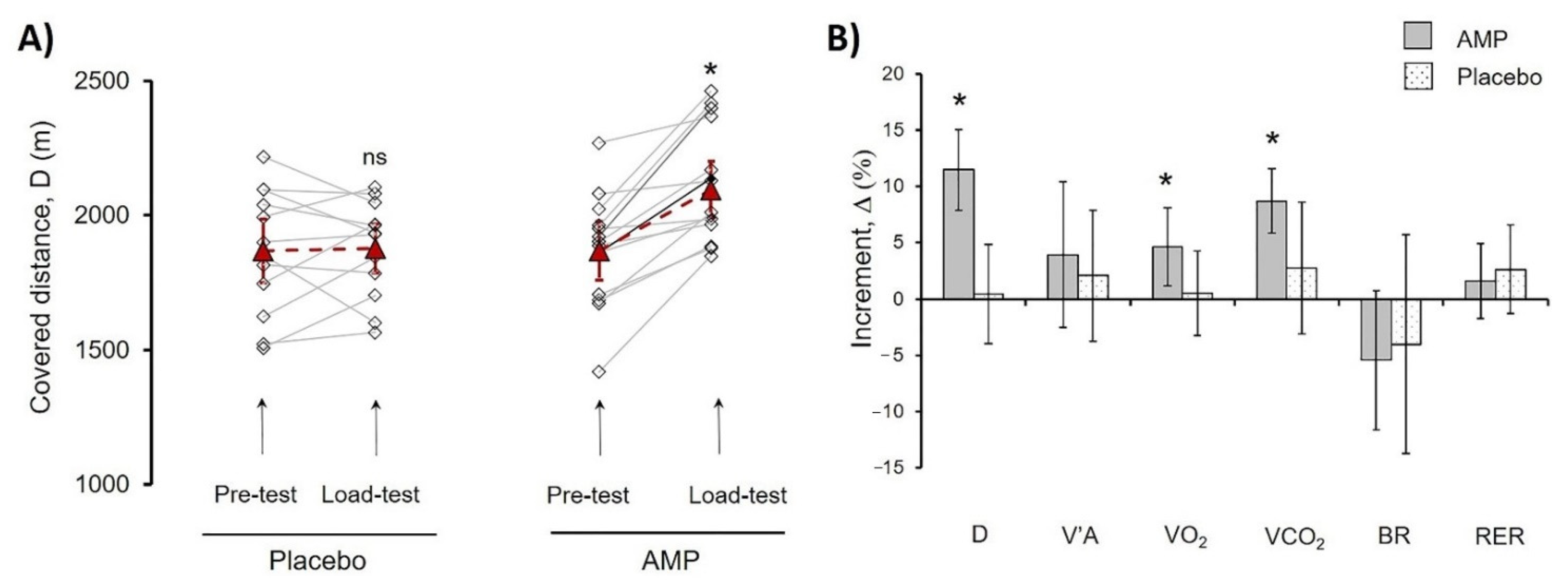
3.1. Ammonium Preconditioning Showed the Beneficial Effects in Physical Exercise Progress
3.2. AMP Reduced the Shifts in the Acid-Base Balance under Physical Exercises
3.3. AMP Did Not Induce Significant Changes in Main Enzymatic Biochemical Blood Parameters
3.4. AMP Increased RBC Count and Hb Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laursen, P.B. Training for intense exercise performance: High-intensity or high-volume training? Scand. J. Med. Sci. Sports 2010, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.; Girard, O.; Mendez-Villanueva, A. Repeated-Sprint Ability—Part II. Sports Med. 2011, 41, 741–756. [Google Scholar] [CrossRef] [PubMed]
- Kilduff, L.P.; Finn, C.V.; Baker, J.S.; Cook, C.J.; West, D.J. Preconditioning Strategies to Enhance Physical Performance on the Day of Competition. Int. J. Sports Physiol. Perform. 2013, 8, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Hostrup, M.; Bangsbo, J. Limitations in intense exercise performance of athletes–Effect of speed endurance training on ion handling and fatigue development. J. Physiol. 2017, 595, 2897–2913. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.G.; Jones, H.; Gregson, W.; Atkinson, G.; Cable, T.N.; Thijssen, D.H.J. Effect of Ischemic Preconditioning on Lactate Accumulation and Running Performance. Med. Sci. Sports Exerc. 2012, 44, 2084–2089. [Google Scholar] [CrossRef]
- Da Mota, G.R.; Marocolo, M. The Effects of Ischemic Preconditioning on Human Exercise Performance: A Counterpoint. Sports Med. 2016, 46, 1575–1576. [Google Scholar] [CrossRef] [PubMed]
- Marocolo, M.; Billaut, F.; Da Mota, G.R. Ischemic Preconditioning and Exercise Performance: An Ergogenic Aid for Whom? Front. Physiol. 2018, 9, 1874. [Google Scholar] [CrossRef]
- Lintz, J.A.; Dalio, M.B.; Joviliano, E.E.; Piccinato, C.E. Ischemic pre and postconditioning in skeletal muscle injury produced by ischemia and reperfusion in rats. Acta Cir. Bras. 2013, 28, 441–446. [Google Scholar] [CrossRef]
- Addison, P.D.; Neligan, P.C.; Ashrafpour, H.; Khan, A.; Zhong, A.; Moses, M.; Forrest, C.R.; Pang, C.Y. Noninvasive remote ischemic preconditioning for global protection of skeletal muscle against infarction. Am. J. Physiol. Circ. Physiol. 2003, 285, H1435–H1443. [Google Scholar] [CrossRef]
- Lee, H.; Schroeder, J.C.; Shah, P.; Babu, S.; Thompson, C.; Belloni, F. Preconditioning with Ischemia or Adenosine Protects Skeletal Muscle from Ischemic Tissue Reperfusion Injury. J. Surg. Res. 1996, 63, 29–34. [Google Scholar] [CrossRef]
- Incognito, A.V.; Burr, J.F.; Millar, P.J. The Effects of Ischemic Preconditioning on Human Exercise Performance. Sports Med. 2016, 46, 531–544. [Google Scholar] [CrossRef]
- Montoye, A.H.; Mitchinson, C.J.; Townsend, O.R.; Nemmers, C.H.; Serkaian, C.N.; Rider, B.C. Ischemic Preconditioning Does Not Improve Time Trial Performance in Recreational Runners. Int. J Exerc. Sci. 2020, 13, 1402–1417. [Google Scholar]
- Barbosa, T.C.; Machado, A.C.; Braz, I.D.; Fernandes, I.A.; Vianna, L.C.; Nobrega, A.C.L.; Silva, B.M. Remote ischemic preconditioning delays fatigue development during handgrip exercise. Scand. J. Med. Sci. Sports 2015, 25, 356–364. [Google Scholar] [CrossRef]
- Tomschi, F.; Niemann, D.; Bloch, W.; Predel, H.-G.; Grau, M. Ischemic Preconditioning Enhances Performance and Erythrocyte Deformability of Responders. Int. J. Sports Med. 2018, 39, 596–603. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, M.C.; Gerosa-Neto, J.; Zanchi, N.E.; Lira, F.S.; Rossi, F.E. Role of metabolic stress for enhancing muscle adaptations: Practical applications. World J. Methodol. 2017, 7, 46–54. [Google Scholar] [CrossRef]
- Birnbaumer, P.; Müller, A.; Tschakert, G.; Sattler, M.C.; Hofmann, P. Performance Enhancing Effect of Metabolic Pre-conditioning on Upper-Body Strength-Endurance Exercise. Front. Physiol. 2018, 9, 963. [Google Scholar] [CrossRef]
- Galloway, S.D.R.; Talanian, J.L.; Shoveller, A.K.; Heigenhauser, G.J.F.; Spriet, L.L. Seven days of oral taurine supplementation does not increase muscle taurine content or alter substrate metabolism during prolonged exercise in humans. J. Appl. Physiol. 2008, 105, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Waldron, M.; Patterson, S.D.; Jeffries, O. Oral taurine improves critical power and severe-intensity exercise tolerance. Amino Acids 2019, 51, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Housh, T.J.; Devries, H.A.; Johnson, G.O.; Evans, S.A.; McDowell, S. The effect of ammonium chloride and sodium bicarbonate ingestion on the physical working capacity at the fatigue threshold. Graefe’s Arch. Clin. Exp. Ophthalmol. 1991, 62, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I.; Hermiston, A.J.; Symons, J.D. Effects of prior exercise or ammonium chloride ingestion on muscular strength and endurance. Med. Sci. Sports Exerc. 1993, 25, 809–814. [Google Scholar] [CrossRef]
- Peart, D.J.; Kirk, R.J.; Hillman, A.R.; Madden, L.A.; Siegler, J.C.; Vince, R.V. The physiological stress response to high-intensity sprint exercise following the ingestion of sodium bicarbonate. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 113, 127–134. [Google Scholar] [CrossRef]
- Egger, F.; Meyer, T.; Such, U.; Hecksteden, A. Effects of Sodium Bicarbonate on High-Intensity Endurance Performance in Cyclists: A Double-Blind, Randomized Cross-Over Trial. PLoS ONE 2014, 9, e114729. [Google Scholar] [CrossRef]
- Siegler, J.C.; Mudie, K.; Marshall, P. The influence of sodium bicarbonate on maximal force and rates of force development in the triceps surae and brachii during fatiguing exercise. Exp. Physiol. 2016, 101, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.J.; Hopkins, W.G.; Gore, C.J. Effects of Acute Alkalosis and Acidosis on Performance. Sports Med. 2011, 41, 801–814. [Google Scholar] [CrossRef]
- Hollidge-Horvat, M.G.; Parolin, M.L.; Wong, D.; Jones, N.L.; Heigenhauser, G.J.F. Effect of induced metabolic alkalosis on human skeletal muscle metabolism during exercise. Am. J. Physiol. Metab. 2000, 278, E316–E329. [Google Scholar] [CrossRef] [PubMed]
- Ament, W.; Verkerke, G.J. Exercise and Fatigue. Sports Med. 2009, 39, 389–422. [Google Scholar] [CrossRef]
- Coelho, W.S.; De Castro, L.V.; Deane, E.; Magno-França, A.; Bassini, A.; Cameron, L.-C. Investigating the Cellular and Metabolic Responses of World-Class Canoeists Training: A Sportomics Approach. Nutrients 2016, 8, 719. [Google Scholar] [CrossRef]
- Schranner, D.; Kastenmüller, G.; Schönfelder, M.; Römisch-Margl, W.; Wackerhage, H. Metabolite Concentration Changes in Humans After a Bout of Exercise: A Systematic Review of Exercise Metabolomics Studies. Sports Med. Open 2020, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Cairns, S.P. Lactic Acid and Exercise Performance. Sports Med. 2006, 36, 279–291. [Google Scholar] [CrossRef]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- Mutch, B.J.; Banister, E.W. Ammonia metabolism in exercise and fatigue: A review. Med. Sci. Sports Exerc. 1983, 15, 41–50. [Google Scholar] [CrossRef]
- Banister, E.W.; Rajendra, W.; Mutch, B.J. Ammonia as an Indicator of Exercise Stress Implications of Recent Findings to Sports Medicine. Sports Med. 1985, 2, 34–46. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Goldfarb, A.H. Anaerobic Exercise and Oxidative Stress: A Review. Can. J. Appl. Physiol. 2004, 29, 245–263. [Google Scholar] [CrossRef]
- Numan, Y.; Jawaid, Y.; Hirzallah, H.; Kusmic, D.; Megri, M.; Aqtash, O.; Amro, A.; Mezughi, H.; Maher, E.; Raru, Y.; et al. Ammonia vs. Lactic Acid in Predicting Positivity of Microbial Culture in Sepsis: The ALPS Pilot Study. J. Clin. Med. 2018, 7, 182. [Google Scholar] [CrossRef]
- Drews, L.; Zimmermann, M.; Westhoff, P.; Brilhaus, D.; Poss, R.E.; Bergmann, L.; Wiek, C.; Brenneisen, P.; Piekorz, R.P.; Mettler-Altmann, T.; et al. Ammonia inhibits energy metabolism in astrocytes in a rapid and glutamate dehydrogenase 2-dependent manner. Dis. Model. Mech. 2020, 13, dmm047134. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Graham, T.E. Glutamate ingestion and its effects at rest and during exercise in humans. J. Appl. Physiol. 2002, 93, 1251–1259. [Google Scholar] [CrossRef]
- Liu, Y.; Lange, R.; Langanky, J.; Hamma, T.; Yang, B.; Steinacker, J.M. Improved training tolerance by supplementation with α-Keto acids in untrained young adults: A randomized, double blind, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2012, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Kamei, Y.; Hatazawa, Y.; Uchitomi, R.; Yoshimura, R.; Miura, S. Regulation of Skeletal Muscle Function by Amino Acids. Nutrients 2020, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Robergs, R.; Hutchinson, K.; Hendee, S.; Madden, S.; Siegler, J. Influence of Pre-Exercise Acidosis and Alkalosis on the Kinetics of Acid-Base Recovery Following Intense Exercise. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Korf, E.A.; Mindukshev, I.V.; Novozhilov, A.V.; Krivchenko, A.I.; Goncharov, N.V. Ammonium Salts Increase Physical Performance and Reduce Blood Lactate Level in Rats in a Model of Forced Swimming. Bull. Exp. Biol. Med. 2020, 168, 610–613. [Google Scholar] [CrossRef]
- Novozhilov, A.V.; Mindukshev, I.V.; Korf, E.A.; Krivchenko, A.I.; Goncharov, N.V. Ammonium Salts Promote Functional Adaptation of Rat Erythrocytes on the Model of Forced Swimming. Bull. Exp. Biol. Med. 2020, 168, 444–448. [Google Scholar] [CrossRef]
- Chen, S.; Minegishi, Y.; Hasumura, T.; Shimotoyodome, A.; Ota, N. Involvement of ammonia metabolism in the improvement of endurance performance by tea catechins in mice. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.M., 3rd; Naughton, J.P.; Haskell, W.L. Physical activity and the prevention of coronary heart disease. Ann. Clin. Res. 1971, 3, 404–432. [Google Scholar] [CrossRef]
- Shookster, D.; Lindsey, B.; Cortes, N.; Martin, J.R. Accuracy of Commonly Used Age-Predicted Maximal Heart Rate Equations. Int. J. Exerc. Sci. 2020, 13, 1242–1250. [Google Scholar]
- Stewart, I.B.; Warburton, D.E.R.; Hodges, A.N.H.; Lyster, D.M.; McKenzie, N.C. Cardiovascular and splenic responses to exercise in humans. J. Appl. Physiol. 2003, 94, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.E.; Bangsbo, J.; Gollnick, P.D.; Juel, C.; Saltin, B. Ammonia metabolism during intense dynamic exercise and recovery in humans. Am. J. Physiol. Metab. 1990, 259, E170–E176. [Google Scholar] [CrossRef]
- Kantanista, A.; Krzysztof, K.; Dopierala, K.; Trinschek, J.; Krol, H.; Wlodarczyk, M.; Łodarczyk, M.; Zielinski, J. Blood lactate, ammonia and kinematic indices during a speed-endurance training session in elite sprinters. TRENDS Sport Sci. 2016, 2, 73–79. [Google Scholar]
- Itoh, H.; Ohkuwa, T. Peak blood ammonia and lactate after submaximal, maximal and supramaximal exercise in sprinters and long-distance runners. Graefe’s Arch. Clin. Exp. Ophthalmol. 1990, 60, 271–276. [Google Scholar] [CrossRef]
- Huizenga, J.R.; Tangerman, A.; Gips, C.H. Determination of Ammonia in Biological Fluids. Ann. Clin. Biochem. Int. J. Lab. Med. 1994, 31, 529–543. [Google Scholar] [CrossRef]
- Sudnitsyna, J.; Gambaryan, S.P.; Krivchenko, A.I.; Mindukshev, I.V. Ammonia/ammonium influx in human erythrocytes. Biologicheskie Membrany 2018, 35, 398–402. [Google Scholar] [CrossRef]
- Yuan, Y.; So, R.; Wong, S.; Chan, K.M. Ammonia threshold-comparison to lactate threshold, correlation to other physiological parameters and response to training. Scand. J. Med. Sci. Sports 2002, 12, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Malek, M.H.; Housh, T.J.; Crouch, L.D.; Johnson, G.O.; Hendrix, C.R.; Beck, T.W.; Mielke, M.; Schmidt, R.J.; Housh, D.J. Plasma Ammonia Concentrations and the Slow Component of Oxygen Uptake Kinetics During Cycle Ergometry. J. Strength Cond. Res. 2008, 22, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Grivennikova, V.G.; Kareyeva, A.V.; Vinogradov, A.D. What are the sources of hydrogen peroxide production by heart mitochondria? Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1797, 939–944. [Google Scholar] [CrossRef]
- Stathis, C.G.; Febbraio, M.A.; Carey, M.F.; Snow, R.J. Influence of sprint training on human skeletal muscle purine nucleotide metabolism. J. Appl. Physiol. 1994, 76, 1802–1809. [Google Scholar] [CrossRef]
- Barta, E.; Babusikova, F. The concentration of ammonia in blood and plasma stored for transfusion. Resuscitation 1982, 10, 135–139. [Google Scholar] [CrossRef]
- Ludewig, U.; Von Wirén, N.; Rentsch, R.; Frommer, W.B. Rhesus factors and ammonium: A function in efflux? Genome Biol. 2001, 2, 1010. [Google Scholar] [CrossRef]
- Hemker, M.B.; Cheroutre, G.; Van Zwieten, R.; Wijk, P.A.M.-V.; Roos, D.; Loos, J.A.; Van Der Schoot, C.E.; Borne, A.E.G.K.V.D. The Rh complex exports ammonium from human red blood cells. Br. J. Haematol. 2003, 122, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Khademi, S. Mechanism of Ammonia Transport by Amt/MEP/Rh: Structure of AmtB at 1.35 A. Science 2004, 305, 1587–1594. [Google Scholar] [CrossRef]
- Ripoche, P.; Bertrand, O.; Gane, P.; Birkenmeier, C.; Colin, Y.; Cartron, J.-P. Human Rhesus-associated glycoprotein mediates facilitated transport of NH3 into red blood cells. Proc. Natl. Acad. Sci. USA 2004, 101, 17222–17227. [Google Scholar] [CrossRef]
- Sudnitsyna, J.S.; Skvertchinskaya, E.A.; Dobrylko, I.A.; Nikitina, E.R.; Krivchenko, A.I.; Gambaryan, S.P.; Mindukshev, I.V. Human erythrocyte ammonium transport is mediated by functional interaction of ammonium (RhAG) and anion (AE1) transporters. Biochem. (Moscow) Suppl. Ser. A Membr. Cell Biol. 2016, 10, 301–310. [Google Scholar] [CrossRef]
- Laub, M.; Hvid-Jacobsen, K.; Hovind, P.; Kanstrup, I.L.; Christensen, N.J.; Nielsen, S.L. Spleen emptying and venous hematocrit in humans during exercise. J. Appl. Physiol. 1993, 74, 1024–1026. [Google Scholar] [CrossRef]
- Stewart, I.B.; McKenzie, D.C. The Human Spleen During Physiological Stress. Sports Med. 2002, 32, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Shephard, R.J. Responses of the human spleen to exercise. J. Sports Sci. 2015, 34, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Pilegaard, H.; Domino, K.; Noland, T.; Juel, C.; Hellsten, Y.; Halestrap, A.P.; Bangsbo, J. Effect of high-intensity exercise training on lactate/H+ transport capacity in human skeletal muscle. Am. J. Physiol. Metab. 1999, 276, E255–E261. [Google Scholar] [CrossRef]
- Sudnitsyna, J.; Skverchinskaya, E.; Dobrylko, I.; Nikitina, E.; Gambaryan, S.; Mindukshev, I. Microvesicle Formation Induced by Oxidative Stress in Human Erythrocytes. Antioxidants 2020, 9, 929. [Google Scholar] [CrossRef]
- Seeger, J.P.; Timmers, S.; Ploegmakers, D.J.; Cable, N.T.; Hopman, M.T.; Thijssen, D.H. Is delayed ischemic preconditioning as effective on running performance during a 5 km time trial as acute IPC? J. Sci. Med. Sport 2017, 20, 208–212. [Google Scholar] [CrossRef]
- Caru, M.; Levesque, A.; LaLonde, F.; Curnier, D. An overview of ischemic preconditioning in exercise performance: A systematic review. J. Sport Health Sci. 2019, 8, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Barrabes, J.A.; Bøtker, H.E.; Davidson, S.M.; Di Lisa, F.; Downey, J.; Engstrom, T.; Ferdinandy, P.; Carbrera-Fuentes, H.A.; Heusch, G.; et al. Ischaemic conditioning and targeting reperfusion injury: A 30 year voyage of discovery. Basic Res. Cardiol. 2016, 111, 1–24. [Google Scholar] [CrossRef]
| Athletes | Age (years) | Weight (kg) | Height (cm) | Experience (years) | Pre-Test (D, m) | Assigned Group for the Load-Test | |
|---|---|---|---|---|---|---|---|
| AMP (D, m) | PL (D, m) | ||||||
| No. 1 | 18 | 80 | 178 | 8 | 1417 | 1417 | |
| No. 2 | 19 | 62 | 174 | 4 | 1508 | 1508 | |
| No. 3 | 18 | 68 | 180 | 8 | 1522 | 1522 | |
| No. 4 | 19 | 66 | 177 | 7 | 1623 | 1623 | |
| No. 5 | 21 | 60 | 171 | 12 | 1674 | 1674 | |
| No. 6 | 19 | 61 | 174 | 6 | 1684 | 1684 | |
| No. 7 | 28 | 83 | 174 | 12 | 1706 | 1706 | |
| No. 8 | 19 | 72 | 179 | 8 | 1746 | 1746 | |
| No. 9 | 20 | 72 | 176 | 7 | 1853 | 1853 | |
| No. 10 | 18 | 72 | 173 | 8 | 1861 | 1861 | |
| No. 11 | 18 | 59 | 178 | 6 | 1861 | 1861 | |
| No. 12 | 25 | 75 | 186 | 12 | 1889 | 1889 | |
| No. 13 | 19 | 70 | 173 | 11 | 1901 | 1901 | |
| No. 14 | 18 | 78 | 182 | 6 | 1901 | 1901 | |
| No. 15 | 19 | 75 | 174 | 11 | 1922 | 1922 | |
| No. 16 | 19 | 83 | 178 | 10 | 1950 | 1950 | |
| No. 17 | 20 | 68 | 178 | 9 | 1962 | 1962 | |
| No. 18 | 19 | 67 | 178 | 9 | 1995 | 1995 | |
| No. 19 | 24 | 74 | 178 | 11 | 2023 | 2023 | |
| No. 20 | 28 | 75 | 178 | 15 | 2039 | 2039 | |
| No. 21 | 20 | 72 | 174 | 8 | 2080 | 2080 | |
| No. 22 | 27 | 82 | 181 | 6 | 2096 | 2096 | |
| No. 23 | 18 | 78 | 182 | 6 | 2096 | 2096 | |
| No. 24 | 18 | 69 | 181 | 6 | 2218 | 2218 | |
| No. 25 | 20 | 72 | 174 | 5 | 2270 | 2270 | |
| n | 25 | 25 | 25 | 25 | 25 | 14 | 11 |
| Mean | 20.4 | 71.7 | 177.2 | 8.4 | 1871.9 | 1871 ns | 1873 ns |
| SD | 3.2 | 6.9 | 3.6 | 2.7 | 218.8 | 206 | 244 |
| CPET Parameter | Pre-Test | Load-Test | ||
|---|---|---|---|---|
| AMP | PL | AMP | PL | |
| D, m | 1871 ± 206 | 1872 ± 244 | 2118 ± 216 * | 1885 ± 186 |
| V’A, L/min | 77.0 ± 14.6 | 79.0 ± 15.9 | 83.2 ± 12.6 | 81.4 ± 13.3 |
| VO2, mL/min | 3292 ± 482 | 3398 ± 456 | 3433 ± 405 * | 3410 ± 382 |
| VCO2, mL/min | 2775 ± 423 | 2903 ± 481 | 3084 ± 397 * | 2946 ± 432 |
| BR, % | 50.4 ± 9.1 | 47.7 ± 11.5 | 48.1 ± 8.7 | 47.4 ± 15.3 |
| RER | 0.84 ± 0.04 | 0.85 ± 0.07 | 0.90 ± 0.04 | 0.86 ± 0.06 |
| Parameter | Pre-Test | Load-Test | ||||||
|---|---|---|---|---|---|---|---|---|
| AMP | PL | AMP | PL | |||||
| Before | After | Before | After | Before | After | Before | After | |
| pH | 7.37 ± 0.04 | 7.29 ± 0.07 * | 7.39 ± 0.02 | 7.31 ± 0.05 * | 7.35 ± 0.03 | 7.30 ± 0.04 * | 7.36 ± 0.04 | 7.28 ± 0.05 * |
| pO2 (mmHg) | 39.6 ± 8.0 | 34.6 ± 6.4 * | 38.5 ± 6.7 | 31.7 ± 8.5 * | 38.4 ± 3.8 | 34.0 ± 7.0 * | 40.3 ± 3.2 | 29.9 ± 7.9 * |
| pCO2 (mmHg) | 49.8 ± 8.4 | 58.3 ± 10.2 * | 46.5 ± 6.3 | 57.6 ± 8.8 * | 50.1 ± 6.6 | 55.7 ± 9.8 * | 51.5 ± 6.7 | 60.4 ± 9.7 * |
| BEb (mM) | 1.81 ± 1.95 | 0.57 ± 2.08 * | 2.51 ± 3.25 | 1.14 ± 2.99 | 1.74 ± 3.27 | −0.42 ± 2.27 | 2.26 ± 2.81 | 0.82 ± 2.54 |
| GLU (mM) | 5.15 ± 0.43 | 5.08 ± 0.86 | 4.95 ± 0.79 | 5.29 ± 0.84 | 4.77 ± 0.69 | 5.64 ± 0.81 * | 4.89 ± 0.81 | 5.05 ± 0.66 |
| LAC (mM) | 3.71 ± 0.76 | 6.53 ± 0.70 * | 3.14 ± 0.79 | 6.30 ± 0.88 * | 3.82 ± 0.62 | 5.69 ± 1.08 * | 3.41 ± 0.44 | 6.18 ± 0.81 * |
| Parameter | Pre-Test | Load-Test | ||
|---|---|---|---|---|
| AMP n = 14 | PL n = 11 | AMP n = 14 | PL n = 11 | |
| Δ RBC × 1012 (cells/L) | 0.10 ± 0.10 * | 0.06 ± 0.11 | 0.34 ± 0.1 * | 0.05 ± 0.05 |
| (%) | (2.0 ± 2.0) | (1.2 ± 2.1) | (6.8 ± 2.0) | (1.0 ± 1.0) |
| Δ HGB (g/L) | 3.8 ± 2.5 * | 2.5 ± 1.4 | 7.8 ± 2.8 * | 1.2 ± 1.6 |
| (%) | (2.6 ± 1.6) | (1.7 ± 1.0) | (5.3 ± 1.9) | (0.8 ± 1.1) |
| ΔpH | −0.08 ± 0.03 * | −0.08 ± 0.03 | −0.05 ± 0.02 * | −0.08 ± 0.02 |
| ΔpO2 (mmHg) | −6.2 ± 5.1 * | −6.5 ± 5.6 | −3.9 ± 2.3 * | −10.7 ± 4.5 |
| ΔpCO2 (mmHg) | 7.7 ± 3.7 * | 10.9 ± 4.3 | 5.5 ± 3.1 * | 10.8 ± 3.2 |
| ΔLAC (mM) | 2.8 ± 0.6 * | 3.2 ± 0.7 | 1.9 ± 0.6 * | 2.8 ± 0.7 |
| Parameter | Pre-Test | Load-Test | ||||||
|---|---|---|---|---|---|---|---|---|
| AMP | PL | AMP | PL | |||||
| Before | After | Before | After | Before | After | Before | After | |
| ALT (U/L) | 25.9 ± 13.7 | 29.1 ± 14.2 * | 23.3 ± 14.4 | 27.2 ± 17.9 * | 24.8 ± 14.1 | 30.3 ± 13.3 * | 24.6 ± 14.9 | 28.7 ± 17.5 * |
| AST (U/L) | 23.1 ± 9.6 | 25.4 ± 9.8 * | 20.3 ± 12.3 | 23.0 ± 12.6 * | 26.0 ± 18.9 | 31.5 ± 21.2 * | 21.2 ± 13.4 | 24.3 ± 12.7 * |
| GGTP (U/L) | 15.9 ± 5.4 | 16.4 ± 5.5 | 14.3 ± 7.83 | 14.9 ± 8.2 | 17.4 ± 5.0 | 19.5 ± 5.5 | 14.8 ± 7.0 | 16.8 ± 8.9 |
| LDH (U/L) | 268 ± 51 | 277 ± 39 | 266 ± 51 | 269 ± 56 | 220 ± 43 | 236 ± 18 | 221 ± 47 | 229 ± 55 |
| Parameter | Pre-Test | Load-Test | ||||||
|---|---|---|---|---|---|---|---|---|
| AMP | PL | AMP | PL | |||||
| Before | After | Before | After | Before | After | Before | After | |
| HGB (g/L) | 152.7 ± 3.1 | 156.5 ± 4.6 | 143.0 ± 2.0 | 146.6 ± 2.2 | 149.5 ± 4.0 | 157.3 ± 3.3 * | 148.0 ± 2.4 | 148.0 ± 2.3 |
| RBC (cells/L) | 5.14 ± 0.12 | 5.24 ± 0.17 | 4.91 ± 0.07 | 4.99 ± 0.11 | 5.02 ± 0.16 | 5.35 ± 0.14 * | 4.98 ± 0.10 | 5.05 ± 0.09 * |
| MCV (fL) | 84.5 ± 0.5 | 84.2 ± 0.5 | 83.5 ± 0.5 | 83.3 ± 0.5 | 84.4 ± 0.4 | 84.4 ± 0.5 | 83.7 ± 0.5 | 83.9 ± 0.6 |
| WBC (cells/L) | 6.8 ± 0.6 | 8.4 ± 0.9 * | 6.8 ± 0.4 | 8.4 ± 0.5 * | 7.0 ± 0.6 | 9.9 ± 0.9 * | 7.6 ± 0.9 | 9.7 ± 1.1 * |
| LYM (cells/L) | 2.1 ± 0.2 | 2.7 ± 0.3 * | 2.2 ± 0.2 | 3.0 ± 0.4 * | 2.0 ± 0.2 | 3.2 ± 0.3 * | 2.1 ± 0.2 | 3.2 ± 0.4 * |
| PLT (cells/L) | 204 ± 29 | 242 ± 24 | 261 ± 12 | 266 ± 28 | 186 ± 43 | 218 ± 41 | 231 ± 34 | 217 ± 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mindukshev, I.; Sudnitsyna, J.; Goncharov, N.V.; Skverchinskaya, E.; Dobrylko, I.; Nikitina, E.; Krivchenko, A.I.; Gambaryan, S. Low-Dose Ammonium Preconditioning Enhances Endurance in Submaximal Physical Exercises. Sports 2021, 9, 29. https://doi.org/10.3390/sports9020029
Mindukshev I, Sudnitsyna J, Goncharov NV, Skverchinskaya E, Dobrylko I, Nikitina E, Krivchenko AI, Gambaryan S. Low-Dose Ammonium Preconditioning Enhances Endurance in Submaximal Physical Exercises. Sports. 2021; 9(2):29. https://doi.org/10.3390/sports9020029
Chicago/Turabian StyleMindukshev, Igor, Julia Sudnitsyna, Nikolay V. Goncharov, Elisaveta Skverchinskaya, Irina Dobrylko, Elena Nikitina, Alexandr I. Krivchenko, and Stepan Gambaryan. 2021. "Low-Dose Ammonium Preconditioning Enhances Endurance in Submaximal Physical Exercises" Sports 9, no. 2: 29. https://doi.org/10.3390/sports9020029
APA StyleMindukshev, I., Sudnitsyna, J., Goncharov, N. V., Skverchinskaya, E., Dobrylko, I., Nikitina, E., Krivchenko, A. I., & Gambaryan, S. (2021). Low-Dose Ammonium Preconditioning Enhances Endurance in Submaximal Physical Exercises. Sports, 9(2), 29. https://doi.org/10.3390/sports9020029









