Muscle Fiber Type Transitions with Exercise Training: Shifting Perspectives
Abstract
:1. Introduction
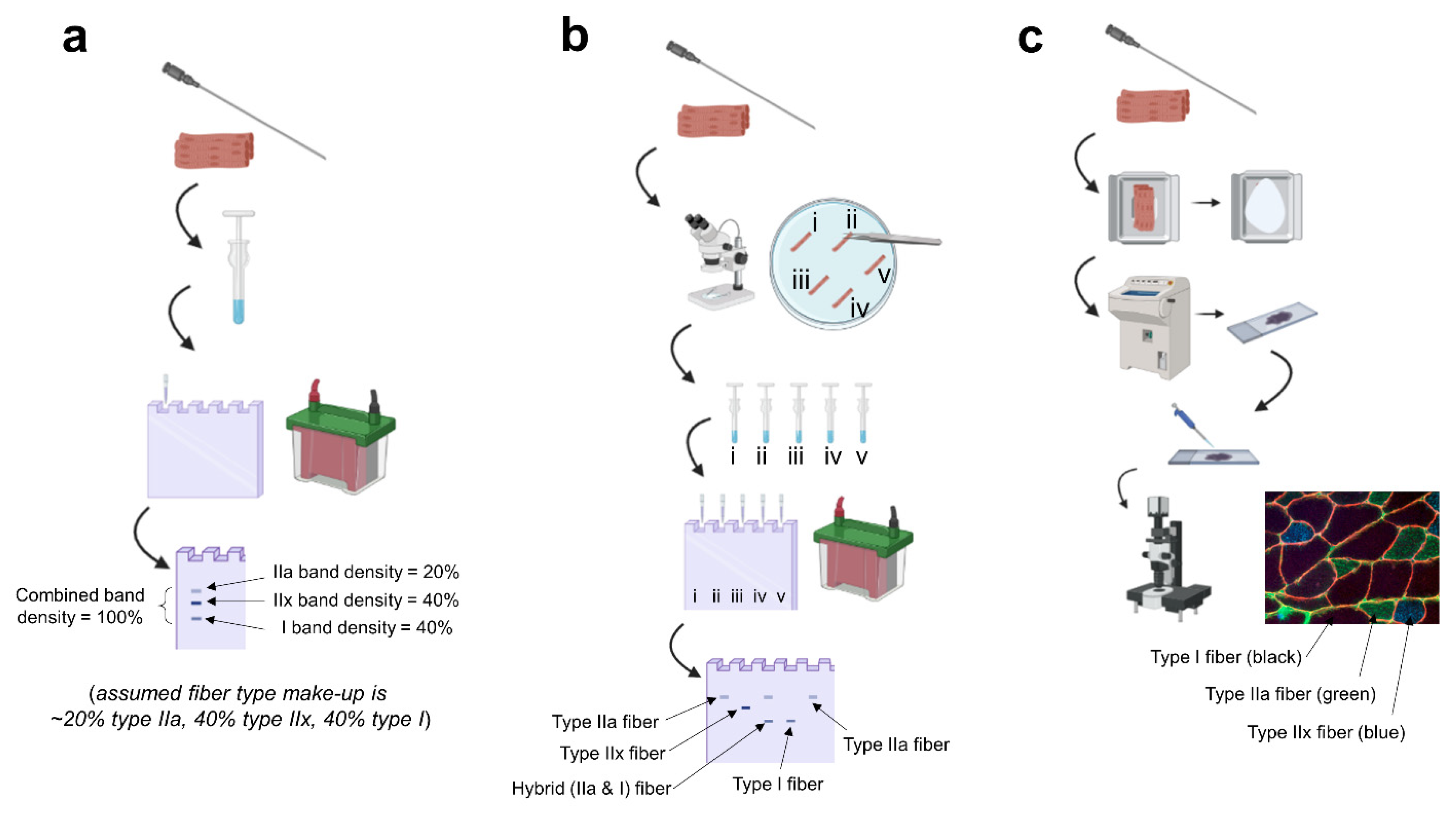
2. Fiber Type Identification Techniques
3. Resistance/Sprint and Power Training
4. Endurance Training
5. Disuse and Type IIx Fiber Overshoot
6. Potential Mechanisms
7. Further Directions and Limitations
8. Practical Applications
- Innate ability will affect how an individual performs at different sports/tasks, and fiber type composition likely plays an important role from a physiological perspective.
- Evidence suggests that muscle fibers have the ability to undergo fiber type transition, from hybrid to pure fibers, and between fiber types. The ability to discern hybrids is necessary to have a high degree of confidence in findings related to fiber type distribution.
- Given the dearth of evidence on the time course of long-term adaptation (>16 weeks), few long-term planning/periodization inferences can be drawn from the current fiber type transition literature. However, it likely would be prudent to maintain some of the force-velocity characteristics of the specific task/sport.
- Tapering practices are useful to allow for a transition back toward a faster fiber type. Some emerging evidence suggests an “overshoot” phenomenon in which type IIx fibers reach post-taper levels above what would be expected with rest alone. This may allow an athlete to be more explosive for a given event. Thus, tapering may present a viable strategy for power and endurance athletes to enhance performance.
- More research will help to elucidate differences in fiber type plasticity in other muscles, regional specificity, long-term adaptability, and other practical considerations, such as nutritional and training strategies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Smerdu, V.; Karsch-Mizrachi, I.; Campione, M.; Leinwand, L.; Schiaffino, S. Type IIx Myosin Heavy Chain Transcripts Are Expressed in Type IIb Fibers of Human Skeletal Muscle. Am. J. Physiol. 1994, 267, C1723–C1728. [Google Scholar] [CrossRef] [PubMed]
- Tihanyi, J.; Apor, P.; Fekete, G. Force-Velocity-Power Characteristics and Fiber Composition in Human Knee Extensor Muscles. Eur. J. Appl. Physiol. Occup. Physiol. 1982, 48, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Tesch, P.A.; Karlsson, J. Muscle Fiber Types and Size in Trained and Untrained Muscles of Elite Athletes. J. Appl. Physiol. (1985) 1985, 59, 1716–1720. [Google Scholar] [CrossRef] [PubMed]
- Serrano, N.; Colenso-Semple, L.M.; Lazauskus, K.K.; Siu, J.W.; Bagley, J.R.; Lockie, R.G.; Costa, P.B.; Galpin, A.J. Extraordinary Fast-Twitch Fiber Abundance in Elite Weightlifters. PLoS ONE 2019, 14, e0207975. [Google Scholar] [CrossRef] [Green Version]
- Trappe, S.; Luden, N.; Minchev, K.; Raue, U.; Jemiolo, B.; Trappe, T.A. Skeletal Muscle Signature of a Champion Sprint Runner. J. Appl. Physiol. (1985) 2015, 118, 1460–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larson, L.; Lioy, J.; Johnson, J.; Medler, S. Transitional Hybrid Skeletal Muscle Fibers in Rat Soleus Development. J. Histochem. Cytochem. 2019, 67, 891–900. [Google Scholar] [CrossRef]
- Roberts, M.D.; Dalbo, V.J.; Sunderland, K.L.; Kerksick, C.M. Electrophoretic Separation of Myosin Heavy Chain Isoforms Using a Modified Mini Gel System. J. Strength Cond. Res. 2012, 26, 3461–3468. [Google Scholar] [CrossRef]
- Medler, S. Mixing It up: The Biological Significance of Hybrid Skeletal Muscle Fibers. J. Exp. Biol. 2019, 222, jeb200832. [Google Scholar] [CrossRef]
- Quiat, D.; Voelker, K.A.; Pei, J.; Grishin, N.V.; Grange, R.W.; Bassel-Duby, R.; Olson, E.N. Concerted Regulation of Myofiber-Specific Gene Expression and Muscle Performance by the Transcriptional Repressor Sox6. Proc. Natl. Acad. Sci. USA 2011, 108, 10196–10201. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M.D.; Brown, J.D.; Company, J.M.; Oberle, L.P.; Heese, A.J.; Toedebusch, R.G.; Wells, K.D.; Cruthirds, C.L.; Knouse, J.A.; Ferreira, J.A.; et al. Phenotypic and Molecular Differences between Rats Selectively Bred to Voluntarily Run High vs. Low Nightly Distances. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R1024–R1035. [Google Scholar] [CrossRef] [Green Version]
- Müntener, M. Variable PH Dependence of the Myosin-ATPase in Different Muscles of the Rat. Histochemistry 1979, 62, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Tobias, I.S.; Galpin, A.J. Moving Human Muscle Physiology Research Forward: An Evaluation of Fiber Type-Specific Protein Research Methodologies. Am. J. Physiol.-Cell Physiol. 2020, 319, C858–C876. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.R.; Hather, B.M.; Baldwin, K.M.; Dudley, G.A. Skeletal Muscle Myosin Heavy Chain Composition and Resistance Training. J. Appl. Physiol. (1985) 1993, 74, 911–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, T.J.; Abernethy, P.J.; Logan, P.A.; Barber, M.; McEniery, M.T. Resistance Training Frequency: Strength and Myosin Heavy Chain Responses to Two and Three Bouts per Week. Eur. J. Appl. Physiol. Occup. Physiol. 1998, 78, 270–275. [Google Scholar] [CrossRef]
- Liu, Y.; Schlumberger, A.; Wirth, K.; Schmidtbleicher, D.; Steinacker, J.M. Different Effects on Human Skeletal Myosin Heavy Chain Isoform Expression: Strength vs. Combination Training. J. Appl. Physiol. (1985) 2003, 94, 2282–2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malisoux, L.; Francaux, M.; Nielens, H.; Theisen, D. Stretch-Shortening Cycle Exercises: An Effective Training Paradigm to Enhance Power Output of Human Single Muscle Fibers. J. Appl. Physiol. 2006, 100, 771–779. [Google Scholar] [CrossRef] [Green Version]
- Andersen, J.L.; Klitgaard, H.; Saltin, B. Myosin Heavy Chain Isoforms in Single Fibres from m. Vastus Lateralis of Sprinters: Influence of Training. Acta Physiol. Scand. 1994, 151, 135–142. [Google Scholar] [CrossRef]
- D’Antona, G.; Lanfranconi, F.; Pellegrino, M.A.; Brocca, L.; Adami, R.; Rossi, R.; Moro, G.; Miotti, D.; Canepari, M.; Bottinelli, R. Skeletal Muscle Hypertrophy and Structure and Function of Skeletal Muscle Fibres in Male Body Builders. J. Physiol. 2006, 570, 611–627. [Google Scholar] [CrossRef]
- Gehlert, S.; Weber, S.; Weidmann, B.; Gutsche, K.; Platen, P.; Graf, C.; Kappes-Horn, K.; Bloch, W. Cycling Exercise-Induced Myofiber Transitions in Skeletal Muscle Depend on Basal Fiber Type Distribution. Eur. J. Appl. Physiol. 2012, 112, 2393–2402. [Google Scholar] [CrossRef]
- Luden, N.; Hayes, E.; Minchev, K.; Louis, E.; Raue, U.; Conley, T.; Trappe, S. Skeletal Muscle Plasticity with Marathon Training in Novice Runners. Scand. J. Med. Sci. Sports 2012, 22, 662–670. [Google Scholar] [CrossRef]
- Vandervoort, A.A.; McComas, A.J. A Comparison of the Contractile Properties of the Human Gastrocnemius and Soleus Muscles. Eur. J. Appl. Physiol. Occup. Physiol. 1983, 51, 435–440. [Google Scholar] [CrossRef]
- Gallagher, P.; Trappe, S.; Harber, M.; Creer, A.; Mazzetti, S.; Trappe, T.; Alkner, B.; Tesch, P. Effects of 84-Days of Bedrest and Resistance Training on Single Muscle Fibre Myosin Heavy Chain Distribution in Human Vastus Lateralis and Soleus Muscles. Acta Physiol. Scand. 2005, 185, 61–69. [Google Scholar] [CrossRef]
- Howald, H.; Hoppeler, H.; Claassen, H.; Mathieu, O.; Straub, R. Influences of Endurance Training on the Ultrastructural Composition of the Different Muscle Fiber Types in Humans. Pflug. Arch. 1985, 403, 369–376. [Google Scholar] [CrossRef]
- Jansson, E.; Sjödin, B.; Tesch, P. Changes in Muscle Fibre Type Distribution in Man after Physical Training: A Sign of Fibre Type Transformation? Acta Physiol. Scand. 1978, 104, 235–237. [Google Scholar] [CrossRef]
- Trappe, S.; Harber, M.; Creer, A.; Gallagher, P.; Slivka, D.; Minchev, K.; Whitsett, D. Single Muscle Fiber Adaptations with Marathon Training. J. Appl. Physiol. (1985) 2006, 101, 721–727. [Google Scholar] [CrossRef] [Green Version]
- Costill, D.L.; Daniels, J.; Evans, W.; Fink, W.; Krahenbuhl, G.; Saltin, B. Skeletal Muscle Enzymes and Fiber Composition in Male and Female Track Athletes. J. Appl. Physiol. 1976, 40, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Costill, D.L.; Fink, W.J.; Pollock, M.L. Muscle Fiber Composition and Enzyme Activities of Elite Distance Runners. Med. Sci. Sports 1976, 8, 96–100. [Google Scholar] [CrossRef]
- Fink, W.J.; Costill, D.L.; Pollock, M.L. Submaximal and Maximal Working Capacity of Elite Distance Runners. Part II. Muscle Fiber Composition and Enzyme Activities. Ann. N. Y. Acad. Sci. 1977, 301, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Vikne, H.; Strøm, V.; Pripp, A.H.; Gjøvaag, T. Human Skeletal Muscle Fiber Type Percentage and Area after Reduced Muscle Use: A Systematic Review and Meta-Analysis. Scand. J. Med. Sci. Sports 2020, 30, 1298–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, L.L.; Andersen, J.L.; Magnusson, S.P.; Suetta, C.; Madsen, J.L.; Christensen, L.R.; Aagaard, P. Changes in the Human Muscle Force-Velocity Relationship in Response to Resistance Training and Subsequent Detraining. J. Appl. Physiol. 2005, 99, 87–94. [Google Scholar] [CrossRef]
- Houmard, J.A.; Scott, B.K.; Justice, C.L.; Chenier, T.C. The Effects of Taper on Performance in Distance Runners. Med. Sci. Sports Exerc. 1994, 26, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Murach, K.A.; Bagley, J.R. Less Is More: The Physiological Basis for Tapering in Endurance, Strength, and Power Athletes. Sports 2015, 3, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Buller, A.J.; Eccles, J.C.; Eccles, R.M. Interactions between Motoneurones and Muscles in Respect of the Characteristic Speeds of Their Responses. J. Physiol. 1960, 150, 417–439. [Google Scholar] [CrossRef]
- Rhee, H.S.; Hoh, J.F.Y. Immunohistochemical Analysis of the Effects of Cross-Innervation of Murine Thyroarytenoid and Sternohyoid Muscles. J. Histochem. Cytochem. 2010, 58, 1057–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, E.R.; Olson, E.N.; Richardson, J.A.; Yang, Q.; Humphries, C.; Shelton, J.M.; Wu, H.; Zhu, W.; Bassel-Duby, R.; Williams, R.S. A Calcineurin-Dependent Transcriptional Pathway Controls Skeletal Muscle Fiber Type. Genes Dev. 1998, 12, 2499–2509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCullagh, K.J.A.; Calabria, E.; Pallafacchina, G.; Ciciliot, S.; Serrano, A.L.; Argentini, C.; Kalhovde, J.M.; Lømo, T.; Schiaffino, S. NFAT Is a Nerve Activity Sensor in Skeletal Muscle and Controls Activity-Dependent Myosin Switching. Proc. Natl. Acad. Sci. USA 2004, 101, 10590–10595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, A.L.; Murgia, M.; Pallafacchina, G.; Calabria, E.; Coniglio, P.; Lømo, T.; Schiaffino, S. Calcineurin Controls Nerve Activity-Dependent Specification of Slow Skeletal Muscle Fibers but Not Muscle Growth. Proc. Natl. Acad. Sci. USA 2001, 98, 13108–13113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Röckl, K.S.C.; Hirshman, M.F.; Brandauer, J.; Fujii, N.; Witters, L.A.; Goodyear, L.J. Skeletal Muscle Adaptation to Exercise Training: AMP-Activated Protein Kinase Mediates Muscle Fiber Type Shift. Diabetes 2007, 56, 2062–2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.-P.; Stephens, T.J.; Murthy, S.; Canny, B.J.; Hargreaves, M.; Witters, L.A.; Kemp, B.E.; McConell, G.K. Effect of Exercise Intensity on Skeletal Muscle AMPK Signaling in Humans. Diabetes 2003, 52, 2205–2212. [Google Scholar] [CrossRef] [Green Version]
- Dreyer, H.C.; Fujita, S.; Cadenas, J.G.; Chinkes, D.L.; Volpi, E.; Rasmussen, B.B. Resistance Exercise Increases AMPK Activity and Reduces 4E-BP1 Phosphorylation and Protein Synthesis in Human Skeletal Muscle. J. Physiol. 2006, 576, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, Y.; Jia, G.; Liu, G.; Zhao, H.; Huang, Z. Arginine Promotes Skeletal Muscle Fiber Type Transformation from Fast-Twitch to Slow-Twitch via Sirt1/AMPK Pathway. J. Nutr. Biochem. 2018, 61, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Flück, M.; Kramer, M.; Fitze, D.P.; Kasper, S.; Franchi, M.V.; Valdivieso, P. Cellular Aspects of Muscle Specialization Demonstrate Genotype–Phenotype Interaction Effects in Athletes. Front. Physiol. 2019, 10, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simoneau, J.A.; Bouchard, C. Genetic Determinism of Fiber Type Proportion in Human Skeletal Muscle. FASEB J. 1995, 9, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Vincent, B.; De Bock, K.; Ramaekers, M.; Van den Eede, E.; Van Leemputte, M.; Hespel, P.; Thomis, M.A. ACTN3 (R577X) Genotype Is Associated with Fiber Type Distribution. Physiol. Genom. 2007, 32, 58–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumagai, H.; Tobina, T.; Ichinoseki-Sekine, N.; Kakigi, R.; Tsuzuki, T.; Zempo, H.; Shiose, K.; Yoshimura, E.; Kumahara, H.; Ayabe, M.; et al. Role of Selected Polymorphisms in Determining Muscle Fiber Composition in Japanese Men and Women. J. Appl. Physiol. (1985) 2018, 124, 1377–1384. [Google Scholar] [CrossRef] [Green Version]
- Borisov, O.; Semenova, E.; Miyamoto-Mikami, E.; Murakami, H.; Zempo, H.; Kostryukova, E.; Kulemin, N.; Larin, A.; Miyachi, M.; Ospanova, E.; et al. A Genome-Wide Association Study of Muscle Fibers Composition Revealed 5 SNPs Associated with Slow-Twitch Fibers. In FEBS Open Bio; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Bagley, J.; Murach, K.; Trappe, S. Microgravity-Induced Fiber Type Shift in Human Skeletal Muscle. Gravit. Space Biol. 2012, 26, 34–40. [Google Scholar]
- Hua, N.; Takahashi, H.; Yee, G.M.; Kitajima, Y.; Katagiri, S.; Kojima, M.; Anzai, K.; Eguchi, Y.; Hamilton, J.A. Influence of Muscle Fiber Type Composition on Early Fat Accumulation under High-Fat Diet Challenge. PLoS ONE 2017, 12, e0182430. [Google Scholar] [CrossRef] [Green Version]
- Korfage, J.A.M.; Van Eijden, T.M.G.J. Regional Differences in Fibre Type Composition in the Human Temporalis Muscle. J. Anat. 1999, 194, 355–362. [Google Scholar] [CrossRef]
- Horwath, O.; Envall, H.; Röja, J.; Emanuelsson, E.B.; Sanz, G.; Ekblom, B.; Apró, W.; Moberg, M. Variablity in Vastus Lateralis Fiber Type Distribution, Fiber Size and Myonuclear Content along and between the Legs. J. Appl. Physiol. 2021. [Google Scholar] [CrossRef]
| Andersen, 1994 | Male Sprinters: N = 6 | Sprint training preparation group, post-competitive season (no comparator) | The training was 2.5–3 h per day, 6 days a week, and consisted of a combination of strength and interval training for 3 months. Strength training was 2.5 days a week and consisted of exercises in the one to eight repetition range with 80–100% of 1 RM. Uphill sprints or interval-running were performed 2.5 days a week. One day was used for jumping exercises or recreational activities, such as basketball. | VL; Biopsies were taken post-3-week deload (which commenced after the track season) and post-intervention period. | Single Fiber Isolation. SDS-PAGE. | Mean changes: MHC I: 10.8% decrease (52.0+/−3.0 to 41.2+/−4.7) MHC I/IIa: 1.2% increase (0.2+/−0.2 to 1.4+/−1) MHC IIa: 17.6% increase (34.7+/−6.1 to 52.3+/−3.6) MHC IIa/IIx: 7.8% decrease (12.9+/−5.0 to 5.1+/−3.1) MHC IIx: 0.2% decrease (0.2+/−0.2 to 0.0) |
| Andersen, 1994 | Male Soccer Players in the Danish National League: N = 14 | High resistance knee extensor strength training group (ST): N = 8 Control group (no RT): N = 6 | The training in the ST group was performed for 12 weeks, 3 times a week. Sessions consisted of four sets of eight repetitions of knee extensions using high resistance loads at velocities of 30–50 degrees per second. | VL; Biopsies were taken pre- and post-intervention. | Single Fiber Isolation. SDS-PAGE. | Mean changes: ST Group: MHC I: 3.1% increase (55.6+/−4.7 to 58.7+/−3.2) MHC I/IIa: 1.7% decrease (4.4+/−1.2 to 2.7+/−1.0) MHC IIa: 3.3% decrease (33.1+/−4.9 to 29.8+/−3.8) MHC IIa/IIx: 1.8% increase (6.7+/−3.2 to 8.5+/−2.2) MHC IIx: 0.1% increase (0.2+/−0.2 to 0.3+/−0.2) CTRL Group: All changes less than 3% except: MHC IIa: 6.2% decrease (27.7+/−5.2 to 21.5+/−6.8) MHC IIa/IIx: 3.9% increase (7.7+/−2.8 to 11.6 +/−4.9) |
| Williamson, 2000 | Untrained Older Males: N = 7 | Progressive resistance training (PRT) (no comparator) | The intervention was 12 weeks in duration, with training sessions 3 times per week. Training consisted of knee extensions at a cadence of 2–3 s for both the concentric and eccentric. The subjects performed three sets; the first two sets were 10 repetitions, and the last set was performed to volitional exhaustion. Nearly 2–3 min of rest was given between sets. All PRT exercise sessions were performed at 80% of one-repetition maximum (1 RM) and reassessed every 2 weeks in order to maintain prescribed intensity. | VL; Biopsies were taken pre- and post-intervention. | Single Fiber Isolation. SDS-PAGE. | The MHC I fiber distribution significantly increased by 10.4% after PRT, whereas the MHC IIa and IIx remained unchanged. Authors speculated the 12-week resistance training protocol might have not been sufficient in length to strengthen and increase the proportion of the denervated/reinnervated fibers often present in untrained older populations; thus, type II fibers would have been adapted at a slower rate than the type I fibers. |
| Williamson, 2001 | Young Untrained: Male: N = 6 Female: N = 6 | Progressive resistance training (PRT) group of men and women compared (same protocol, no comparator) | Same protocol as Williamson, 2000. | VL; Biopsies were taken pre- and post-intervention. | Single Fiber Isolation. SDS-PAGE. | Refer to Table 2 of Williamson, 2001 for a breakdown of the relative dominance of hybrid single fibers. Mean changes: Total hybrid fibers: 19% decrease in women, 29% decrease in men. MHC IIa increased 24% in men and 27% in women. Very little change in type I in either group. |
| Widrick, 2002 | Untrained Males: N = 6 | Resistance training (no comparator) | A total of 36 exercise sessions were performed at a frequency of 3 times per week on nonconsecutive days. Exercises for the lower body consisted of squats, knee extension, knee flexion, and calf raise. Upper body exercise consisted of bench press, lat pull down, shoulder press, triceps press, biceps curl, seated row, and abdominal exercise. Three sets of 5–10 of the exercises listed above (divided approximately equally between those targeting the upper and lower body). During all sessions, the training resistance was adjusted so that subjects were able to complete only the specified number of repetitions, plus or minus one repetition. | VL; Biopsies were taken pre- and post-intervention. | Single Fiber Isolation. SDS-PAGE. | Mean changes: Type I: 0% decrease (42–42) I/IIa: 4% decrease (4–0) IIa: 25% increase (30–55) IIa/IIx: 19% decrease (22–3) IIx: 3% decrease (3–0) |
| Malisoux, 2005 | Untrained Males: N = 8 | Stretch shortening cycle (SSC) exercise program | SSC exercise training program consisting of 24 sessions was performed 3 times per week for a total of 5228 jumps. Exercises included static jump, vertical countermovement jump, drop jump (height of 40 cm), double-leg triple jump, single-leg triple jump, double-leg hurdle jump, and single-leg hurdle jumps. The participants were instructed to perform all jumps at a maximal effort. The number of jumps was progressively increased during the first 4 weeks such that initial sessions were 20 min long and were 45 min at the end of the training period. | VL; Biopsies were taken pre- and post-intervention. | Single Fiber Isolation. SDS-PAGE. | Mean changes: MHC I: 0.8% decrease (30.0+/−4.9 to 29.2+/−4.1) MHC I/IIa: 3.1% increase (1.9+/−0.5 to 5.0+/−1.4) MHC IIa: 7.2% increase (33.4+/−5.2 to 40.6+/−4.2) MHC IIa/IIx: 4.7% decrease (26.9 +/−1.9 to 22.2+/−4.3) MHC IIx: 4.4% decrease (7.0+/−3.0 to 2.6 +/−1.9) MHC I/IIa/IIx: 0.4% decrease (0.8+/−0.4 to 0.4 +/−0.2) |
| Trappe, 2006 | Relatively Untrained: Male: N = 4 Female: N = 3 | Marathon Training (no comparator) | The training program was 4 days a week divided into two phases: a 13-week training period (10% increase in running volume per week), followed by a 3-week taper period. Compared with the last week of training (week 13), running volume was reduced by 25% in week 14, 47% in week 15, and 80% the week before the marathon. | Lateral gastrocnemius; Biopsies taken before the 16-week training plan, after 13 weeks of run training, and after 3 weeks of taper and marathon. Single muscle fiber MHC isoform experiments were only conducted before the training program and after the taper. | Single Fiber Isolation. SDS-PAGE. | Mean changes: Type I: 8% increase Type I/IIa: 5% decrease Type IIa: 2% increase Type IIa/x: 4% decrease Type I/IIax: 5% decrease Total hybrids: 11% decrease |
| Luden, 2011 | Novice Runners: Male: N = 3 Female: N = 3 | Marathon training (no Comparator) | Same protocol as Trappe, 2006. | VL, Biopsies were taken pre-training, following 13 weeks of run training, and again following 3 weeks of reduced training after a marathon (26.2 miles; 42.2 km). These time points are referred to as T1, T2, and T3, respectively. | Single Fiber Isolation. SDS-PAGE. | Soleus: T1 to T3 MHC I: 5.1% increase (71.1+/−6.1 to 76.2+/−4.7) MHC I/IIa: 0.3% decrease (6.4+/−1.7 to 6.1 +/−1.6%) MHC IIa: 4% decrease (21.4+/−4.8 to 17.4+/−3.9) MHC IIa/IIx: 0.8% decrease (1.1 +/−0.8 to 0.3) MHC IIx: Undetectable at both time points. VL: T1 to T3 MHC I: 6% increase (42.6+/−8.6 to 48.6+/−7.1) MHC I/IIa: 3.1% increase (5.1 +/−1.3 to 8.2 +−3.0) MHC IIa: 4.3% decrease (40.1+/−6.8 to 35.8 +/−4.4) MHC IIa/IIx: 5.5% decrease (11.9+/−2.9 to 6.4+/−2.4) MHC IIx: 1% increase (0 to 1+/−1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plotkin, D.L.; Roberts, M.D.; Haun, C.T.; Schoenfeld, B.J. Muscle Fiber Type Transitions with Exercise Training: Shifting Perspectives. Sports 2021, 9, 127. https://doi.org/10.3390/sports9090127
Plotkin DL, Roberts MD, Haun CT, Schoenfeld BJ. Muscle Fiber Type Transitions with Exercise Training: Shifting Perspectives. Sports. 2021; 9(9):127. https://doi.org/10.3390/sports9090127
Chicago/Turabian StylePlotkin, Daniel L., Michael D. Roberts, Cody T. Haun, and Brad J. Schoenfeld. 2021. "Muscle Fiber Type Transitions with Exercise Training: Shifting Perspectives" Sports 9, no. 9: 127. https://doi.org/10.3390/sports9090127
APA StylePlotkin, D. L., Roberts, M. D., Haun, C. T., & Schoenfeld, B. J. (2021). Muscle Fiber Type Transitions with Exercise Training: Shifting Perspectives. Sports, 9(9), 127. https://doi.org/10.3390/sports9090127








