Extraction Chromatography Materials Prepared with HDEHP on Different Inorganic Supports for the Separation of Gadolinium and Terbium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Solid Extractants (HDEHP + Supports Silanized)
2.1.1. Support
2.1.2. Hydrophobization of Supports
2.1.3. Loading of Silanized Supports with HDEHP
2.2. Extraction Capacity of Solid Extractants
2.2.1. Radiotracer Production
2.2.2. Column Packing
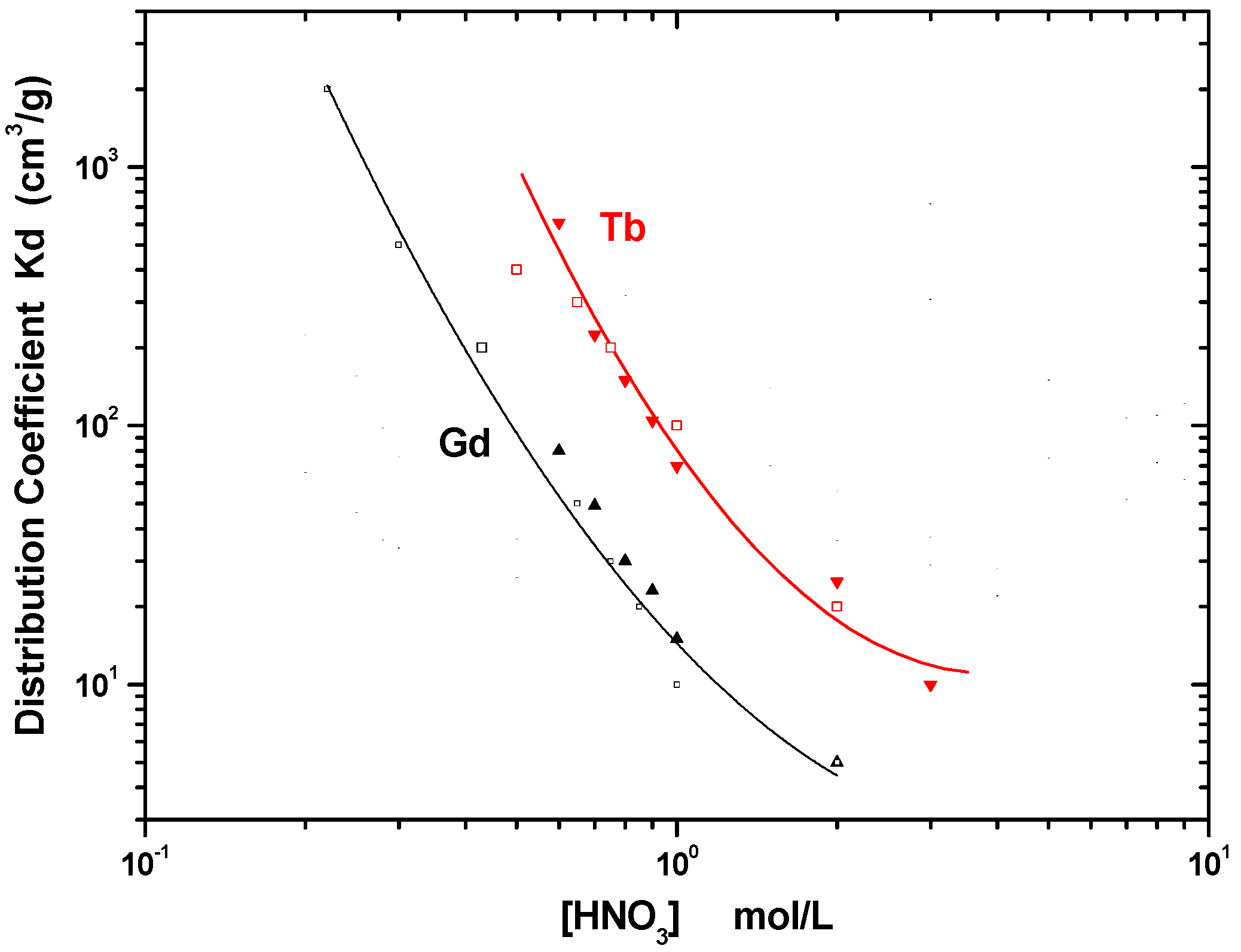
2.2.3. Distribution Coefficients
2.3. Characterization of Solid Extractants
3. Results
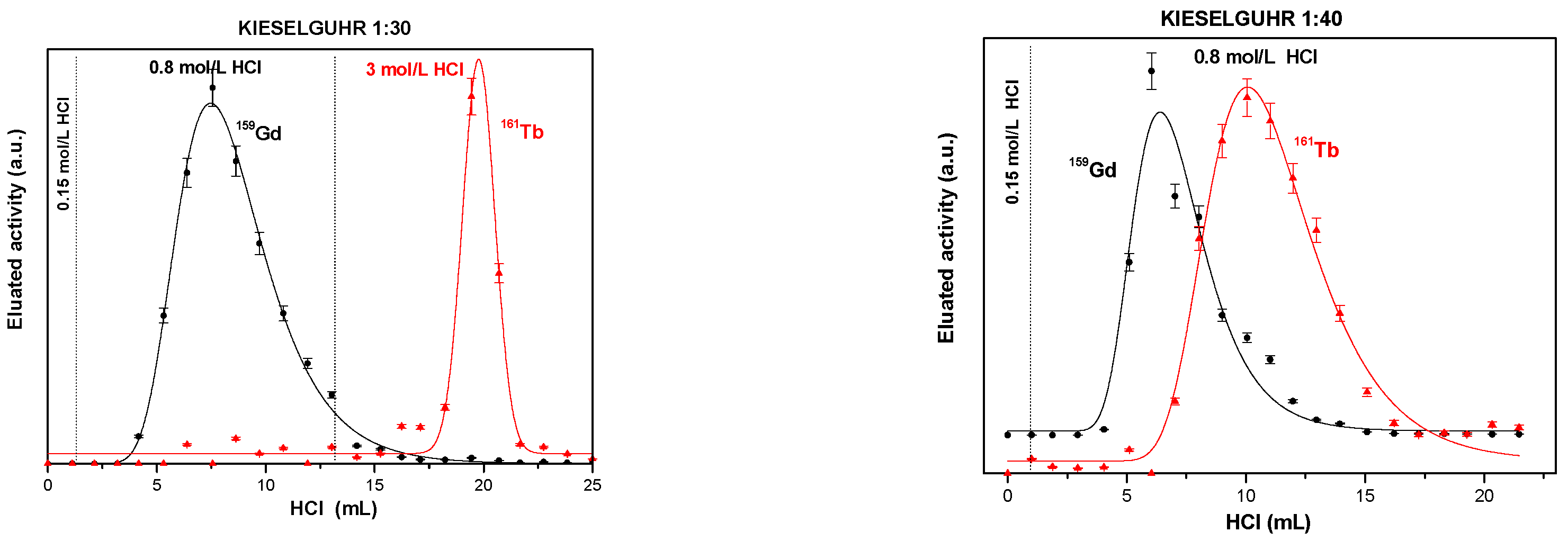
3.1. Solid Extractant Prepared with Kieselguhr
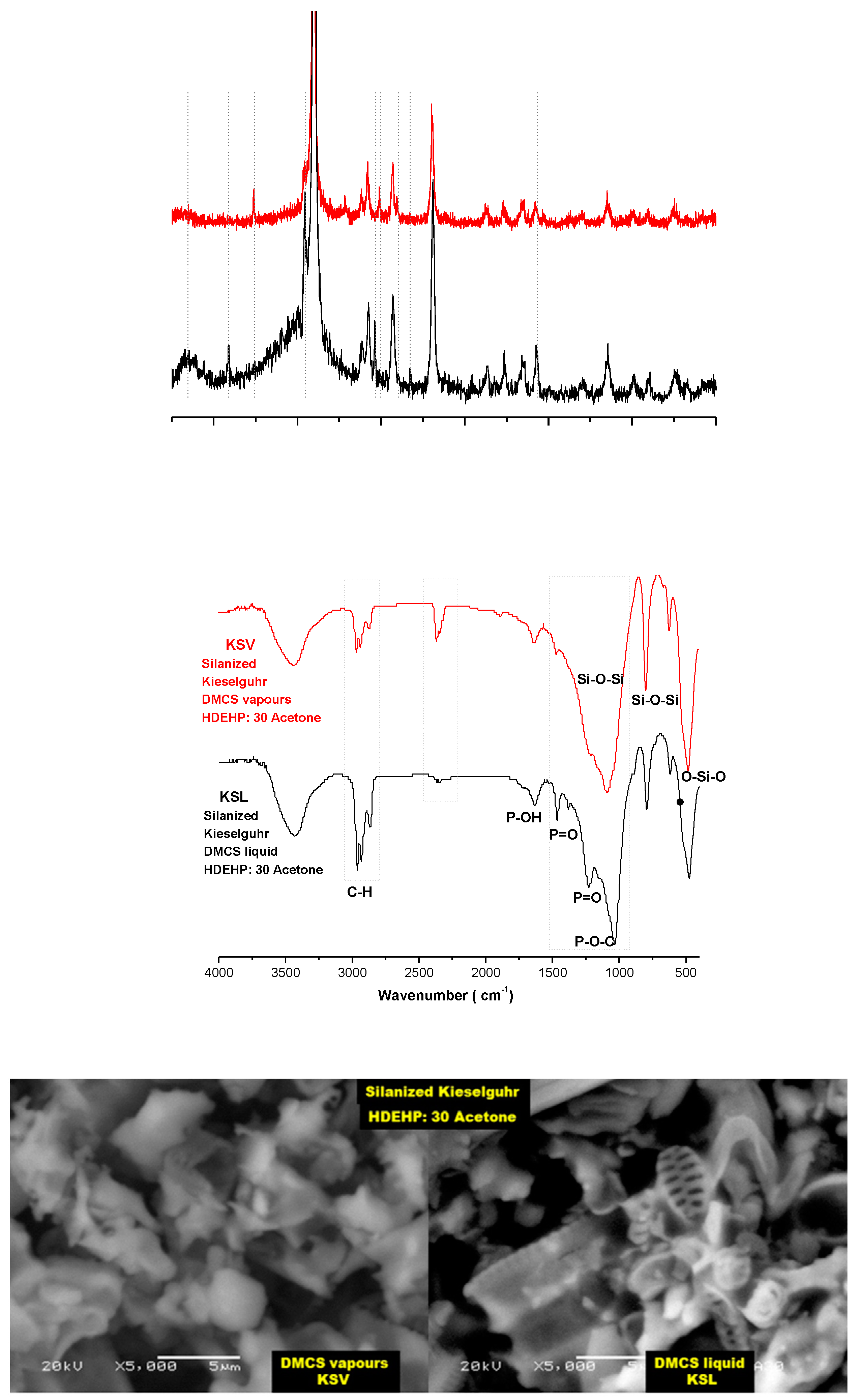
3.1.1. Silanization of Kieselguhr
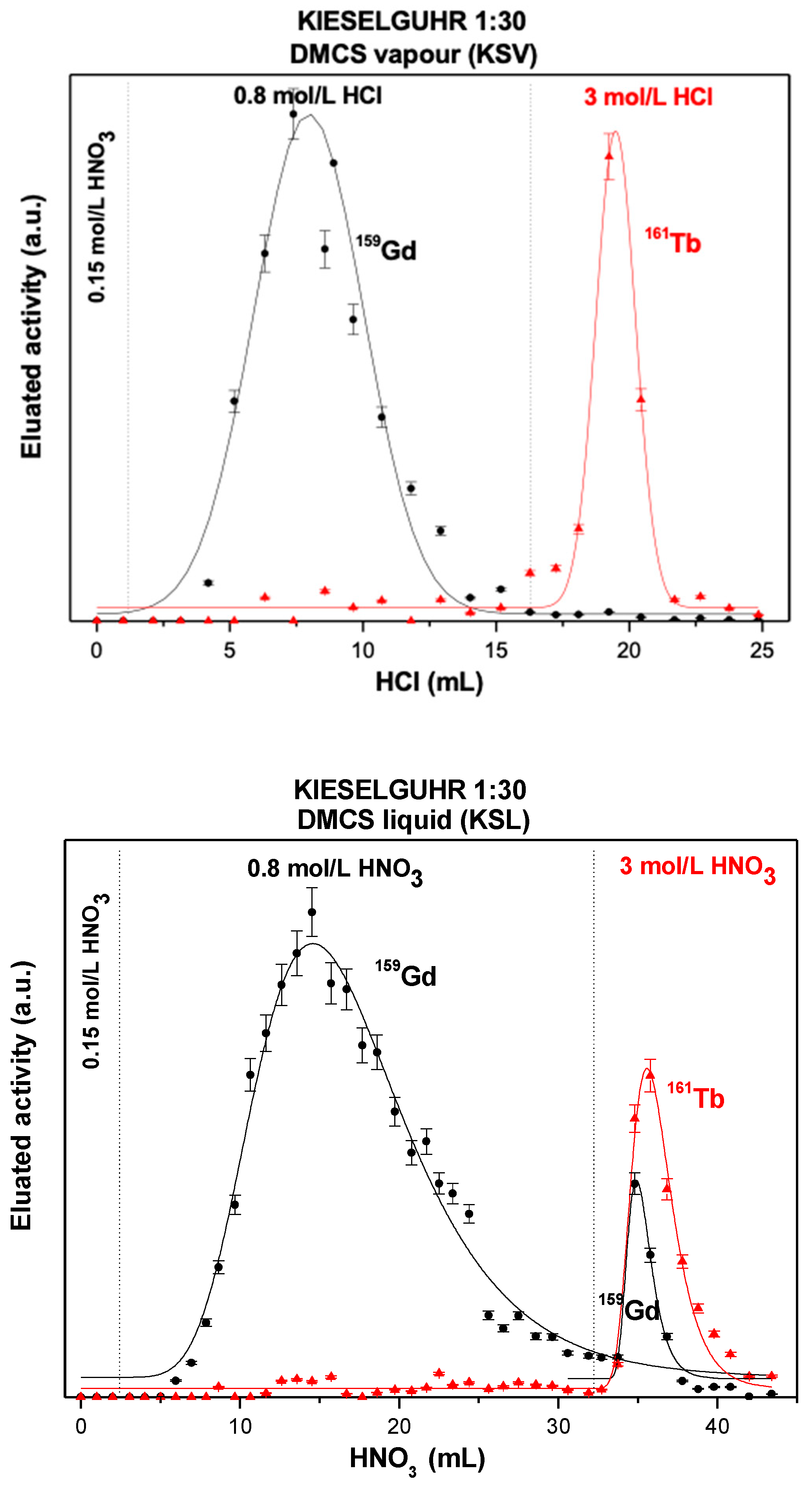
3.1.2. HDEHP-Impregnated Kieselguhr Silanized with DMCS Vapors
3.1.3. HDEHP-Impregnated Kieselguhr Silanized with DMCS Liquid
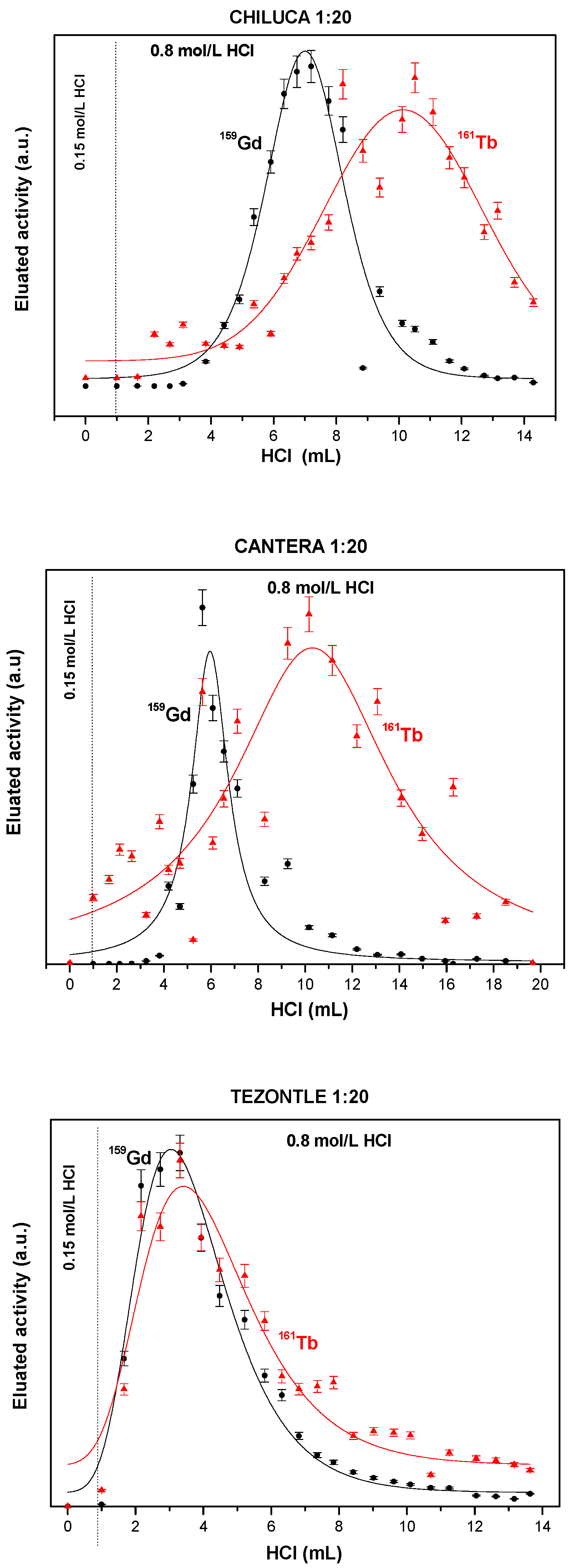
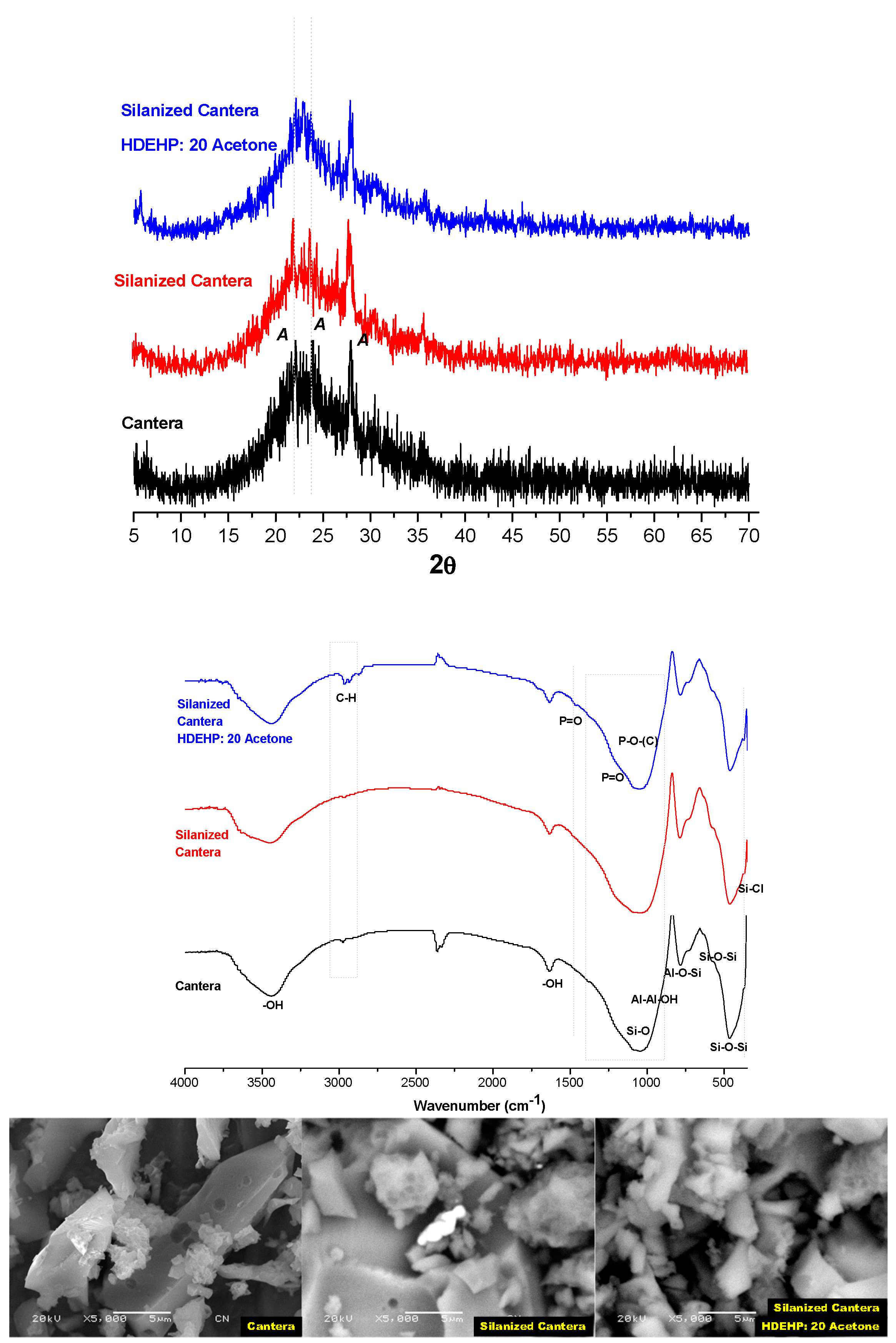
3.2. Solid Extractant Prepared with Volcanic Rocks
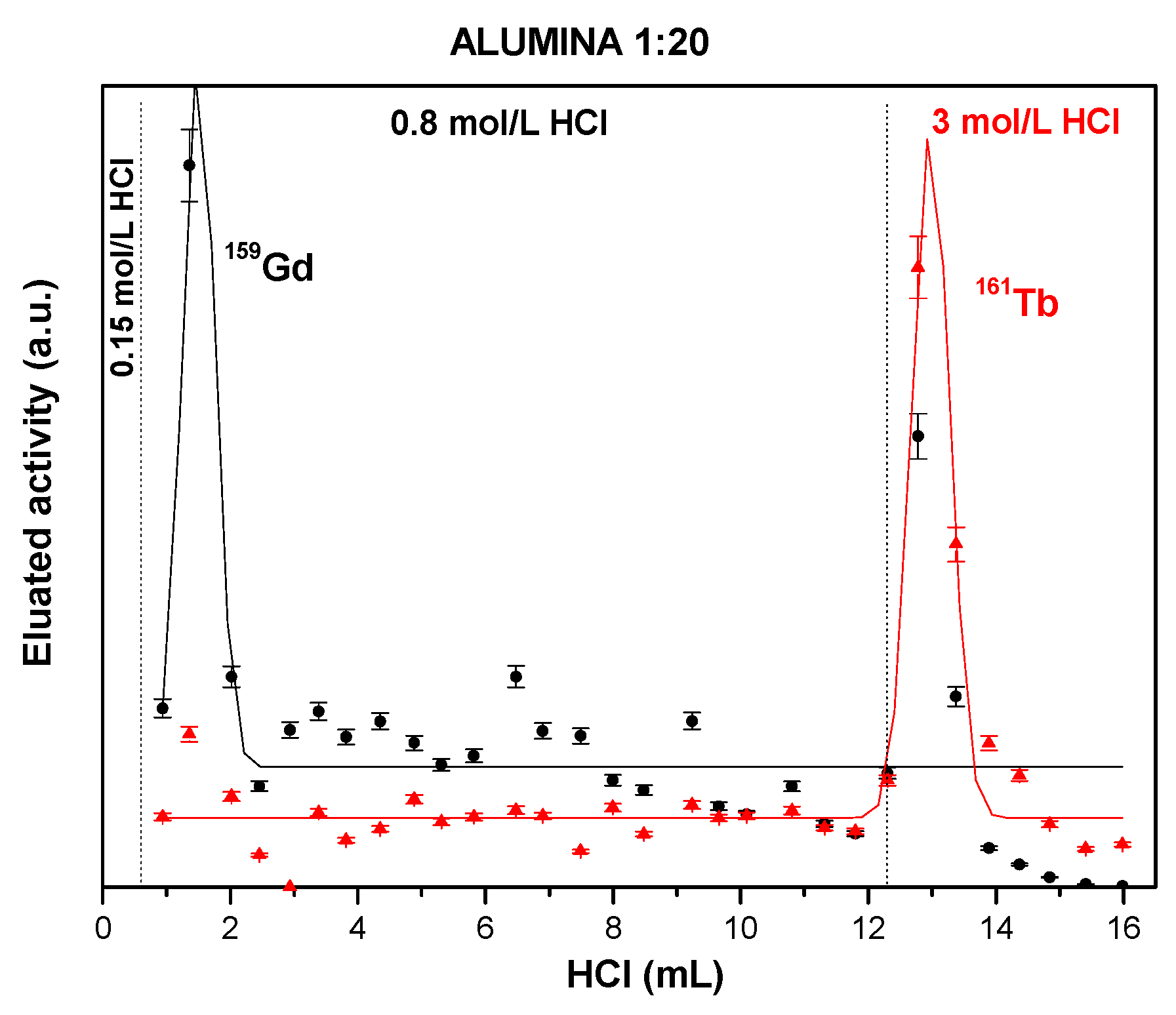
3.3. Solid Extractant Prepared with Alumina
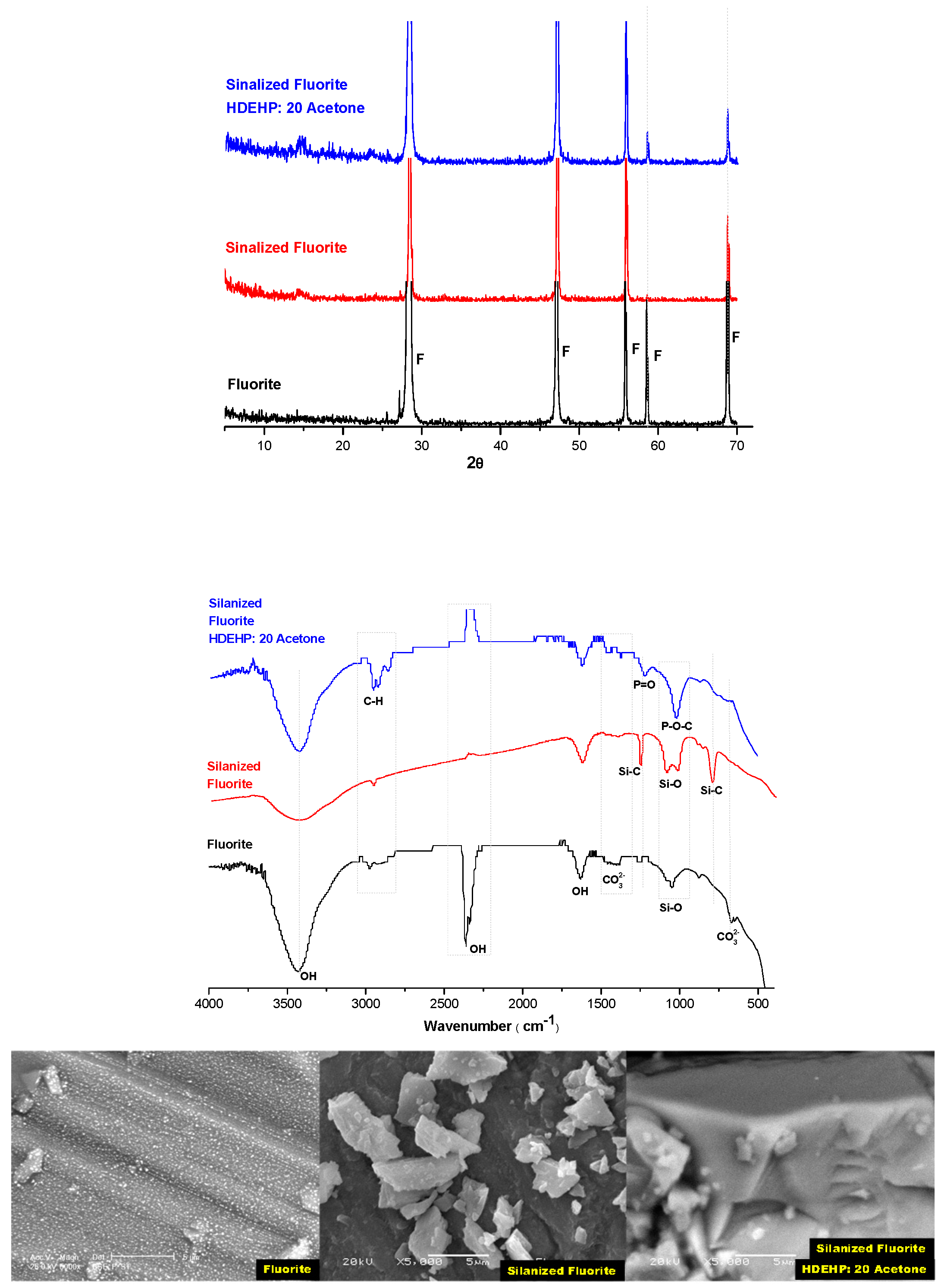
3.4. Solid Extractant Prepared with Fluorite
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dietz, M.L.; Horwitz, E.P.; Bond, A.H. Extraction Chromatography: Progress and Opportunities; ACS Symposium Series Vol. 716, Symposium Proceedings: Progress in Metal Ion Separation and Preconcentration; American Chemical Society: Washington, DC, USA, 1999; pp. 234–250. [Google Scholar] [CrossRef]
- Horwitz, E.P.; McAlister, D.R. Extraction Chromatography Versus Solvent Extraction: How similar are they? Sep. Sci. Technol. 2006, 41, 2163–2182. [Google Scholar] [CrossRef]
- Braun, T.; Ghersini, G. (Eds.) Extraction Chromatrography; Journal of Chromatograpy Library-Volume 2; Elservier: Amsterdam, The Netherlands, 1975. [Google Scholar]
- Monroy-Guzman, F.; Jaime Salinas, E.; Jimenez Barreiro, F.; Vera Treviño, A.L. Radiolanthanides device production. World J. Nucl. Sci. Technol. 2015, 5, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Alfred, G. (Ed.) Handbook of Rare Earth Elements: Analytics; Walter de Gruyter GmbH: Berlin, Germany, 2017. [Google Scholar]
- Shu, Q.; Khayambashi, A. Studies on adsorption of rare earth elements from nitric acid solution with macroporous silica-based bis(2-ethylhexyl)phosphoric acid impregnated polymeric adsorbent. Adsorpt. Sci. Technol. 2018, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Lehto, J.; Hou, X. Chemistry and Analysis of Radionuclides; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; ISBN 978-3-527-32658-7. [Google Scholar]
- Monroy-Guzman, F.; Jaime Salinas, E. Separation of micro-macrocomponent systems: 149Pm–Nd, 161Tb-Gd, 166Ho-Dy and 177Lu-Yb BY extraction chromatography. J. Mex. Chem. Soc. 2015, 59, 143–150. [Google Scholar] [CrossRef]
- Van de Voorde, M.; Van Hecke, K.; Cardinaels, T.; Binnemans, K. Radiochemical processing of nuclear-reactor-produced radiolanthanides for medical applications. Coord. Chem. Rev. 2019, 382, 103–125. [Google Scholar] [CrossRef]
- Kabay, N.; Cortina, J.L.; Trochimczuk, A.; Streat, M. Solvent-impregnated resins (SIRs)—Methods of preparation and their applications Reactive & Functional. Polymers 2010, 70, 484–496. [Google Scholar]
- Sochacka, R.J.; Siekierski, S. Reversed-phase partition chromatography with di-(2-ethylhexyl) orthophosphoric acid as the stationary phase: Part I. Separation of rare earths. J. Chromatogr. A 1964, 16, 376–384. [Google Scholar] [CrossRef]
- Yizhong, Q.; Weiming, S.; Nuping, P.; Qiaozhen, L. Quick separation and determination of Pm-147m~ in water by HDEHP-KeL-F chromatography. China Nuclear Sci. Technol. Rep. 1980, 0, 81. [Google Scholar]
- Larsen, N. High-pressure liquid chromatography of irradiated nuclear fuel: Separation of neodymium for burn-up determination. J. Radioanal. Nucl. Chem. 1979, 52, 85–91. [Google Scholar] [CrossRef]
- Klug, C.L. Preparation and Characterization of Extraction Chromatography Resins Using N-Donor Extractants for Trivalent Actinide and Lanthanide Separations. Ph.D. Theses, University of Nevada, Reno, NV, USA, 2010. [Google Scholar]
- Xizhen, Q.; Xianwen, R. Use of HDEHP-PVDF columns in extraction chromatography:Ⅰ.Principles of the sequential separation scheme for fission products analyses. Radialization Prot. 1981, 4, 28–32. [Google Scholar]
- Winchester, J.W. A Preliminary Investigación of a Chromatographic Column Separation of Rare Earths Using di(2-ethylhexyl)phosphoric Acid; Report X-822; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1958; pp. 1–7. [Google Scholar]
- Martin, D.B.; Pope, D.G. Separation of tervalent lanthanides from actinides by extraction chromatography. Anal. Chem. 1982, 54, 2552–2556. [Google Scholar] [CrossRef]
- Wei, Y.; Kumagai, M.; Takashima, Y.; Modolo, G.; Odoj, R. Studies on the separation of minor actinides from high-level wastes by extraction chromatography using novel solica-based extraction resins. Nucl. Technol. 2000, 132, 413–423. [Google Scholar] [CrossRef] [Green Version]
- McAlister, D.R.; Horwitz, E.P. Characterization of extraction of chromatographic materials containing bis(2-ethyl-1-hexyl)phosphoric acid, 2-etyhl-1-hexyl (2- ethyl-1-hexyl) phosphonic acid, and bis(2,4,4-trimethyl-1-pentyl) phosphinic acid. Solv. Extr. Ion Exch. 2007, 25, 757–769. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, S.; Zhang, S.; Zhou, J.; Ning, S.; Wang, X.; Wei, Y. Separation of scandium from the other rare earth elements with a novel macro-porous silica-polymer based adsorbent HDEHP/SiO2-P. Hydrometallurgy 2019, 185, 117–124. [Google Scholar] [CrossRef]
- Sasaki, T.; Uchiyama, S.; Fujiwara, K.; Sugo, T.; Umeno, D.; Saito, K. Similarity of rare earth extraction by acidic extractant bis(2-ethylhexyl) phosphate (HDEHP) supported on a dodecylamino-group-containing graft chain and by HDEHP dissolved in dodecane. Kagaku Kogaku Ronbun. 2014, 40, 404–409. [Google Scholar] [CrossRef]
- Momen, M.A.; Dietz, M.L. High-capacity extraction chromatographic materials based on polysulfone microcapsules for the separation and preconcentration of lanthanides from aqueous solution. Talanta 2019, 197, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, E.R.; Jackson, J.A.; Shafer, J.C. A Survey of Extraction Chromatographic f-Element Separations Developed by E. P. Horwitz. Solv. Extr. Ion Exch. 2020, 38, 251–289. [Google Scholar] [CrossRef]
- Cortina, J.L.; Miralles, N.; Aguilar, M.; Sastre, A.M. Solvent impregnated resins containing di(2-ethylhexyl)phosphoric acid II. Study of the distribution equilibria of Zn(II), Cu(II), and Cd(II). Solv. Extr. Ion Exch. 1994, 12, 371–391. [Google Scholar] [CrossRef]
- Horwitz, E.P.; Bloomquist, C.A.A. Chemical separations for super-heavy element searches in irradiated uranium targets. J. Inorg. Nucl. Chem. 1975, 37, 425–434. [Google Scholar] [CrossRef]
- Huang, C.H. Rare Earth Coordination Chemistry; Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Lutynski, M.; Sakiewicz, P.; Lutynska, S. Characterization of Diatomaceous Earth and Halloysite Resources of Poland. Minerals 2019, 9, 670. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Ortiz, M.; Hernández-Padrón, G.; Bernal, R.; Cruz-Vázquez, C.; Castaño, V.M. Nanocrystalline mimetic opals: Synthesis and comparative characterization vs. natural stones. Int. J. Basic Appl. Sci. 2015, 4, 238. [Google Scholar]
- Cefarelli, A.O.; Ferrario, M.E.; Almandoz, G.O.; Atencio, A.G.; Akselman, R.; Vernet, M. Diversity of the diatom genus Fragilariopsis in the Argentine Sea and Antarctic waters: Morphology, distribution and abundance. Polar Biol. 2010, 33, 1463–1484. [Google Scholar] [CrossRef] [Green Version]
- Correchera, V.; Garcia-Guinea, J.; Bustillo, M.A.; Garcia, R. Study of the thermoluminescence emission of a natural α-cristobalite. Radiat. Eff. Defect. S. 2009, 164, 59–67. [Google Scholar] [CrossRef]
- Emam, S.; Gasser, M.S.; El-Hefny, N.E.; Mahmoud, W.H.; Aly, H.F. Sorption of certain lanthanides by kieselguhr loaded with Cyanex 572 from sulfate medium. J. Rare Earth. 2020, 38, 299–305. [Google Scholar] [CrossRef]
- Wang, L.; Lu, A.; Xiao, Z.; Ma, J.; Li, Y. Modification of nano-fibriform silica by dimethyldichlorosilane. Appl. Surf. Sci. 2009, 255, 7542–7546. [Google Scholar] [CrossRef]
- Monroy-Guzman, F. Comportement a l’echelle des indicateurs des elements zirconium, hafnium, et l’element 104, niobium, tantale et protactinium (element 105) en milieux tres complexants. Ph.D. Thesis, University of Paris, Paris, France, 1997. [Google Scholar]
- Herter, A. Etude de l’influence de paramètres géométriques et cinétiques sur les courbes d’élution en chromatographie d’échange d’ions. In Rapport de fin de Cycle CES TNRP, CRN; Strasbourg, France, 1996. [Google Scholar]
- Hayes, R.; Ahmed, A.; Edge, T.; Zhang, H. Core-shell particles: Preparation, fundamentals and applications in high performance liquid chromatography. J. Chromatogr. A 2014, 1357, 36–52. [Google Scholar] [CrossRef] [Green Version]
- Al-Anber, M.A. Adsorption of ferric ions onto natural feldspar: Kinetic modeling and adsorption isotherm. Int. J. Environ. Sci. Technol. 2015, 12, 139–150. [Google Scholar] [CrossRef] [Green Version]
- Ritz, M.; Vaculíková, L.; Plevová, E.; Matýsek, D.; Mališ, J. Determination of chlorite, muscovite, albite and quartz in claystones and clay shales by infrared spectroscopy and partial least-squares regression. Acta Geodyn. Geomater. 2012, 9, 511–520. [Google Scholar]
- Jovanovski, G.; Makreski, P. Minerals from Macedonia. XXX. Complementary use of Vibrational Spectroscopy and X-Ray Powder Diffraction for spectra-structural study of some cyclo-, phyllo- and tectosilicate minerals. A Review. Maced. J. Chem. Chem. En. 2016, 35, 125–155. [Google Scholar] [CrossRef] [Green Version]
- Boal, D.; Ozin, G.A. Vibrational spectra, vibrational analysis and stereochemistries of the pentacoordinate molecules SiC13X - N(CH3)3 and SiC13X. N(CD3)3 where X = H, F, Br, or I. Can. J. Chem. 1973, 51, 609–617. [Google Scholar] [CrossRef]
- Romero Toledo, R.; Ruíz Santoyo, V.; Moncada Sánchez, D.; Martínez Rosales, M. Effect of aluminum precursor on physicochemical properties of Al2O3 by hydrolysis/precipitation method. Nova Sci. 2018, 10, 1–11. [Google Scholar] [CrossRef]
- Pakizeh, M.; Moghadam, A.N.; Omidkhah, M.R.; Namvar-Mahboub, M. Preparation and characterization of dimethyldichlorosilane modified SiO2/PSf nanocomposite membrane. Korean J. Chem. Eng. 2013, 30, 751–760. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, Z.; Gao, H.; Zheng, R.; Jin, Y.; Niu, C. Flotation studies of fluorite and barite with sodium petroleum sulfonate and sodium hexam- etaphosphate. J. Mater. Res. Technol. 2018. [Google Scholar] [CrossRef]
- Huang, L.; Zeng, Q.; Hu, L.; Hu, Y.; Zhong, Z.; He, Z. The Contribution of Long-Terms Static Interactions Between Minerals and Flotation Reagents for the Separation of Fluorite and Calcite. Minerals 2019, 9, 699. [Google Scholar] [CrossRef] [Green Version]
- Sasmaz, A.; Kryuchenko, N.; Zhovinsky, E.; Suyarko, V.; Konakci, N.; Akgul, B. Major, trace and rare earth element (REE) geochemistry of different colored fluorites in the Bobrynets region, Ukraine. Ore. Geol. Rev. 2018, 102, 338–350. [Google Scholar] [CrossRef]
- Al Hameed, Z.; Saleem, J.; Hussain, S.S.; Ghani, A.A.; Lal, H. Study of indigenous fluorspar as metallurgical flux. J. Eng. Appl. Sci. 2017, 36, 1. [Google Scholar]
- Chrusciel, J.J.; Fejdys, M.; Fortuniak, W. Synthesis, Characterization and Microstructure of New Liquid Poly(methylhydrosiloxanes) Containing Branching Units SiO4/2. Polymers 2018, 10, 484. [Google Scholar] [CrossRef] [Green Version]




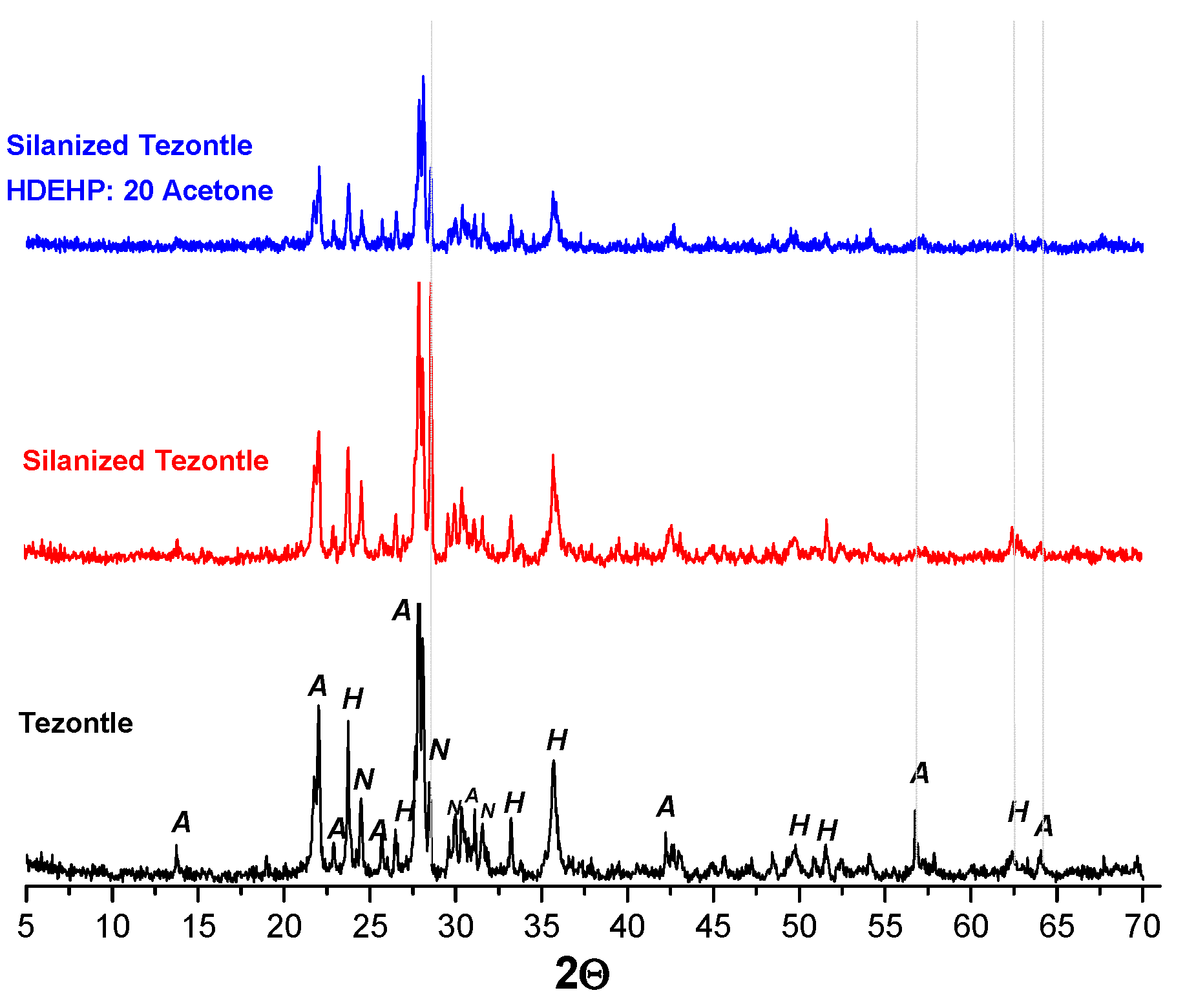
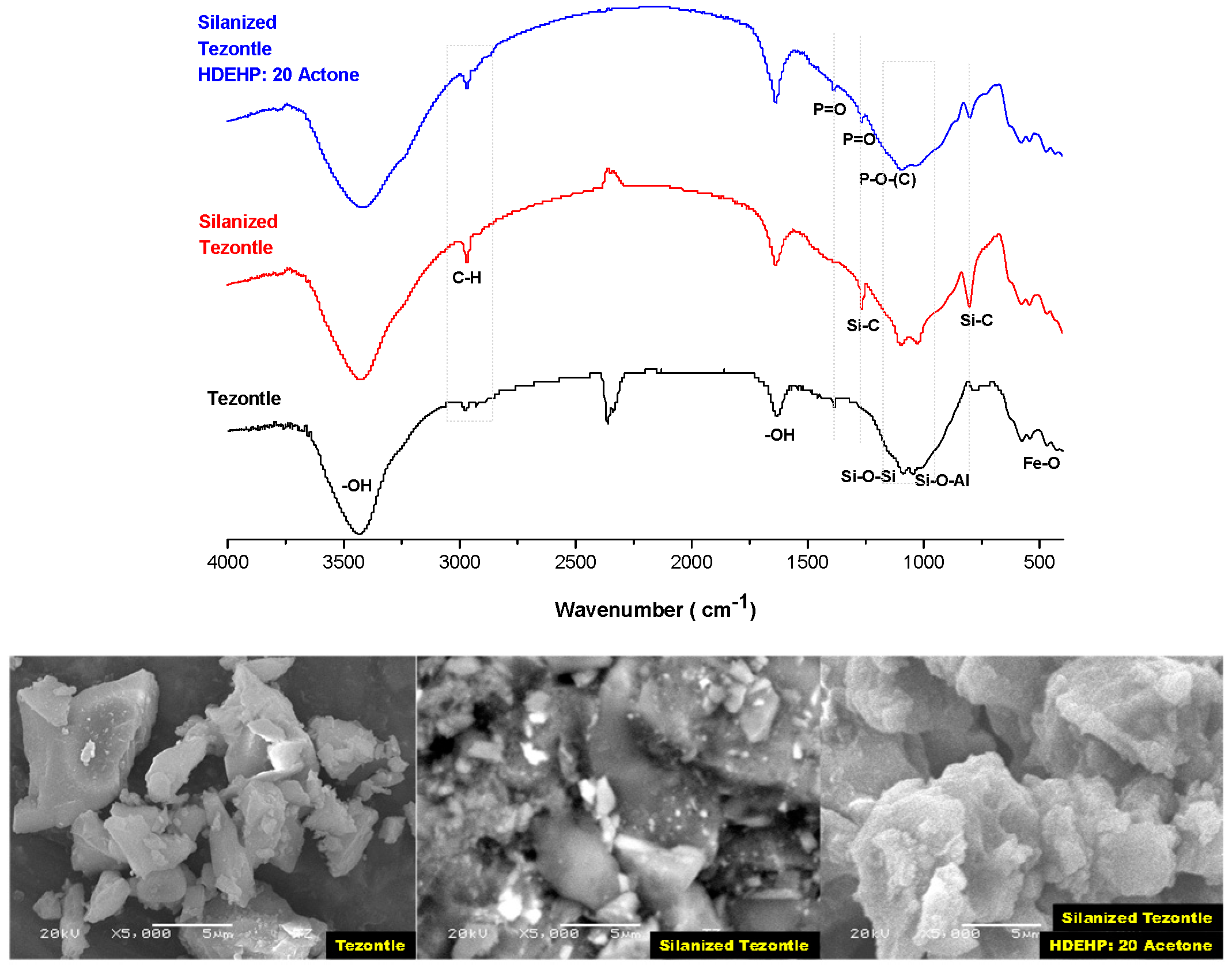
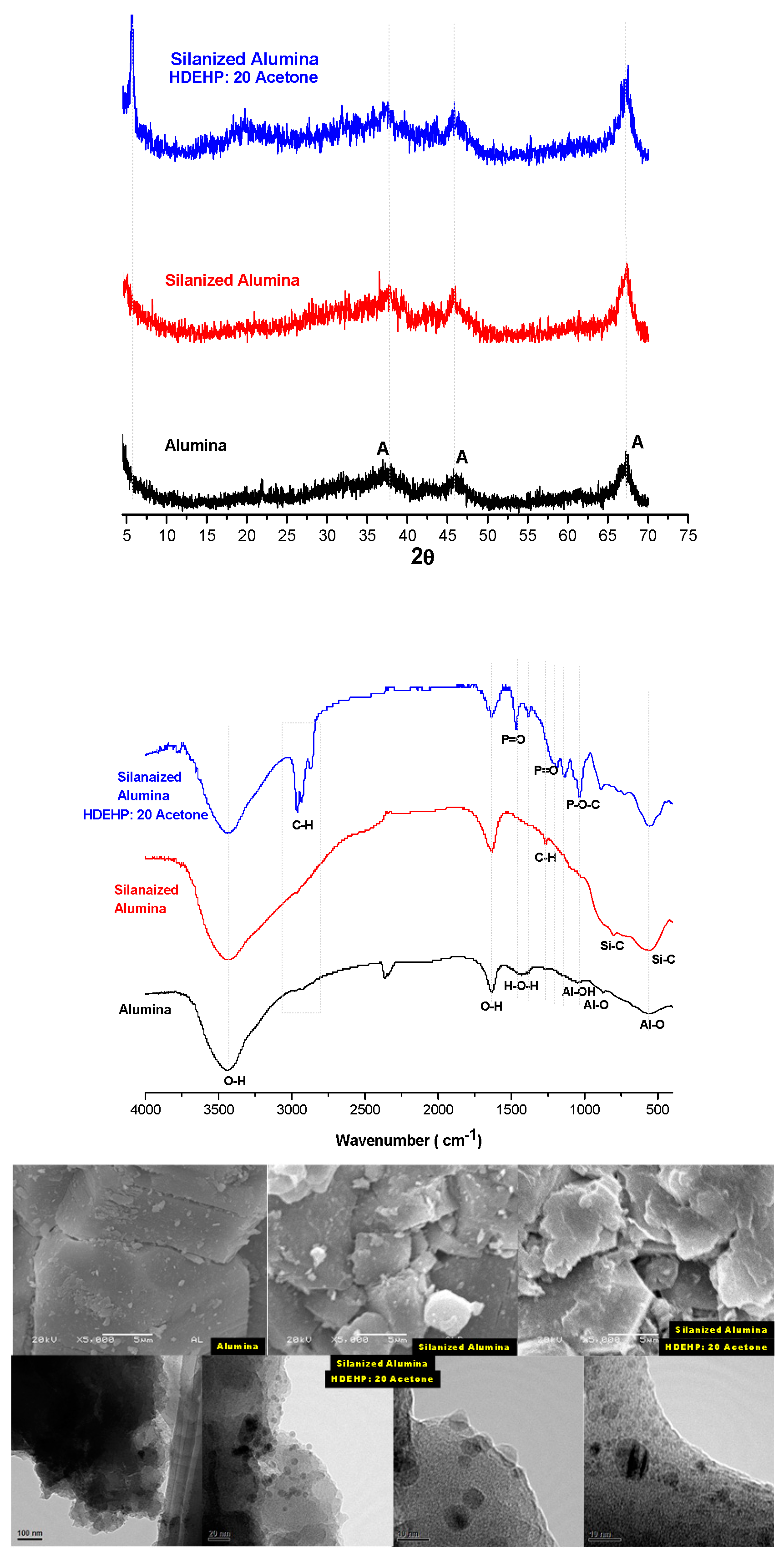


| Support | Silanization Method |
Loading of HDEHP Technique | HDEHP/Acetone |
|---|---|---|---|
| Kieselguhr | DMCS solution | Evaporation | 1:4 |
| Kieselguhr | DMCS solution | Evaporation | 1:8 |
| Kieselguhr | DMCS solution | Evaporation | 1:15 |
| Kieselguhr | DMCS solution | Evaporation | 1:20 |
| Kieselguhr | DMCS solution | Evaporation | 1:30 |
| Kieselguhr | DMCS vapors | Packed column | 1:4 |
| Kieselguhr | DMCS vapors | Packed column | 1:8 |
| Kieselguhr | DMCS vapors | Packed column | 1:15 |
| Kieselguhr | DMCS vapors | Packed column | 1:20 |
| Kieselguhr | DMCS vapors | Packed column | 1:30 |
| Kieselguhr | DMCS vapors | Packed column | 1:40 |
| Alumina | DMCS vapors | Packed column | 1:20 |
| Fluorite | DMCS vapors | Packed column | 1:20 |
| Tezontle | DMCS vapors | Packed column | 1:20 |
| Cantera | DMCS vapors | Packed column | 1:20 |
| Chiluca | DMCS vapors | Packed column | 1:20 |
| Target | Nuclear Reaction |
|---|---|
| 152Gd 0.2% | 152Gd(n, γ)153Gd(241.6 d)→ 153Eu (stable) |
| 154Gd 2.18% | 154Gd(n, γ) 155Gd(stable) |
| 155Gd 14.8% | 155Gd(n, γ) 156Gd(stable) |
| 156Gd 20.47% | 156Gd(n, γ) 157Gd(stable) |
| 157Gd 15.6% | 157Gd(n, γ) 158Gd(stable) |
| 158Gd 24.84% | 158Gd(n, γ) 159Gd(18.6 h) → 149Tb(stable) |
| 160Gd 21.86% | 160Gd(n, γ)161Gd(3.7 min) → 161Tb*(17.6 h) → 161Dy (stable) |
| Solid Extractant | g HDEHP/ g Support | KdGd HCl (0.8 mol/L) | KdTb HCl (3 mol/L) | Separation Resolution | FWHM Gd (mL) | FWHM Tb (mL) | Surface Area (m2/g) | Total Pore Volume (cm3/g) |
|---|---|---|---|---|---|---|---|---|
| Kieselguhr/DMCS vapor/ HDEHP: 4 Acetone | 0.1016 ± 0.0036 | 8.5 ± 1.3 | 2.8 ± 0.7 | 0.59 ± 0.02 | 8.78 ± 0.36 | 1.65 ± 0.06 | 2.8064 ± 0.0281 | 0.6445 ± 0.019 |
| Kieselguhr/DMCS vapor/ HDEHP: 8 Acetone | 0.1045 ± 0.0037 | 5.9 ± 1.4 | 1.5 ± 0.4 | 1.02 ± 0.03 | 7.82 ± 0.64 | 1.78 ± 0.07 | 2.8064 ± 0.0281 | 0.6445 ± 0.019 |
| Kieselguhr/DMCS vapor/ HDEHP: 15 Acetone | 0.1138 ± 0.0040 | 3.2 ± 0.9 | 1.8 ± 0.6 | 0.77 ± 0.02 | 4.65 ± 0.15 | 1.87(9.6) ± 0.14 | 2.8064 ± 0.0281 | 0.6445 ± 0.019 |
| Kieselguhr/DMCS vapor/ HDEHP: 20 Acetone | 0.1051 ± 0.0037 | 4.0 ± 0.8 | 1.3 ± 0.4 | 1.23 ± 0.04 | 4.64 ± 0.17 | 1.44 ± 0.04 | 2.8064 ± 0.0281 | 0.6445 ± 0.019 |
| Kieselguhr/DMCS vapor/ HDEHP: 30 Acetone | 0.1070 ± 0.0038 | 4.8 ± 0.9 | 4.8 ± 1.2 | 1.2 ± 0.04 | 4.84 ± 0.26 | 1.7 ± 0.39 | 2.8064 ± 0.0281 | 0.6445 ± 0.019 |
| Kieselguhr/DMCS vapor/HDEHP: 40 Acetone | 0.0474 ± 0.0017 | 3.4 ± 1.0 | 5.7 1 ± 1.6 | 0.29 ± 0.01 | 3.65 ± 0.38 | 5.2 ± 0.32 | 2.8064 ± 0.0281 | 0.6445 ± 0.019 |
| Kieselguhr/DMCS liquid/HDEHP: 30 Acetone | 0.0888 ± 0.0031 | 9.9 2 ± 1.7 | 2.7 3 ± 0.9 | 0.92 ± 0.03 | 10.99 ± 0.65 | 3.12 ± 0.17 | 2.8064 ± 0.0281 | 0.6445 ± 0.019 |
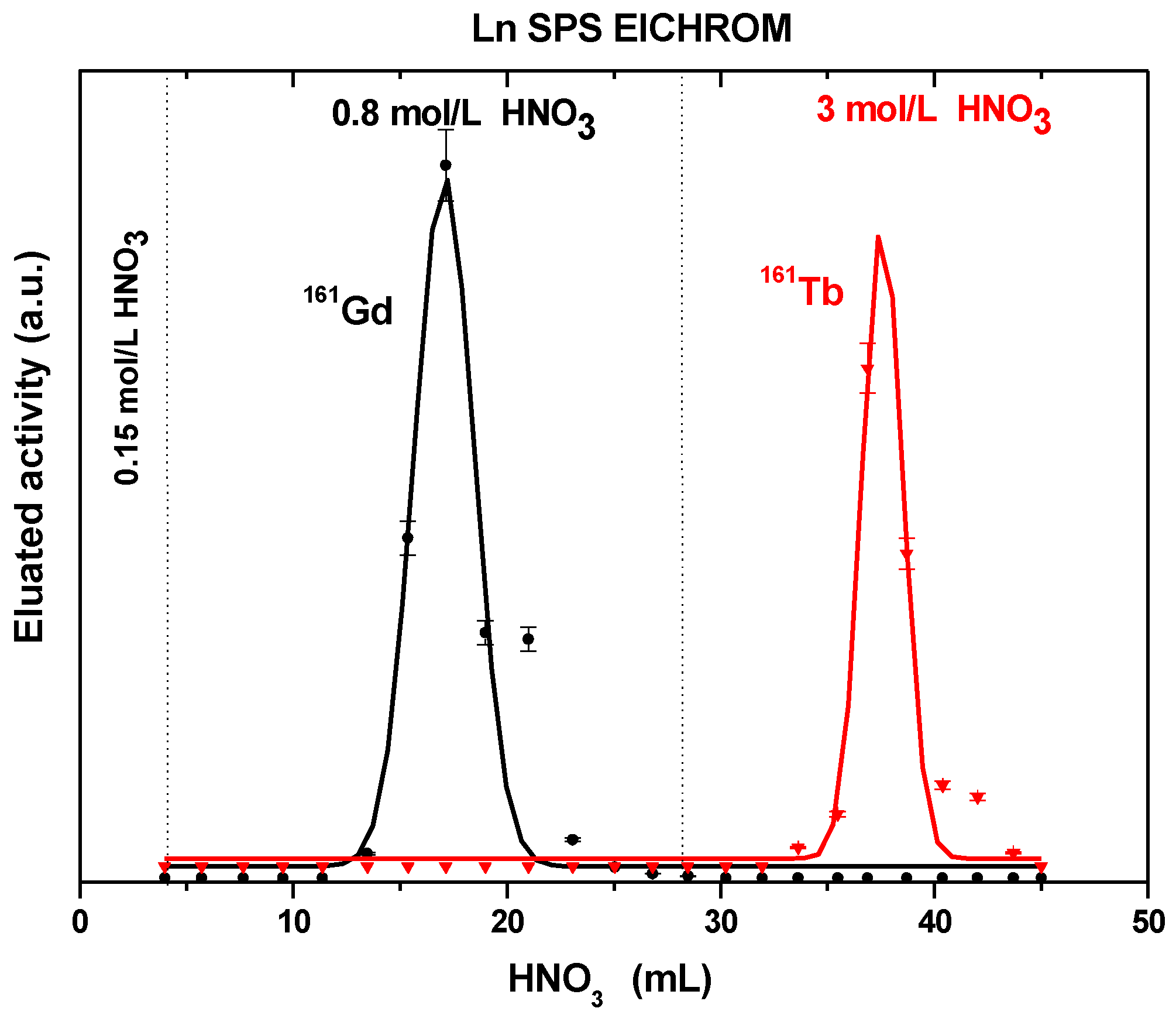
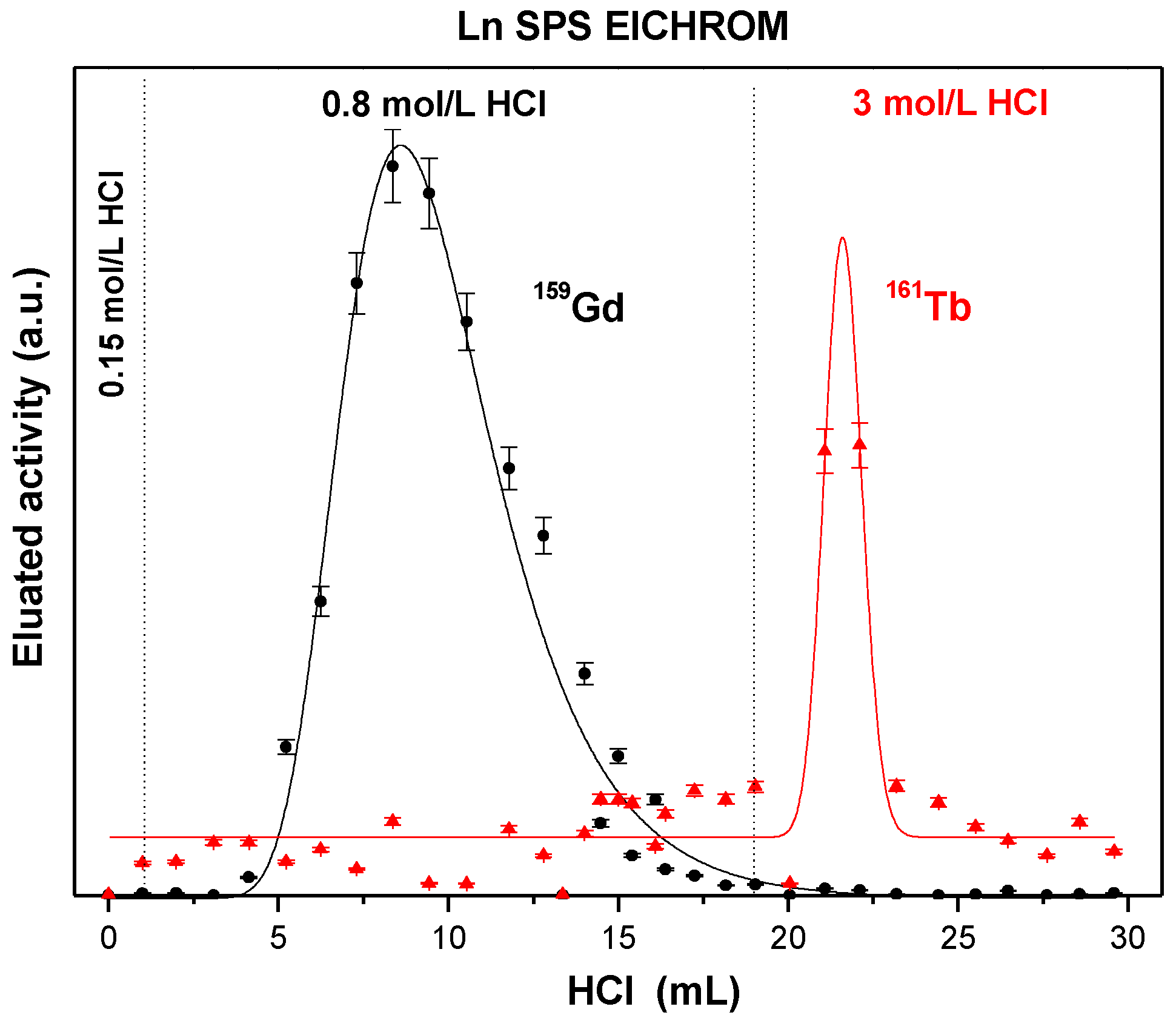
| Ln SPS eluted with HCl | – | 6.7 ± 1.9 | 2.2 ± 0.8 | 0.98 ± 0.03 | 5.39 ± 0.22 | 0.64 ± 0.08 | – | – |
| Ln SPS eluted with HNO3 | – | 14.3 ± 2.2 | 8.0 ± 2.2 | 1.58 ± 0.05 | 3.29 ± 0.19 | 2.23 ± 0.12 | – | -– |
| Alumina/DMCS vapor/ HDEHP: 20 Acetone | 0.0711 ± 0.0025 | 14 ± 2.1 | 13 ± 2.9 | 3.15 ± 0.1 | 0.59 ± 0.06 | 0.69 ± 0.04 | 193.1729 ± 1.9327 | 0.8864 ± 0.0227 |
| Chiluca/DMCS vapor/ HDEHP: 20 Acetone | 0.0100 ± 0.0004 | 4.9 ± 1.1 | 7.4 1 ± 1.5 | 0.27 ± 0.01 | 2.94 ± 0.18 | 5.90 ± 0.23 | 2.1730 ± 0.0217 | 0.0025 ± 0.00012 |
| Cantera/DMCS vapor/ HDEHP: 20Acetone | 0.0273 ± 0.001 | 7.9 ± 1.4 | 12.3 1 ± 2.5 | 0.31 ± 0.01 | 2.15 ± 0.32 | 7.6 ± 1.39 | 5.8283 ± 0.0583 | 0.0650 ± 0.0026 |
| Tezontle/DMCS vapor/ HDEHP: 20Acetone | 0.0193 ± 0.0007 | 2.8 ± 0.7 | 3.2 1 ± 1.2 | 0.03 ± 0.003 | 3.35 ± 0.29 | 3.79 ± 0.36 | 1.7738 ± 0.0177 | 0.0036 ± 0.00012 |
| Fluorite/DMCS vapor/ HDEHP: 20Acetone | 0.0102 ± 0.0004 | 1.6 ± 0.6 | 1.7 1 ± 0.6 | 0.03 ± 0.003 | 0.98 ± 0.06 | 1.02 ± 0.19 | 0.0204 ± 0.0002 | 0.0002 ± 0.00001 |
| Kieselgurhr (K) HDEHP:Acetone | Cantera (CA) | Chiluca (CH) | Tezontle (TE) | Al2O3 (AL) | CaF2 (FL) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Element | K | DMCS Vapour (KSV) | KSV 1:4 | KSV 1:8 | KSV 1:15 | KSV 1:20 | KSV 1:30 | KSV 1:40 | DMCS Liquid (KSL) | KSL 1:30 | CA | DMCS Vapour (CAS) | CAS 1:20 | CH | DMCS Vapour (CHS) | CHS 1:20 | TE | DMCS Vapour (TES) | TES 1:20 | AL | DMCS Vapour (TES) | 1:20 | FL | DMCS Vapour (FL) | 1:20 |
| Si | 31.1 ± 0.34 | 34.85 ± 0.46 | 31.26 ± 0.28 | 32.81 ± 0.35 | 40.25 ± 0.38 | 31.32 ± 0.36 | 30.13 ± 0.31 | 43.31 ± 0.41 | 36.03 ± 0.44 | 24.27 ± 0.29 | 30.23 ± 0.29 | 32.57 ± 0.31 | 28.53 ± 0.26 | 24.45 ± 0.27 | 23.95 ± 0.25 | 20.52 ± 0.21 | 21.98 ± 0.21 | 23.94 ± 0.23 | 21.74 ± 0.20 | 3.13 ± 0.09 | 3.10 ± 0.08 | 3.02 ± 0.07 | |||
| C | 22.1 ± 0.62 | 22.89 ± 0.79 | 25.30 ± 0.51 | 24.10± 0.61 | 14.04 ± 0.61 | 25.86 ± 0.69 | 25.90 ± 0.62 | 12.80 ± 0.62 | 16.41 ± 00.77 | 34.88 ± 0.59 | 12.35 ± 0.56 | 11.22 ± 0.61 | 19.08 ± 0.54 | 22.50 ± 0.62 | 22.85 ± 0.63 | 20.07 ± 0.56 | 8.51 ± 0.08 | 7.97 ± 0.09 | 6.75 ± 0.09 | 17.53 ± 0.34 | 15.45 ± 0.38 | 39.37 ± 0.43 | 7.62 ± 0.26 | 7.57 ± 0.25 | 7.51 ± 0.22 |
| O | 42.8 ± 0.49 | 39.15 ± 0.60 | 36.43 ± 0.39 | 37.35 ± 0.47 | 40.86 ± 0.44 | 38.19 ± 0.50 | 39.73 ± 0.46 | 40.11 ± 0.44 | 44.15 ± 0.57 | 35.75 ± 0.52 | 40.38 ± 0.42 | 42.53 ± 0.43 | 39.70 ± 0.41 | 39.76 ± 0.41 | 41.57 ± 0.47 | 40.87 ± 0.44 | 51.24 ± 0.41 | 49.53 ± 0.46 | 49.09 ± 0.42 | 43.31 ± 0.45 | 41.94 ± 0.47 | 32.85 ± 0.5 | 3.51 ± 0.88 | 3.48 ± 0.87 | 3.25 ± 0.84 |
| P | 2.52 ± 0.09 | 2.36 ± 0.11 | 1.19 ± 0.09 | 1.25 ± 0.08 | 1.07 ± 0.07 | 3.16 ± 0.11 | 0.69 ± 0.06 | 0.2 ± 0.04 | 2.90 ± 0.1 | ||||||||||||||||
| Na | 0.82 ± 0.07 | 0.58 ± 0.08 | 0.60 ± 0.05 | 0.69 ± 0.07 | 0.68 ± 0.07 | 0.56 ± 0.06 | 0.58 ± 0.05 | 0.69 ± 0.07 | 0.79 ± 0.09 | 0.45 ± 0.07 | 1.54 ± 0.07 | 1.82 ± 0.08 | 1.60 ± 0.07 | 2.67 ± 0.08 | 2.28 ± 0.07 | 2.57 ± 0.07 | 1.88 ± 0.06 | 2.12 ± 0.07 | 1.72 ± 0.07 | ||||||
| Al | 0.63 ± 0.06 | 0.65 ± 0.08 | 0.57 ± 0.05 | 0.50 ± 0.06 | 0.65 ± 0.06 | 0.54 ± 0.05 | 0.47 ± 0.05 | 0.69 ± 0.06 | 0.66 ± 0.08 | 0.48 ± 0.06 | 10.90 ± 0.13 | 6.44 ± 0.1 | 5.54 ± 0.08 | 6.98 ± 0.12 | 5.56 ± 0.09 | 5.73 ± 0.08 | 6.49 ± 0.06 | 7.58 ± 0.07 | 6.75 ± 0.09 | 39.16 ± 0.53 | 38.84 ± 0.45 | 22.99 ± 0.51 | 1.91 ± 0.07 | 1.90 ± 0.07 | 1.88 ± 0.07 |
| S | 0.44 ± 0.06 | 0.37 ± 0.07 | 0.82 ± 0.05 | 0.38 ± 0.06 | 0.49 ± 0.06 | 0.33 ± 0.05 | 0.4 ± 0.04 | 0.46 ± 0.06 | 0.31 ± 0.07 | 0.12 ± 0.05 | |||||||||||||||
| K | 3.65 ± 0.08 | 4.04 ± 0.08 | 3.59 ± 0.07 | 1.51 ± 0.04 | 2.16 ± 0.06 | 1.20 ± 0.04 | 0.91 ± 0.07 | 1.12 ± 0.07 | 1.18 ± 0.07 | ||||||||||||||||
| Ca | 0.82 ± 0.07 | 0.61 ± 0.08 | 1.71 ± 0.06 | 0.82 ± 0.07 | 0.88 ± 0.07 | 0.72 ± 0.06 | 0.68 ± 0.05 | 0.82 ± 0.07 | 0.75 ± 0.08 | 0.28 ± 0.06 | 0.27 ± 0.04 | 0.42 ± 0.05 | 0.35 ± 0.04 | 1.41 ± 0.04 | 0.76 ± 0.04 | 1.36±0.04 | 2.22 ± 0.07 | 3.31 ± 0.07 | 4.36 ± 0.06 | 41.28 ± 0.51 | 40.98 ± 0.47 | 41.19 ± 0.5 | |||
| Fe | 0.86 ± 0.13 | 0.90 ± 0.16 | 0.78 ± 0.10 | 0.98 ± 0.13 | 0.97 ± 0.12 | 1.11 ± 0.12 | 0.92 ± 0.1 | 1.11 ± 0.13 | 0.89 ± 0.15 | 0.60 ± 0.12 | 0.67 ± 0.06 | 0.84 ± 0.08 | 0.66 ± 0.06 | 0.40 ± 0.06 | 0.65 ± 0.06 | 0.37±0.05 | 4.33 ± 0.07 | 2.29 ± 0.06 | 5.12 ± 0.07 | ||||||
| Ti | 0.12 ± 0.06 | 0.12 ± 0.05 | 0.31 ± 0.06 | 0.22 ± 0.04 | 0.1±0.03 | 0.54 ± 0.04 | 0.42 ± 0.03 | 0.48 ± 0.03 | |||||||||||||||||
| Cl | 0.14 ± 0.04 | 0.26 ± 0.04 | – | 0.63 ± 0.05 | 1.02 ± 0.05 | 3.76 ± 0.07 | 1.88 ± 0.04 | 0.71 ± 0.05 | 0.55 ± 0.04 | ||||||||||||||||
| F | 42.56 ± 0.54 | 42.26 ± 0.53 | 42.6 ± 0.54 | ||||||||||||||||||||||
| Mg | 1.94 ± 0.06 | 1.52 ± 0.07 | 1.77 ± 0.06 | ||||||||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monroy-Guzman, F.; De la Cruz Barba, C.d.C.; Jaime Salinas, E.; Garibay-Feblés, V.; Nava Entzana, T.N. Extraction Chromatography Materials Prepared with HDEHP on Different Inorganic Supports for the Separation of Gadolinium and Terbium. Metals 2020, 10, 1390. https://doi.org/10.3390/met10101390
Monroy-Guzman F, De la Cruz Barba CdC, Jaime Salinas E, Garibay-Feblés V, Nava Entzana TN. Extraction Chromatography Materials Prepared with HDEHP on Different Inorganic Supports for the Separation of Gadolinium and Terbium. Metals. 2020; 10(10):1390. https://doi.org/10.3390/met10101390
Chicago/Turabian StyleMonroy-Guzman, Fabiola, Celia del Carmen De la Cruz Barba, Edgar Jaime Salinas, Vicente Garibay-Feblés, and Tobias Noel Nava Entzana. 2020. "Extraction Chromatography Materials Prepared with HDEHP on Different Inorganic Supports for the Separation of Gadolinium and Terbium" Metals 10, no. 10: 1390. https://doi.org/10.3390/met10101390
APA StyleMonroy-Guzman, F., De la Cruz Barba, C. d. C., Jaime Salinas, E., Garibay-Feblés, V., & Nava Entzana, T. N. (2020). Extraction Chromatography Materials Prepared with HDEHP on Different Inorganic Supports for the Separation of Gadolinium and Terbium. Metals, 10(10), 1390. https://doi.org/10.3390/met10101390





