Precipitation and Growth Simulation of γ′ Phase in Single Crystal Superalloy DD6 with Multiphase-Field Method and Explicit Nucleation Algorithm
Abstract
1. Introduction
2. Modeling
2.1. Multiphase-Field Model
2.2. Explicit Nucleation Algorithm
2.3. Parameters
3. Results and Discussion
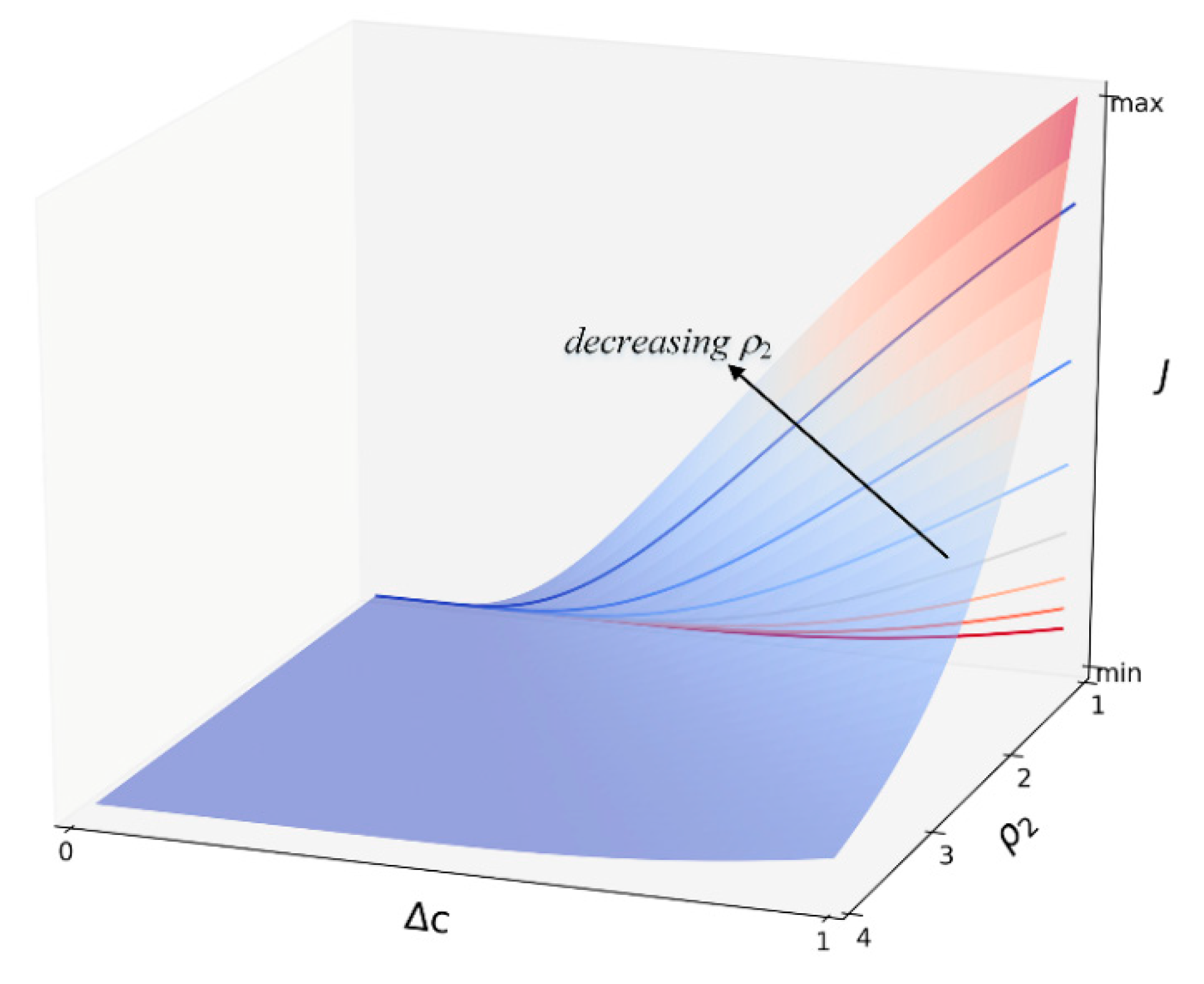
3.1. Nucleation Law of γ′ Phase
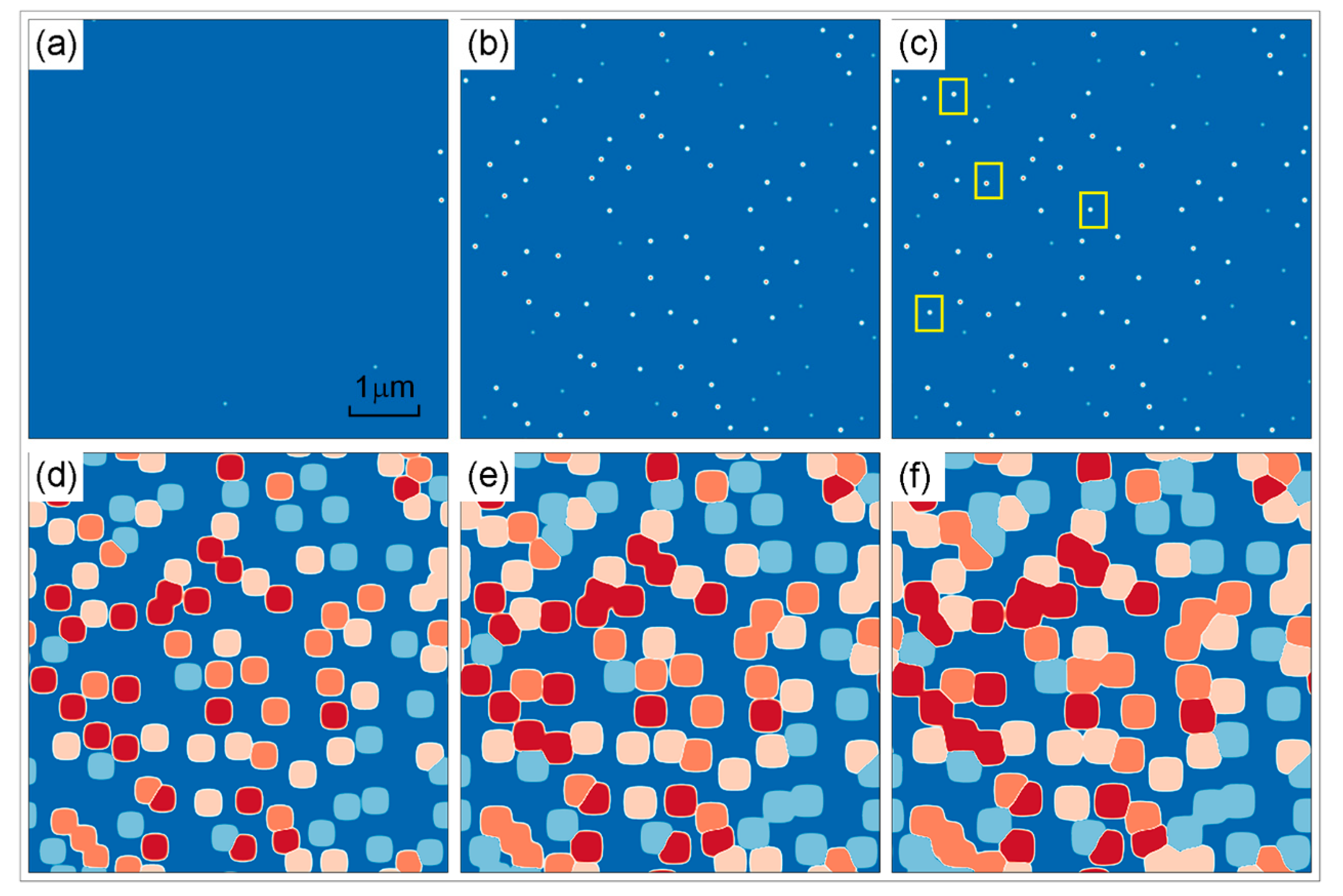
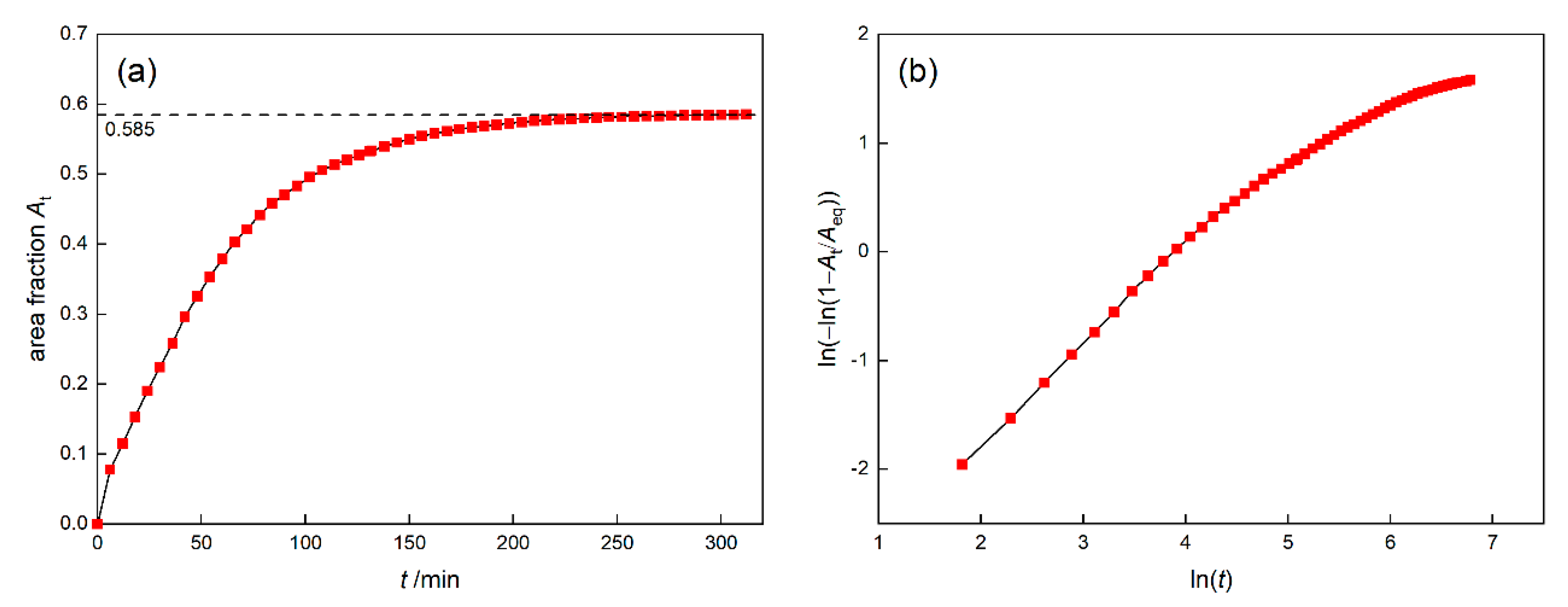
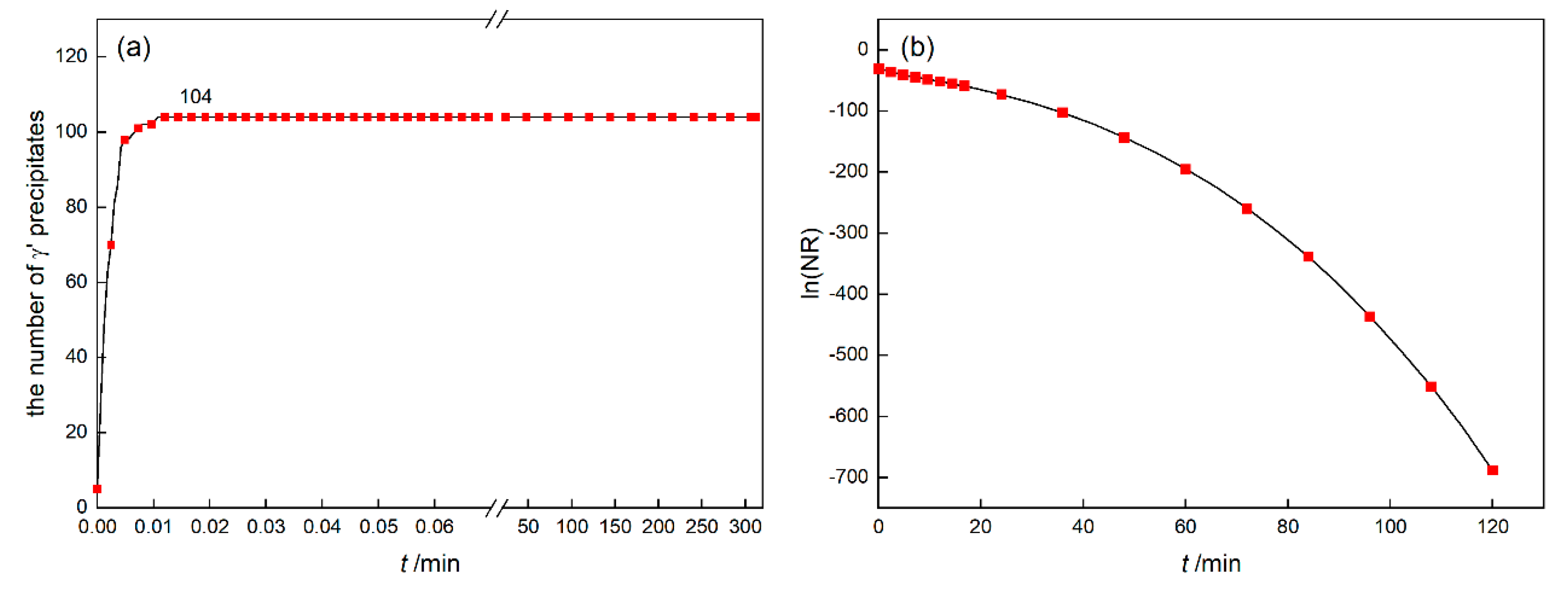
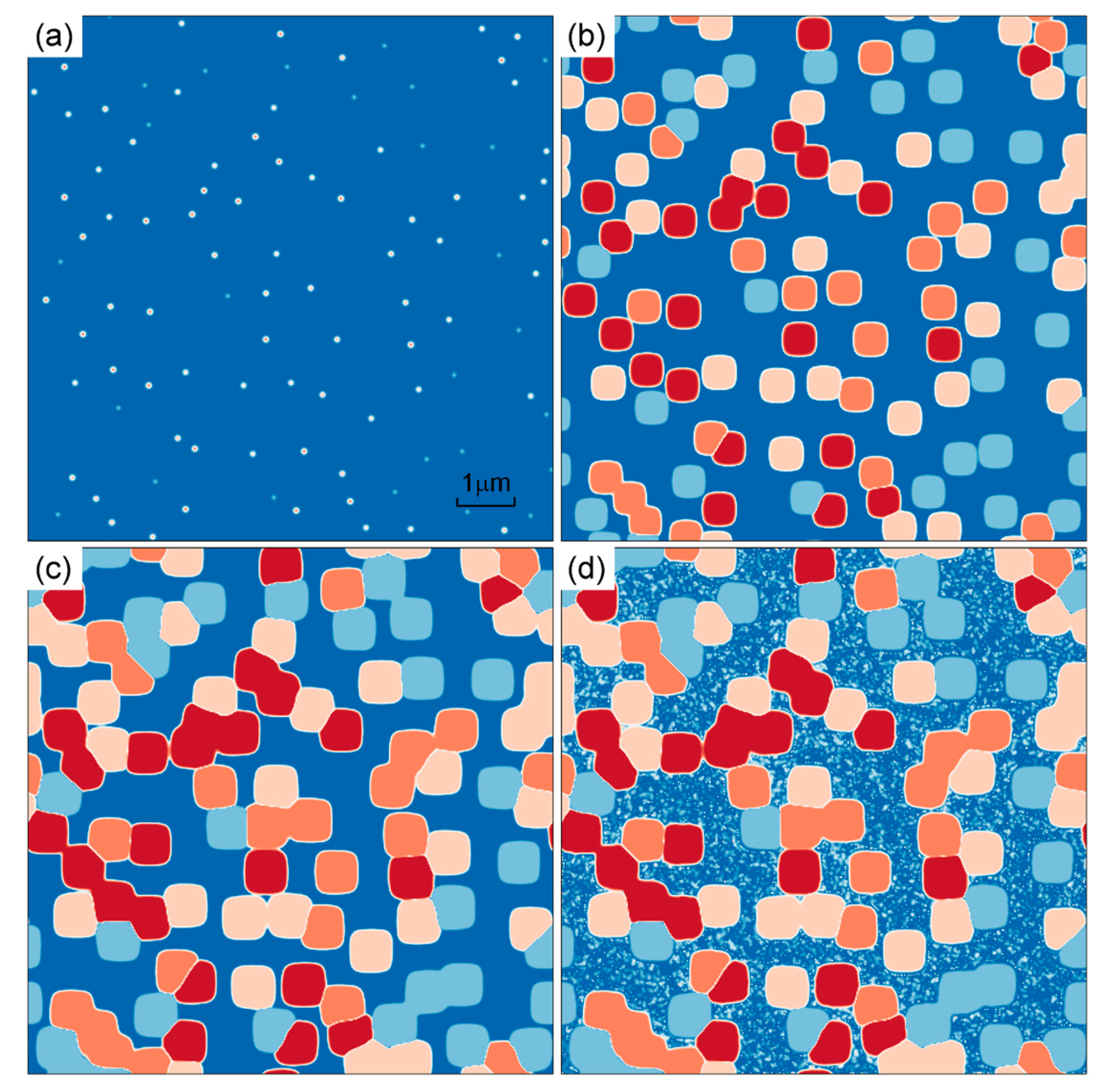
3.1.1. Isothermal Simulation
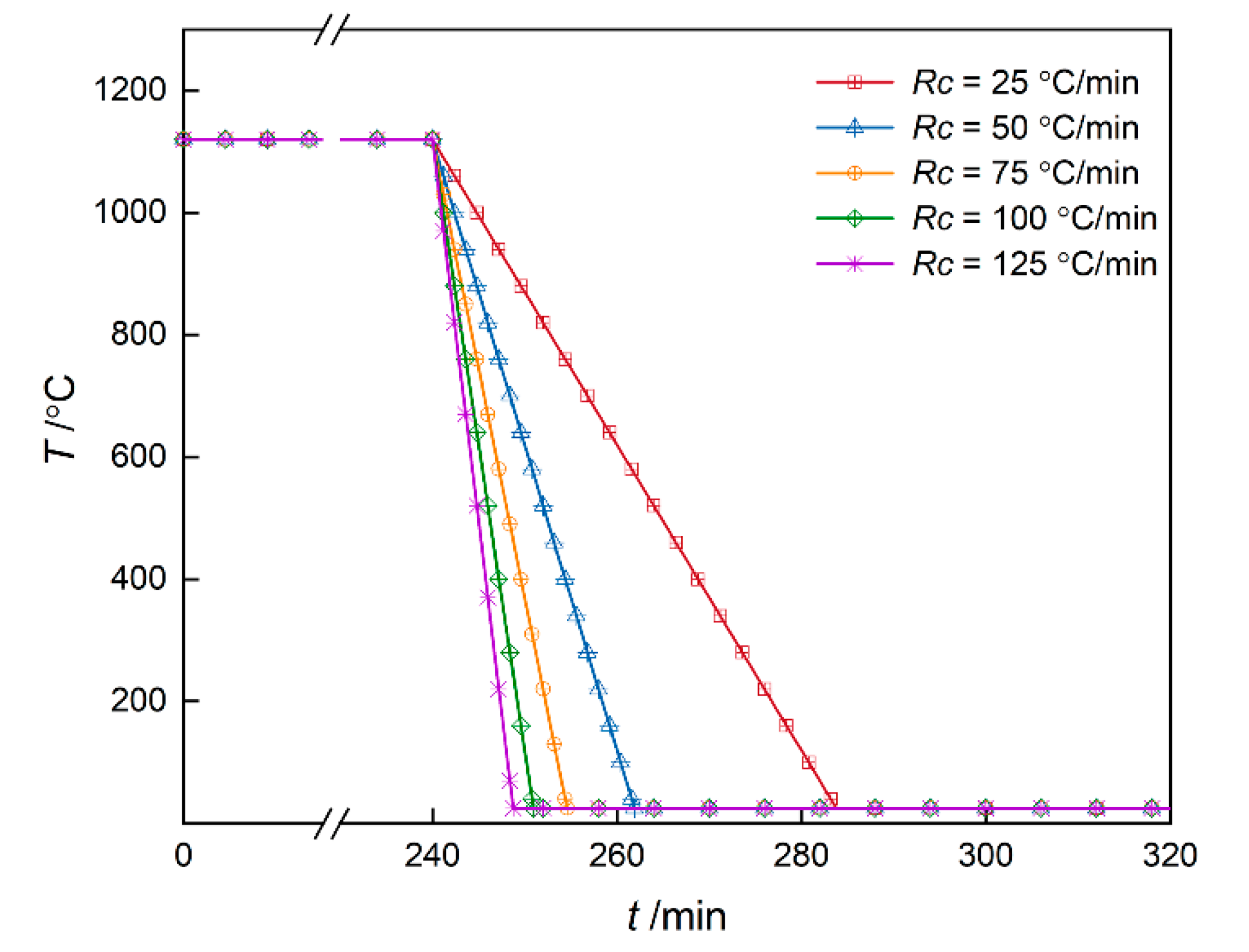
3.1.2. Non-Isothermal Simulation
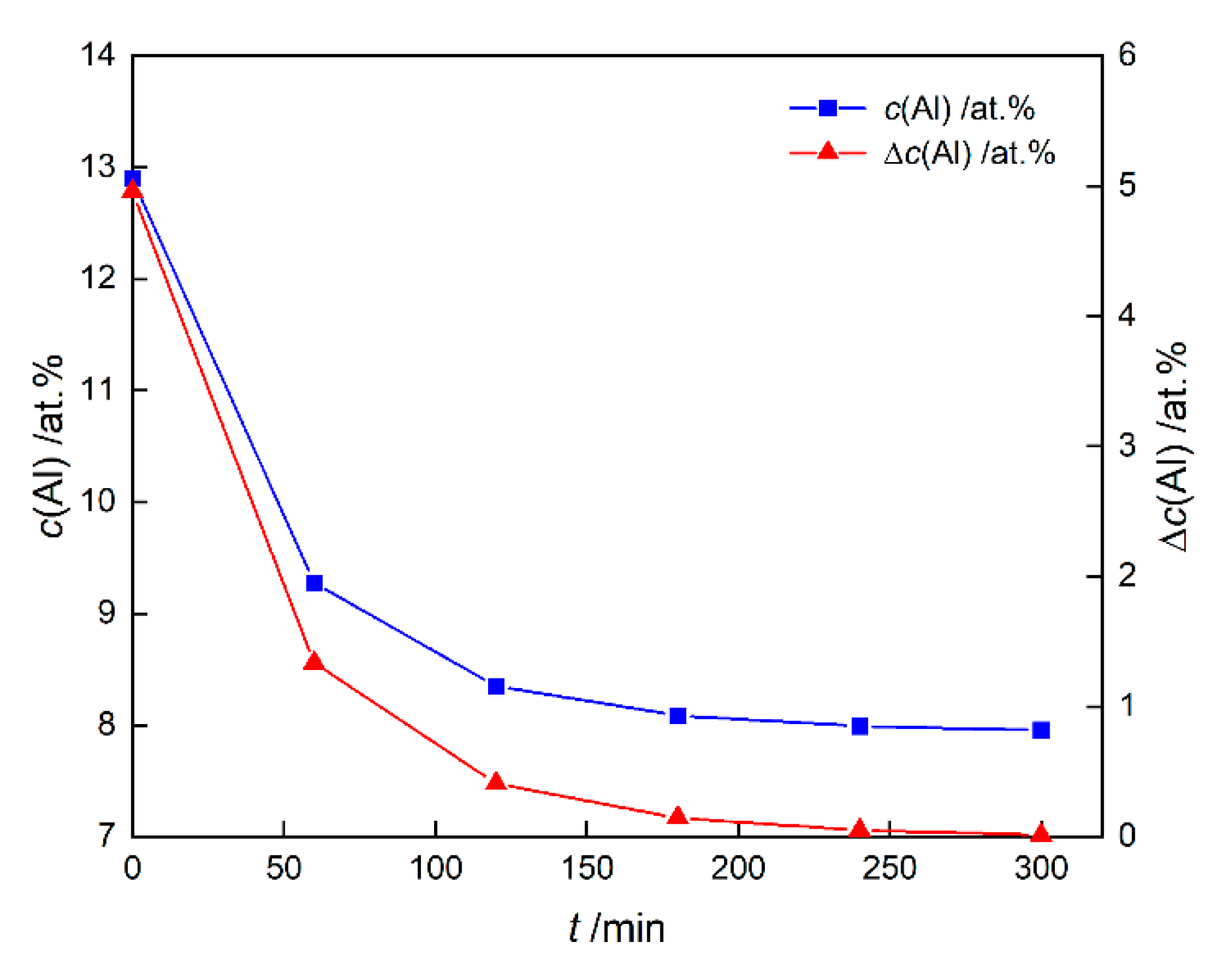
3.2. Driving Force of γ′ Growth
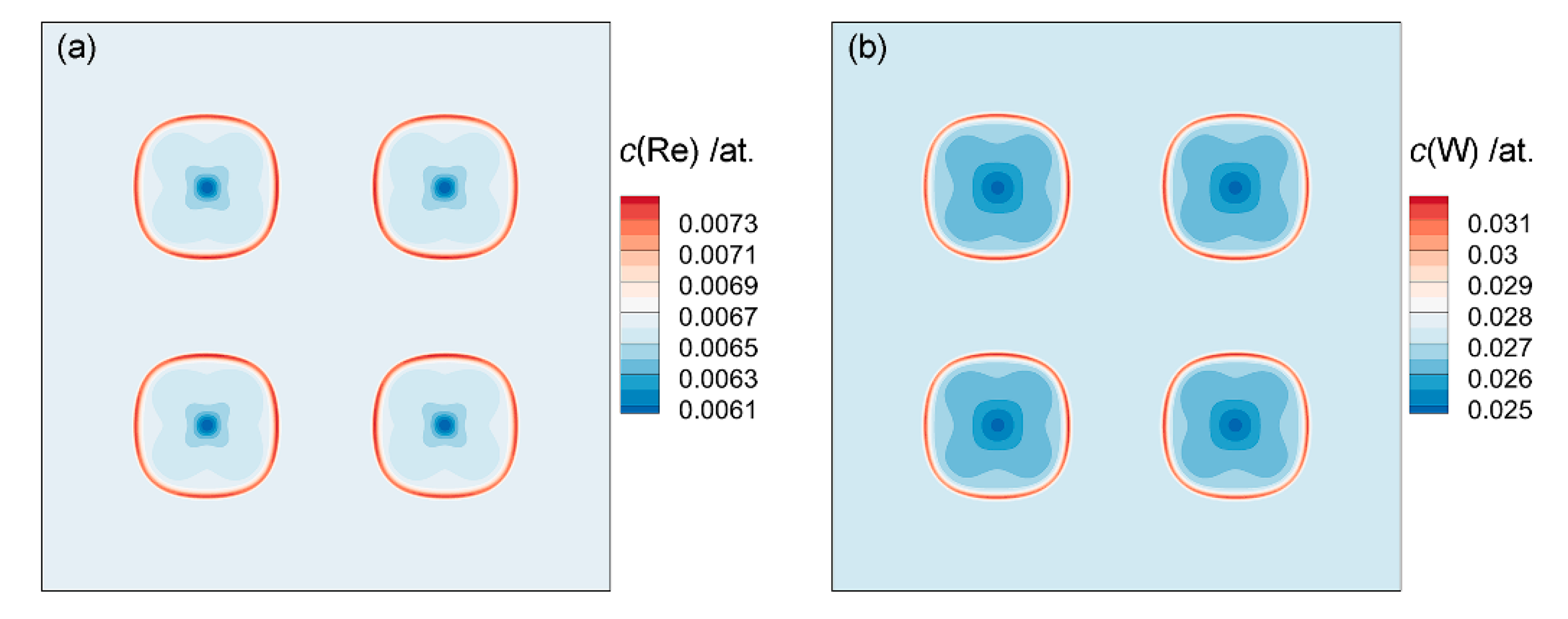
3.2.1. Chemical Driving Force
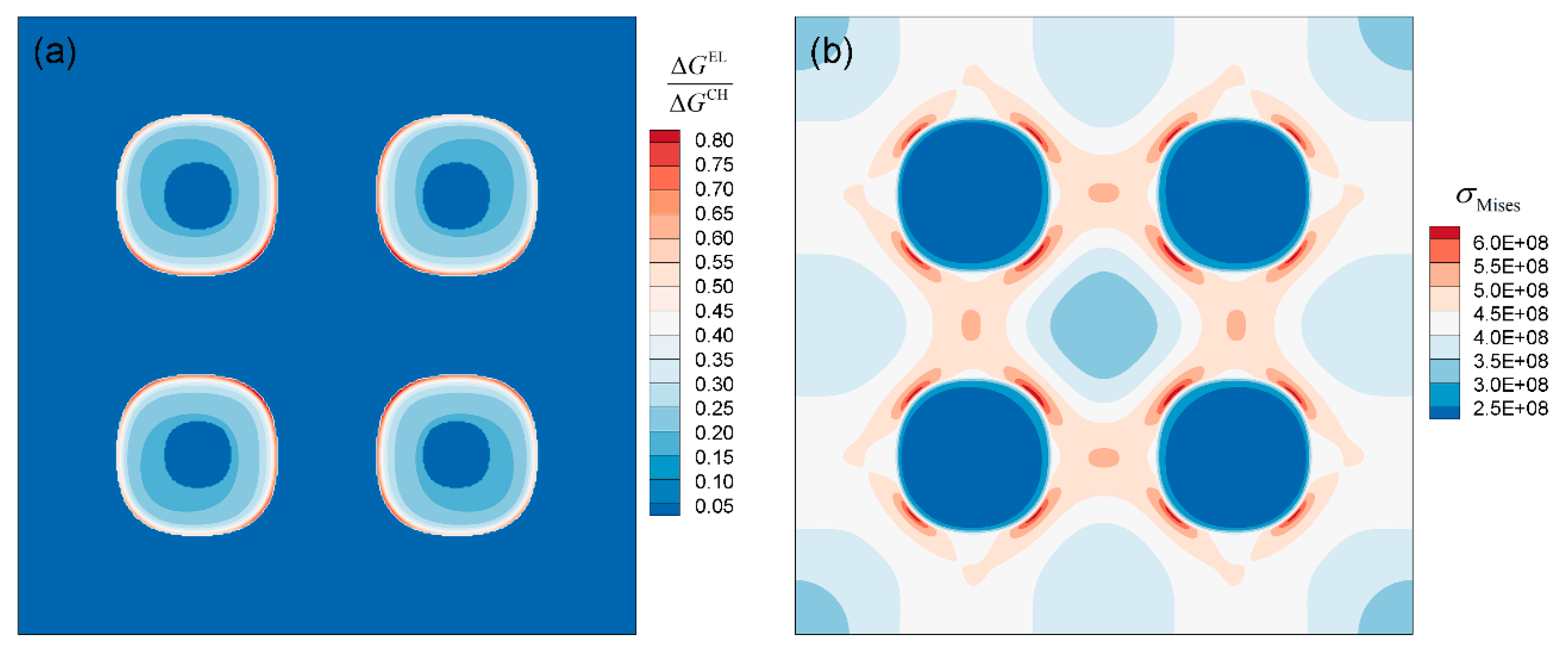
3.2.2. Elastic Driving Force
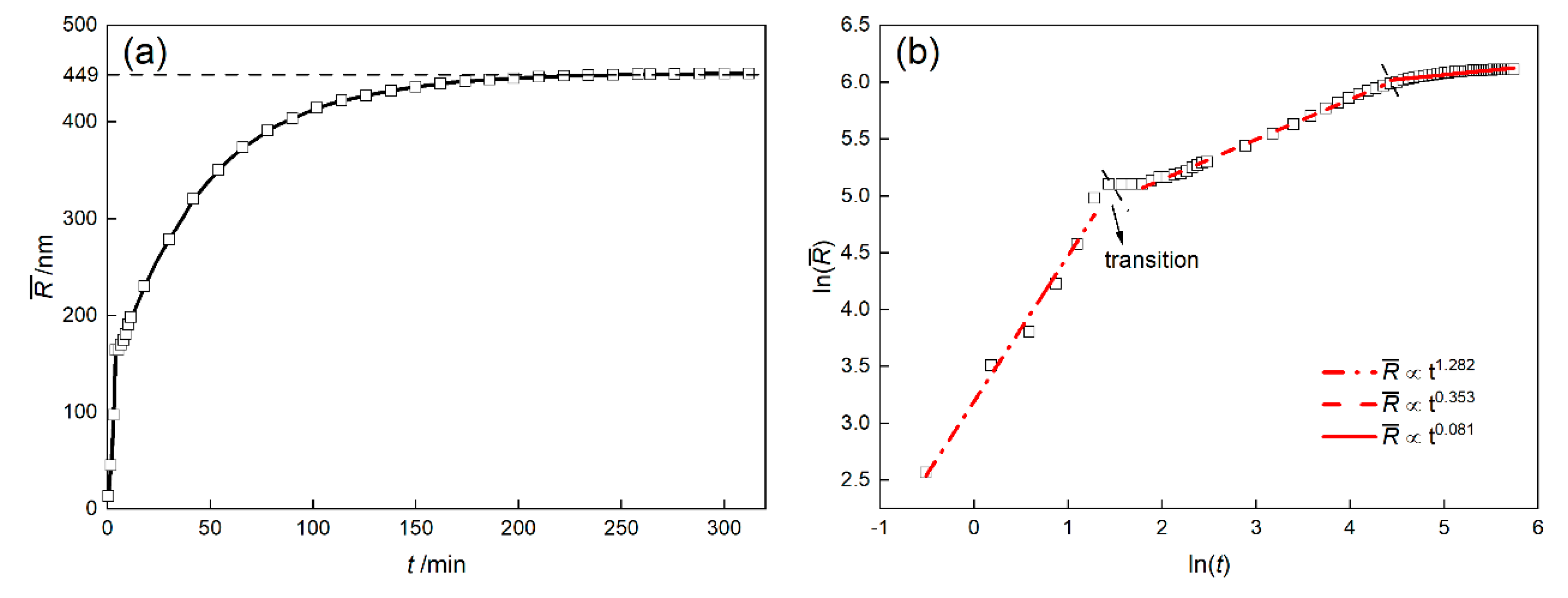
3.3. Growth Law of γ′ Phase
4. Conclusions
- (1)
- The multicomponent multiphase-field model coupled with explicit nucleation algorithm in this paper provides a good method for studying the microstructure evolutions of precipitation and growth phenomena in multicomponent elastic-inhomogeneous systems.
- (2)
- At 1120 °C/4 h isothermal conditions, the Avrami exponent of γ′ nucleation will decrease gradually, which is affected by the “soft impingement” phenomenon.
- (3)
- During the 25–125 °C/min cooling process, bimodal distribution composed of cuboidal γ′ precipitates and fine secondary γ′ precipitates is formed. With the increase in cooling rate, the number of γ′ precipitates increases first and then decreases, which reflects the competition of undercooling and diffusion.
- (4)
- The element Al contributes most of the chemical driving force for the γ′ growth, followed by Ta, while Re and W gather at the γ/γ′ phase interface to inhibit the growth of γ′ phase. Due to the lattice misfit and the difference in elastic constants between γ and γ′ phases, the stress and elastic driving force are concentrated in <111> direction of the γ′ phase, which causes γ′ phases to have a cuboidal shape.
- (5)
- The well-known relationship cannot be simply applied to the growth of γ′ phase in Ni-based superalloys due to the effects of elasticity and element diffusion. According to the multi-phase field simulation results, the growth of γ′ phase can be divided into rapid growth, coarsening and quasi-static coarsening stages.
Author Contributions
Funding
Conflicts of Interest
References
- Shi, Z.X.; Liu, S.Z.; Li, J.R. Rejuvenation Heat Treatment of the Second-Generation Single-Crystal Superalloy DD6. Acta Metall. Sin. 2015, 28, 1278–1285. [Google Scholar] [CrossRef]
- Su, X.; Sun, C.; Xu, Q.; Liu, B. Thermodynamic Simulation Calculation on Precipitation Behavior of Equilibrium Phases in a Low Re-bearing Nickel-based Single Crystal Superalloy. Rare Metal Mater. Eng. 2017, 46, 3699–3714. [Google Scholar]
- Kitashima, T.; Harada, H. A new phase-field method for simulating γ′ precipitation in multicomponent nickel-base superalloys. Acta Mater. 2009, 57, 2020–2028. [Google Scholar] [CrossRef]
- Wen, Y.H.; Simmons, J.P.; Shen, C.; Woodward, C.; Wang, Y. Phase-field modeling of bimodal particle size distributions during continuous cooling. Acta Mater. 2003, 51, 1123–1132. [Google Scholar] [CrossRef]
- Boussinot, G.; Finel, A.; Bouar, Y.L. Phase-field modeling of bimodal microstructures in nickel-based superalloys. Acta Mater. 2009, 57, 921–931. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, J.; Wei, H.; Gui, W. Influence of cooling rate on the formation of bimodal microstructures in nickel-base superalloys during continuous two-step aging. Comput. Mater. Sci. 2018, 149, 14–20. [Google Scholar] [CrossRef]
- Cottura, M.; Le Bouar, Y.; Appolaire, B.; Finel, A. Rôle of elastic inhomogeneity in the development of cuboidal microstructures in Ni-based superalloys. Acta Mater. 2015, 94, 15–25. [Google Scholar] [CrossRef]
- Yang, M.; Wei, H.; Zhang, J.; Zhao, Y.; Jin, T.; Liu, L.; Sun, X.F. Phase-field study on effects of antiphase domain and elastic energy on evolution of γ′ precipitates in nickel-based superalloys. Comput. Mater. Sci. 2017, 129, 211–219. [Google Scholar] [CrossRef]
- Steinbach, I.; Apel, M. Multi phase field model for solid state transformation with elastic strain. Phys. D 2006, 217, 153–160. [Google Scholar] [CrossRef]
- Grosdidier, T.; Hazotte, A.; Simon, A. Precipitation and dissolution processes in γ/γ′ single crystal nickel-based superalloys. Mater. Sci. Eng. A 1998, 256, 183–196. [Google Scholar] [CrossRef]
- Yu, J.; Li, J.; Shi, Z.; Wang, X.; Liu, S.; Zhao, J. Precipitation of Secondary gamma’ Phase of DD6 Single Crystal Superalloy. Rare Metal Mater. Eng. 2013, 42, 1654–1658. [Google Scholar]
- Wang, Y.; Banerjee, D.; Su, C.C.; Khachaturyan, A.G. Field kinetic model and computer simulation of precipitation of L12 ordered intermetallics from f.c.c. solid solution. Acta Mater. 1998, 46, 2983–3001. [Google Scholar] [CrossRef]
- Eiken, J.; Böttger, B.; Steinbach, I. Multiphase-field approach for multicomponent alloys with extrapolation scheme for numerical application. Phys. Rev. E 2006, 73, 066–122. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xu, Q.; Liu, B. A high precision extrapolation method in multiphase-field model for simulating dendrite growth. J. Cryst. Growth 2018, 490, 25–34. [Google Scholar] [CrossRef]
- Guo, W.; Steinbach, I.; Somsen, C.; Eggeler, G. On the effect of superimposed external stresses on the nucleation and growth of Ni4Ti3 particles: A parametric phase field study. Acta Mater. 2011, 59, 3287–3296. [Google Scholar] [CrossRef]
- Biner, S.B. Solving Phase-Field Models with Fourier Spectral Methods. In Programming Phase-Field Modeling; Springer Cham: Idaho Falls, ID, USA, 2017; pp. 99–168. [Google Scholar]
- Simmons, J.P.; Shen, C.; Wang, Y. Phase field modeling of simultaneous nucleation and growth by explicitly incorporating nucleation events. Scr. Mater. 2000, 43, 935–942. [Google Scholar] [CrossRef]
- Simmons, J.P.; Wen, Y.H.; Shen, C.; Wang, Y.Z. Microstructural development involving nucleation and growth phenomena simulated with the Phase Field method. Mater. Sci. Eng. A 2004, 365, 136–143. [Google Scholar] [CrossRef]
- Ardell, A.J. The effects of elastic interactions on precipitate microstructural evolution in elastically inhomogeneous nickel-base alloys. Philos. Mag. 2014, 94, 2101–2130. [Google Scholar] [CrossRef]
- Zhu, J.Z.; Wang, T.; Zhou, S.H.; Liu, Z.K.; Chen, L.Q. Quantitative interface models for simulating microstructure evolution. Acta Mater. 2004, 52, 833–840. [Google Scholar] [CrossRef]
- Manga, V.R.; Shang, S.L.; Wang, W.Y.; Wang, Y.; Liang, J.; Crespi, V.H.; Liu, Z.K. Anomalous phonon stiffening associated with the (111) antiphase boundary in L12 Ni3Al. Acta Mater. 2015, 82, 287–294. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Yang, Y.; Yang, G.; Zhou, Y. Phase-field simulation of the effect of interactions between ordered domains on transformation kinetics in precipitation. Model. Simul. Mater. Sci. Eng. 2008, 16, 025004. [Google Scholar] [CrossRef]
- Jiang, X.W.; Wang, D.; Xie, G.; Li, H.; Lou, L.; Zhang, J. The Effect of Long-Term Thermal Exposure on the Microstructure and Stress Rupture Property of a Directionally Solidified Ni-Based Superalloy. Metall. Mater. Trans. A 2014, 45, 6016–6026. [Google Scholar] [CrossRef]
- Rafiei, M.; Mirzadeh, H.; Malekan, M.; Sohrabi, M.J. Homogenization kinetics of a typical nickel-based superalloy. J. Alloys Compd. 2019, 793, 277–282. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. I. General Theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, Phase Change, and Microstructure Kinetics of Phase Change. III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Pradell, T.; Crespo, D.; Clavaguera, N.; Clavaguera-Mora, M.T. Diffusion controlled grain growth in primary crystallization: Avrami exponents revisited. J. Phys. Condens. Matter 1998, 10, 3833–3844. [Google Scholar] [CrossRef]
- Asta, M.; Beckermann, C.; Karma, A.; Kurz, W.; Napolitano, R.; Plapp, M.; Purdy, G.; Rappaz, M.; Trivedi, R. Solidification microstructures and solid-state parallels: Recent developments, future directions. Acta Mater. 2009, 57, 941–971. [Google Scholar] [CrossRef]
- Gránásy, L.; Rátkai, L.; Szállás, A.; Korbuly, B.; Tóth, G.I.; Környei, L.; Pusztai, T. Phase-Field Modeling of Polycrystalline Solidification: From Needle Crystals to Spherulites-A Review. Metall. Mater. Trans. A 2014, 45, 1694–1719. [Google Scholar] [CrossRef]
- Mao, J.; Chang, K.M.; Yang, W.H.; Ray, K.; Vaze, S.P.; Furrer, D.U. Cooling precipitation and strengthening study in powder metallurgy superalloy U720LI. Metall. Mater. Trans. A 2001, 32, 2441–2452. [Google Scholar] [CrossRef]
- Mushongera, L.T.; Fleck, M.; Kundin, J.; Wang, Y.; Emmerich, H. Effect of Re on directional γ′-coarsening in commercial single crystal Ni-base superalloys: A phase field study. Acta Mater. 2015, 93, 60–72. [Google Scholar] [CrossRef]
- Kundin, J.; Mushongera, L.; Goehler, T.; Emmerich, H. Phase-field modeling of the γ′-coarsening behavior in Ni-based superalloys. Acta Mater. 2012, 60, 3758–3772. [Google Scholar] [CrossRef]
- Slyozov, V.V.; Lifshitz, I.M.; Slyozov, V.V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids 1961, 19, 35–50. [Google Scholar] [CrossRef]
- Wang, T.; Sheng, G.; Liu, Z.K.; Chen, L.Q. Coarsening kinetics of γ′ precipitates in the Ni–Al–Mo system. Acta Mater. 2008, 56, 5544–5551. [Google Scholar] [CrossRef]
- Fratzl, P.; Penrose, O.; Lebowitz, J.L. Modelling of Phase Separation in Alloys with Coherent Elastic Misfit. J. Stat. Phys. 1999, 95, 1429–1503. [Google Scholar] [CrossRef]
- Maebashi, T.; Doi, M. Coarsening behaviours of coherent γ′ and γ precipitates in elastically constrained Ni–Al–Ti alloys. Mater. Sci. Eng. A 2004, 373, 72–79. [Google Scholar] [CrossRef]
- Wu, X.C.; Li, Y.S.; Huang, M.Q.; Liu, W.; Hou, Z.Y. Precipitation kinetics of ordered γ′ phase and microstructure evolution in a Ni-Al alloy. Mater. Chem. Phys. 2016, 182, 125–132. [Google Scholar] [CrossRef]





| Element | Al | Co | Cr | Mo | Re | Ta | W | Ni |
|---|---|---|---|---|---|---|---|---|
| c (wt.%) | 5.6 | 9 | 4.3 | 2 | 2 | 7.5 | 8 | Bal. |
| c (at.%) | 12.8987 | 9.4911 | 5.1396 | 1.2956 | 0.6675 | 2.5760 | 2.7043 | Bal. |
| Element | Al | Co | Cr | Mo | Re | Ta | W |
|---|---|---|---|---|---|---|---|
| (at.%) | 7.9412 | 13.3236 | 9.4536 | 2.5110 | 1.2770 | 0.8859 | 3.8499 |
| (at.%) | 16.4819 | 6.7210 | 2.0216 | 0.4172 | 0.2270 | 3.7976 | 1.8763 |
| (×10−14) | 5.2629 | 0.9834 | 0.9463 | 0.5264 | 0.0017 | 0.6619 | 0.0054 |
| (×10−14) | 0.6688 | 0.0033 | 0.0007 | 0.0033 | 0.0033 | 0.0070 | 0.0032 |
| Elastic Constants | Phase | Value (GPa) |
|---|---|---|
| C11 | γ | |
| γ′ | ||
| C12 | γ | |
| γ′ | ||
| C44 | γ | |
| γ′ |
| Parameters | Value |
|---|---|
| Interface mobility = (m4·J−1 s−1) | 1 × 10−9 |
| Interface energy (J·m−2) | 0.02 [20] |
| APB energy (J·m−2) | 0.2 [21] |
| Molar volume Vm (m3·mol−1) | 7.1 × 10−6 [20] |
| Grid number nx = ny | 400 |
| Grid size Δx (μm) | 0.015 |
| Interface width η (μm) | 0.04 |
| Time step Δt (s) | 9.619 × 10−4 |
| Nucleation parameter ρ1 | 1.1 × 10−14 |
| Nucleation parameter ρ2 | 3 [4] |
| Nucleation parameter ρ3 (°C−1) | 10−4 [4] |
| Nucleation parameter | 4.66 × 10−4 |
| Nucleation parameter Q | 8.87 × 104 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Zhang, Y. Precipitation and Growth Simulation of γ′ Phase in Single Crystal Superalloy DD6 with Multiphase-Field Method and Explicit Nucleation Algorithm. Metals 2020, 10, 1346. https://doi.org/10.3390/met10101346
Xu Q, Zhang Y. Precipitation and Growth Simulation of γ′ Phase in Single Crystal Superalloy DD6 with Multiphase-Field Method and Explicit Nucleation Algorithm. Metals. 2020; 10(10):1346. https://doi.org/10.3390/met10101346
Chicago/Turabian StyleXu, Qingyan, and Yaqian Zhang. 2020. "Precipitation and Growth Simulation of γ′ Phase in Single Crystal Superalloy DD6 with Multiphase-Field Method and Explicit Nucleation Algorithm" Metals 10, no. 10: 1346. https://doi.org/10.3390/met10101346
APA StyleXu, Q., & Zhang, Y. (2020). Precipitation and Growth Simulation of γ′ Phase in Single Crystal Superalloy DD6 with Multiphase-Field Method and Explicit Nucleation Algorithm. Metals, 10(10), 1346. https://doi.org/10.3390/met10101346






