Novel Multicomponent B2-Ordered Aluminides: Compositional Design, Synthesis, Characterization, and Thermal Stability
Abstract
:1. Introduction
2. Compositional Design
2.1. Matching Elements Identification
2.2. Pseudo-Binary Approach for Entropy Maximization
2.3. Phase Diagrams from CALPHAD
3. Materials and Methods
4. Results and Discussion
4.1. Characterization of Multicomponent B2 Aluminides
4.1.1. Structural Characterization Using X-ray Diffraction Techniques
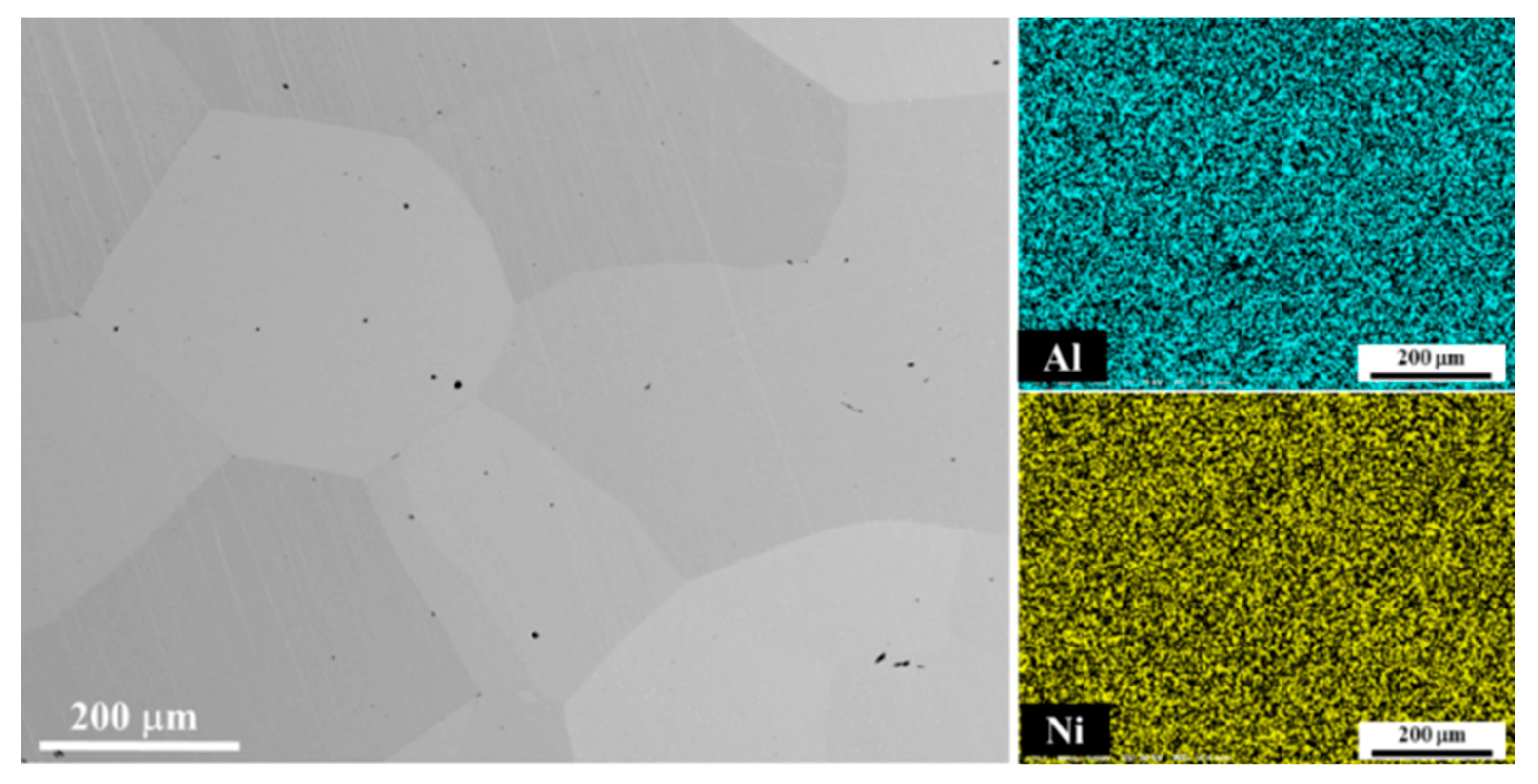
4.1.2. Microstructure and Composition Analysis
4.1.3. High Resolution STEM of Quinary and Senary B2 Aluminides
4.2. Thermal Analysis (DTA) and Heat Treatment
5. Conclusions
- Multicomponent B2-ordered aluminide compositions, namely, Al(CoNi), Al(CoFe), Al(FeNi), Al(CoFeNi), Al(CoFeMnNi), and Al(CoCuFeMnNi), were designed through a heuristic phase diagram inspection, a pseudo-binary approach, and a CALPHAD analysis.
- Multicomponent single-phase B2-ordered aluminides were synthesized successfully through arc melting followed by suction casting.
- Multiple characterization techniques were utilized to investigate the microstructure of the alloys. All the B2 aluminides were observed to be single phase, and a homogeneous distribution of elements was verified by SEM-EDS elemental maps.
- The XRD results indicate the presence of superlattice reflections, substantiating the presence of the B2 phase in all the alloys. The order parameter, L, was calculated until the ternary alloys and found to be highest for Al(CoFe) (L = 0.96) and is lowest for Al(FeNi) (L = 0.74).
- The order parameter for the alloys with a higher number of elements (quinary to senary) was not determined, as the standard database was not available. However, the ratio of the superlattice to fundamental reflections (I100/I110) was used to approximate the order parameter in higher-order systems (quaternary, quinary, and senary alloys), and the maximum order parameter in senary alloy was found to be 0.98.
- TEM results confirmed the presence of B2 ordering in the alloys. The STEM-EDS elemental maps show a slight segregation of Mn along grain boundaries. All the elements were observed to be uniformly distributed inside the grains.
- The thermal stability of multicomponent B2 aluminides was investigated at 1373 and 1073 K. The B2 phase was observed to be stable even after prolonged annealing up to 200 h.
- The phase evolution and the stability in the present B2 aluminides were in accordance with the assumptions made using the matching element method as well as CALPHAD predictions.
- The experimental results indicate that the strategies followed for composition design were very effective in designing multicomponent single-phase B2 alloys.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diao, H.Y.; Feng, R.; Dahmen, K.A.; Liaw, P.K. Fundamental deformation behavior in high-entropy alloys: An overview. Curr. Opin. Solid State Mater. Sci. 2017, 21, 252–266. [Google Scholar] [CrossRef]
- Li, Z.; Raabe, D. Strong and Ductile Non-equiatomic High-Entropy Alloys: Design, Processing, Microstructure, and Mechanical Properties. JOM 2017, 69, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Sriharitha, R.; Murty, B.S.; Kottada, R.S. Alloying, thermal stability and strengthening in spark plasma sintered AlxCoCrCuFeNi high entropy alloys. J. Alloy. Compd. 2014, 583, 419–426. [Google Scholar] [CrossRef]
- Zhao, J.H.; Ji, X.L.; Shan, Y.P.; Fu, Y.; Yao, Z. On the microstructure and erosion-corrosion resistance of AlCrFeCoNiCu high-entropy alloy via annealing treatment. Mater. Sci. Technol. U.K. 2016, 32, 1271–1275. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, J.; Li, J.S.; Kou, H.C.; Niu, S.Z.; Zhu, S.Y.; Yang, J.; Liu, W.M. Dry-sliding tribological properties of AlCoCrFeNiTi0.5 high-entropy alloy. Rare Met. 2017, 1–7. [Google Scholar] [CrossRef]
- Butler, T.M.; Weaver, M.L. Oxidation behavior of arc melted AlCoCrFeNi multi-component high-entropy alloys. J. Alloy. Compd. 2016, 674, 229–244. [Google Scholar] [CrossRef]
- Chokshi, A.H. High temperature deformation in fine grained high entropy alloys. Mater. Chem. Phys. 2017. [Google Scholar] [CrossRef]
- Yeh, J.W. Recent progress in high-entropy alloys. Ann. Chim. Sci. Des Mater. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Lin, S.-J.; Chin, T.-S.; Gan, J.-Y.; Chen, S.-K.; Shun, T.-T.; Tsau, C.-H.; Chou, S.-Y. Formation of simple crystal structures in Cu-Co-Ni-Cr-Al-Fe-Ti-V alloys with multiprincipal metallic elements. Met. Mater. Trans. A 2004, 35, 2533–2536. [Google Scholar] [CrossRef]
- Murty, B.S.; Ranganathan, S.; Yeh, J.W.; Bhattacharjee, P.P. High-Entropy Alloys; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128160671. [Google Scholar]
- Yeh, J.W.; Liaw, P.K.; Gao, M.C.; Zhang, Y. (Eds.) High-Entropy Alloys: Fundamentals and Applications; Springer International Publishing: Cham, Germany, 2016; ISBN 978-3-319-27013-5. [Google Scholar]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhý, A.; Pradeep, K.G.; Kuběnová, M.; Raabe, D.; Eggeler, G.; George, E.P. Decomposition of the single-phase high-entropy alloy CrMnFeCoNi after prolonged anneals at intermediate temperatures. Acta Mater. 2016, 112, 40–52. [Google Scholar] [CrossRef] [Green Version]
- Laplanche, G.; Berglund, S.; Reinhart, C.; Kostka, A.; Fox, F.; George, E.P. Phase stability and kinetics of σ-phase precipitation in CrMnFeCoNi high-entropy alloys. Acta Mater. 2018, 161, 338–351. [Google Scholar] [CrossRef]
- Glienke, M.; Vaidya, M.; Gururaj, K.; Daum, L.; Tas, B.; Rogal, L.; Pradeep, K.G.; Divinski, S.V.; Wilde, G. Grain boundary diffusion in CoCrFeMnNi high entropy alloy: Kinetic hints towards a phase decomposition. Acta Mater. 2020, 195, 304–316. [Google Scholar] [CrossRef]
- Li, C.; Zhao, M.; Li, J.C.; Jiang, Q. B2 structure of high-entropy alloys with addition of Al. J. Appl. Phys. 2008, 104. [Google Scholar] [CrossRef]
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-entropy alloys by mechanical alloying: A review. J. Mater. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Garlapati, M.M.; Vaidya, M.; Karati, A.; Mishra, S.; Bhattacharya, R.; Murty, B.S. Influence of Al content on thermal stability of nanocrystalline AlxCoCrFeNi high entropy alloys at low and intermediate temperatures. Adv. Powder Technol. 2020, 31, 1985–1993. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Yang, H.; Zhang, M.; Ma, S.; Qiao, J. Microstructure and wear properties of nitrided AlCoCrFeNi high-entropy alloy. Mater. Chem. Phys. 2017, 210, 233–239. [Google Scholar] [CrossRef]
- Moravcik, I.; Cizek, J.; Gavendova, P.; Sheikh, S.; Guo, S.; Dlouhy, I. Effect of heat treatment on microstructure and mechanical properties of spark plasma sintered AlCoCrFeNiTi0.5 high entropy alloy. Mater. Lett. 2016, 174, 53–56. [Google Scholar] [CrossRef]
- Nagase, T.; Takemura, M.; Matsumuro, M.; Maruyama, T. Solidification Microstructure of AlCoCrFeNi2.1 Eutectic High Entropy Alloy Ingots. Mater. Trans. 2018, 59, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Reddy, T.S.; Wani, I.S.; Bhattacharjee, T.; Reddy, S.R.; Saha, R.; Bhattacharjee, P.P. Severe plastic deformation driven nanostructure and phase evolution in a Al0.5CoCrFeMnNi dual phase high entropy alloy. Intermetallics 2017, 91, 150–157. [Google Scholar] [CrossRef]
- Tsai, C.W.; Tsai, M.H.; Yeh, J.W.; Yang, C.C. Effect of temperature on mechanical properties of Al0.5CoCrCuFeNi wrought alloy. J. Alloy. Compd. 2010, 490, 160–165. [Google Scholar] [CrossRef]
- Butler, T.M.; Alfano, J.P.; Martens, R.L.; Weaver, M.L. High-Temperature Oxidation Behavior of Al-Co-Cr-Ni-(Fe or Si) Multicomponent High-Entropy Alloys. JOM 2015, 67, 246–259. [Google Scholar] [CrossRef]
- Westbrook, J.H.; Fleischer, R.L. Basic Mechanical Properties and Lattice Defects of Intermetallic Compounds; John Wiley & Sons Ltd: New York, NY, USA, 2000; ISBN 0471611751. [Google Scholar]
- Miracle, D.B. The physical and mechanical properties of NiAl. Acta Met. Mater. 1993, 41, 649–684. [Google Scholar] [CrossRef]
- Baker, I. A review of the mechanical properties of B2 compounds. Mater. Sci. Eng. A 1995, 192–193, 1–13. [Google Scholar] [CrossRef]
- Girifalco, L.A. Vacancy concentration and diffusion in order-disorder alloys. J. Phys. Chem. Solids 1964, 24, 323–333. [Google Scholar] [CrossRef]
- Mohan Muralikrishna, G.; Vaidya, M.; Murty, B.S.; Divinski, S.V.; Wilde, G. Tracer diffusion in ordered pseudo-binary multicomponent aluminides. Scr. Mater. 2020, 178, 227–231. [Google Scholar] [CrossRef]
- Muralikrishna, G.M.; Esin, V.A.; Kulkarni, K.N.; Murty, B.S.; Wilde, G.; Divinski, S.V. Atomic transport in B2-ordered Al(Fe,Ni) alloys: Tracer-interdiffusion couple approach. Intermetallics 2020, 126. [Google Scholar] [CrossRef]
- Kim, K.B.; Warren, P.J.; Cantor, B. Formation of Metallic Glasses in Novel (Ti33Zr33Hf33)100-x-y(Ni50Cu50)xAly Alloys. Mater. Trans. 2003, 44, 411–413. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.; Chen, S.L.; Zhu, J.; Cao, W.S.; Kattner, U.R. An understanding of high entropy alloys from phase diagram calculations. Calphad Comput. Coupling Phase Diagr. 2014, 45, 1–10. [Google Scholar] [CrossRef]
- Massalski, T.B.; Okamoto, H. Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Russel Township, OH, USA, 1990. [Google Scholar]
- Alloy Phase Diagrams. ASM Handbook; ASM International: Russel Township, OH, USA, 1992; Volume 3. [Google Scholar]
- Saunders, N.; Miodownic, A.P. (Eds.) CALPHAD Calculation of Phase Diagrams: A Comprehensive Guide; Pergamon Materials Series; Pergamon: Oxford, UK, 1998; Volume 1, ISBN 0080421296. [Google Scholar]
- Mao, H.; Chen, H.C.Q. TCHEA1: A Thermodynamic Database Not Limited for “High Entropy“ Alloys. J. Phase Equilibria Diffus. 2017, 38. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Mao, H.; Chen, Q. Database development and Calphad calculations for high entropy alloys: Challenges, strategies, and tips. Mater. Chem. Phys. 2018, 210, 279–290. [Google Scholar] [CrossRef]
- Rogal, L.; Bobrowski, P.; Körmann, F.; Divinski, S.; Stein, F.; Grabowski, B. Computationally-driven engineering of sublattice ordering in a hexagonal AlHfScTiZr high entropy alloy. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, Y.; Grabowski, B.; Körmann, F. Ab initio phase stabilities and mechanical properties of multicomponent alloys: A comprehensive review for high entropy alloys and compositionally complex alloys. Mater. Charact. 2019, 147, 464–511. [Google Scholar] [CrossRef]
- Li, Z.; Körmann, F.; Grabowski, B.; Neugebauer, J.; Raabe, D. Ab initio assisted design of quinary dual-phase high-entropy alloys with transformation-induced plasticity. Acta Mater. 2017, 136, 262–270. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction; Pearson Education: London, UK, 1978; ISBN 0201610914. [Google Scholar]
- Tsai, D.S.; Chin, T.S.; Hsu, S.E.; Hung, M.P. A Simple Method for the Determination of Lattice Parameters from Powder X-ray Diffraction Data. Mater. Trans. JIM 1989, 30, 474–479. [Google Scholar] [CrossRef]
- Paul, A.; Divinski, S.V. Handbook of Solid State Diffusion: DIffusion Fundamentals and Techniques; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 449–517. ISBN 9780128042878. [Google Scholar]
- Pike, L.M.; Anderson, I.M.; Liu, C.T.; Chang, Y.A. Site occupancies, point defect concentrations, and solid solution hardening in B2 (Ni,Fe)Al. Acta Mater. 2002, 50, 3859–3879. [Google Scholar] [CrossRef]















| S. No. | Alloy | a | L | ||
|---|---|---|---|---|---|
| 1 | AlNi | 0.2870 | 0.147 | 0.176 | 0.92 |
| 2 | Al(CoFe) | 0.2880 | 0.166 | 0.180 | 0.96 |
| 3 | Al(FeNi) | 0.2890 | 0.096 | 0.176 | 0.74 |
| 4 | Al(CoNi) | 0.2871 | 0.121 | 0.181 | 0.82 |
| 5 | Al(CoFeNi) | 0.2867 | 0.145 | - | * 0.91 |
| 6 | Al(CoFeMnNi) | 0.2900 | 0.159 | - | * 0.95 |
| 7 | Al(CoCuFeMnNi) | 0.2917 | 0.169 | - | * 0.98 |
| Alloy | Al | Co | Cu | Fe | Mn | Ni |
|---|---|---|---|---|---|---|
| AlNi | 51 ± 0.2 | - | - | - | - | 49 ± 0.2 |
| Al(CoNi) | 51 ± 0.2 | 24 ± 0.1 | - | - | - | 25 ± 0.2 |
| Al(FeNi) | 52 ± 0.1 | - | - | 24 ± 0.2 | - | 24 ± 0.2 |
| Al(CoFe) | 51 ± 0.2 | 25 ± 0.4 | - | 24 ± 0.6 | - | - |
| Al(CoFeNi) | 50 ± 0.6 | 16 ± 0.3 | - | 17 ± 0.3 | - | 17 ± 0.5 |
| Al(CoFeMnNi) | 50 ± 0.3 | 12 ± 0.1 | - | 12 ± 0.2 | 12 ± 0.2 | 12 ± 0.1 |
| Al(CoCuFeMnNi) | 50 ± 0.5 | 9 ± 0.3 | 9 ± 0.4 | 11 ± 0.5 | 10 ± 0.1 | 10 ± 0.1 |
| S.No. | Alloy | Tmelting (K) | |
|---|---|---|---|
| Calculated | Measured | ||
| 1 | AlNi | 1954 | 1911 |
| 2 | Al(CoFe) | 1725 | 1781 |
| 3 | Al(FeNi) | 1763 | 1774 |
| 4 | Al(CoNi) | 1984 | - |
| 5 | Al(CoFeNi) | 1864 | 1845 |
| 6 | Al(CoFeMnNi) | 1730 | 1725 |
| 7 | Al(CoCuFeMnNi) | 1676 | 1771 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muralikrishna, G.M.; Esther, A.C.M.; Guruvidyathri, K.; Watermeyer, P.; Liebscher, C.H.; Kulkarni, K.N.; Wilde, G.; Divinski, S.V.; Murty, B.S. Novel Multicomponent B2-Ordered Aluminides: Compositional Design, Synthesis, Characterization, and Thermal Stability. Metals 2020, 10, 1411. https://doi.org/10.3390/met10111411
Muralikrishna GM, Esther ACM, Guruvidyathri K, Watermeyer P, Liebscher CH, Kulkarni KN, Wilde G, Divinski SV, Murty BS. Novel Multicomponent B2-Ordered Aluminides: Compositional Design, Synthesis, Characterization, and Thermal Stability. Metals. 2020; 10(11):1411. https://doi.org/10.3390/met10111411
Chicago/Turabian StyleMuralikrishna, G. Mohan, A. Carmel Mary Esther, K. Guruvidyathri, Philipp Watermeyer, Christian H. Liebscher, Kaustubh N. Kulkarni, Gerhard Wilde, Sergiy V. Divinski, and B. S. Murty. 2020. "Novel Multicomponent B2-Ordered Aluminides: Compositional Design, Synthesis, Characterization, and Thermal Stability" Metals 10, no. 11: 1411. https://doi.org/10.3390/met10111411
APA StyleMuralikrishna, G. M., Esther, A. C. M., Guruvidyathri, K., Watermeyer, P., Liebscher, C. H., Kulkarni, K. N., Wilde, G., Divinski, S. V., & Murty, B. S. (2020). Novel Multicomponent B2-Ordered Aluminides: Compositional Design, Synthesis, Characterization, and Thermal Stability. Metals, 10(11), 1411. https://doi.org/10.3390/met10111411








