Investigating the Aluminothermic Process for Producing Ferrotitanium Alloy from Ilmenite Concentrate
Abstract
:1. Introduction
2. Thermodynamic Calculation
- (1)
- The FeTiO3 concentrate used in the thermodynamic calculation consists of FeTiO3 only without any impurities.
- (2)
- The amount of Al required to reduce 100% of the FeTiO3 is one equivalent amount.
- (3)
- The recovery ratio of Ti and Fe in the FeTi alloy phase was 60% and 100%, respectively, and the Ti in the slag was present in the form of TiO. This assumption was only utilized for calculating the maximum temperature of the system. Herein, the recovery ratios were selected based on the previously reported results [16,24].
- (4)
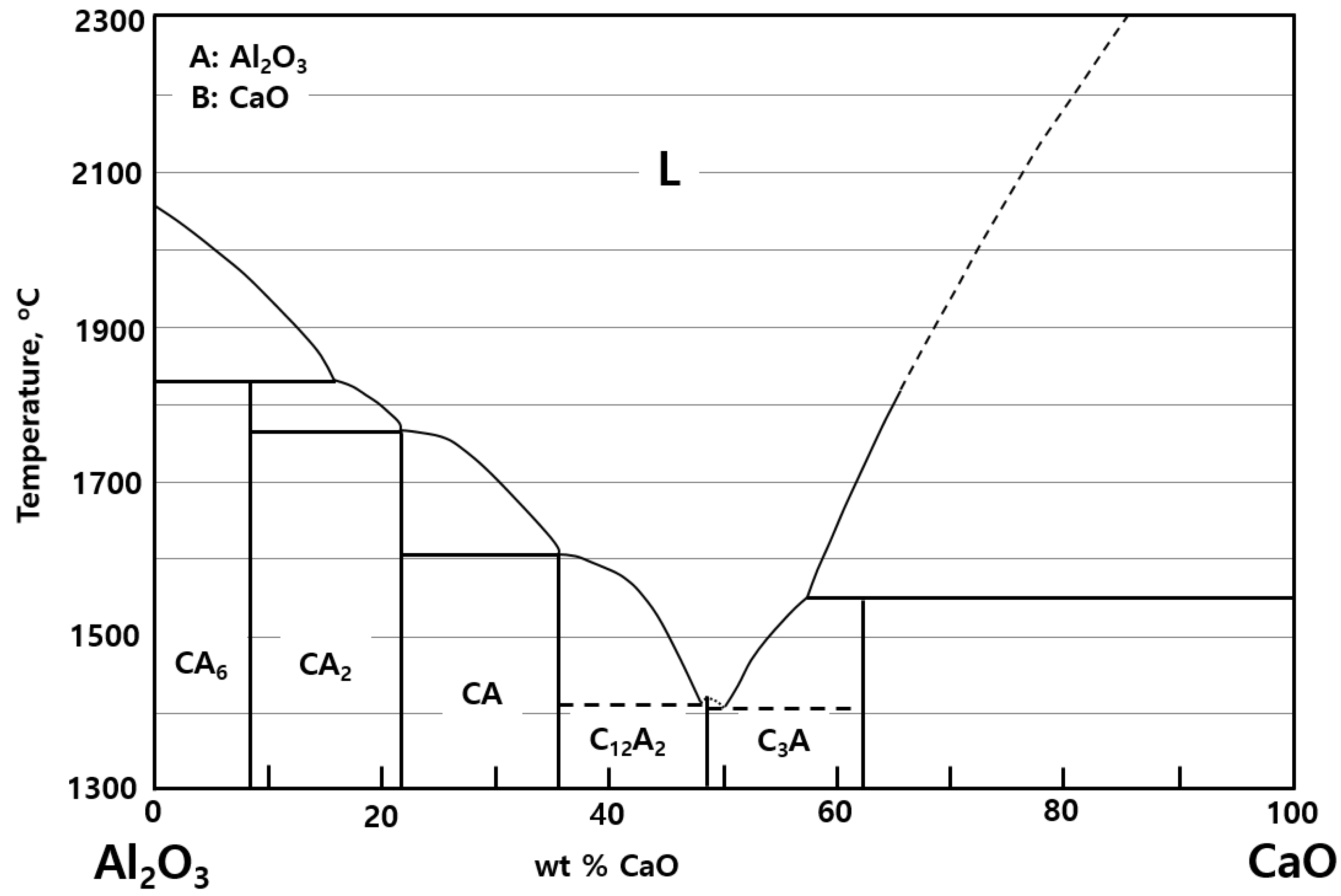
- The mixing ratio of slag-forming agent (CaO) was set to 30 wt % of CaO with a melting temperature of approximately 1730 °C or lower based on the Al2O3–CaO binary slag system. This assumption was only utilized for calculating the maximum temperature of the system.
- (5)
- The heat losses were 30% of the reaction heat in the system.
- (6)
- The activity coefficient of all the compounds considered herein was one.
3. Experimental Method
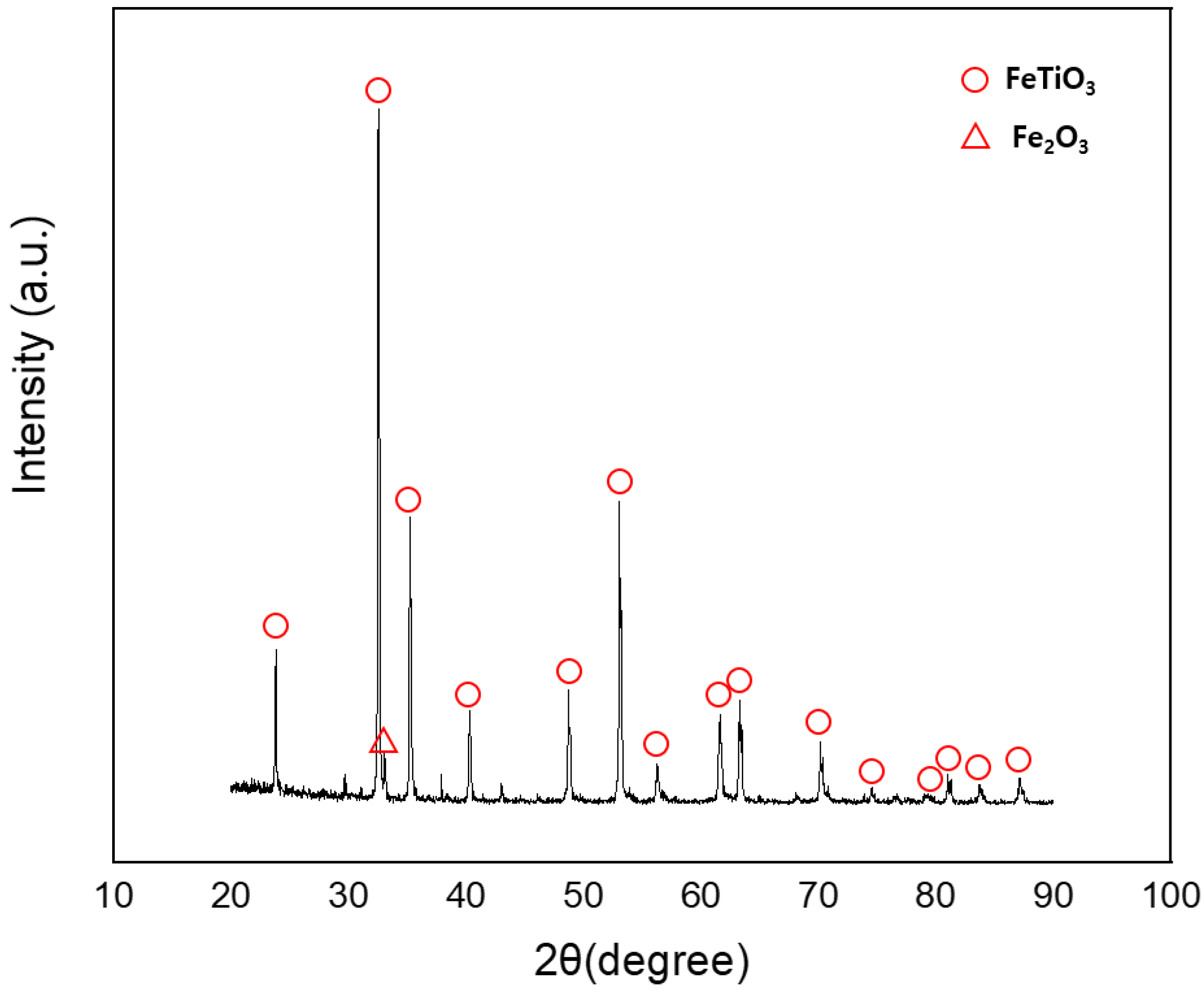
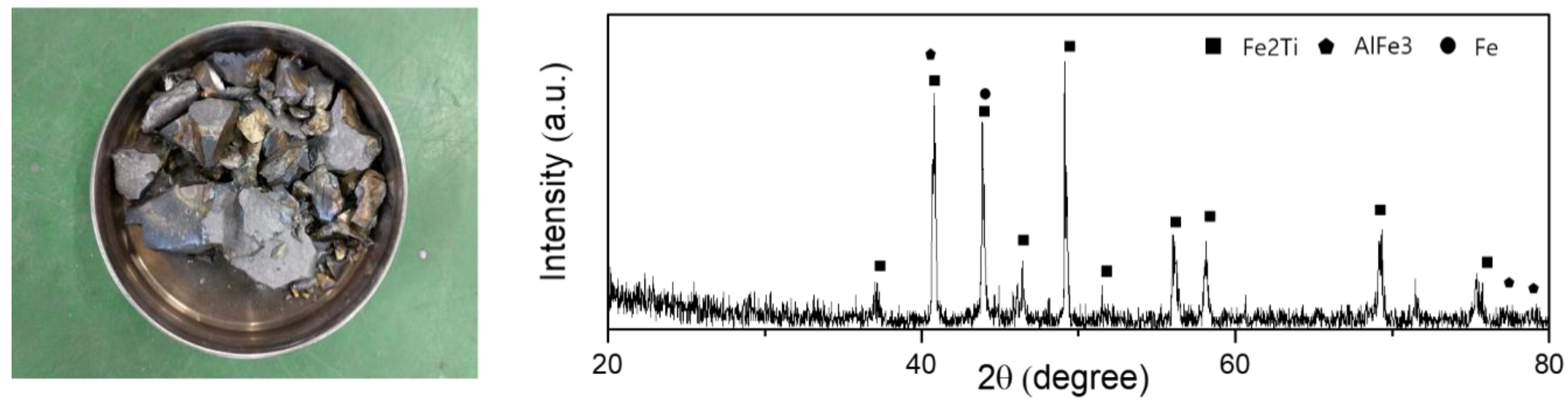
4. Results and Discussion
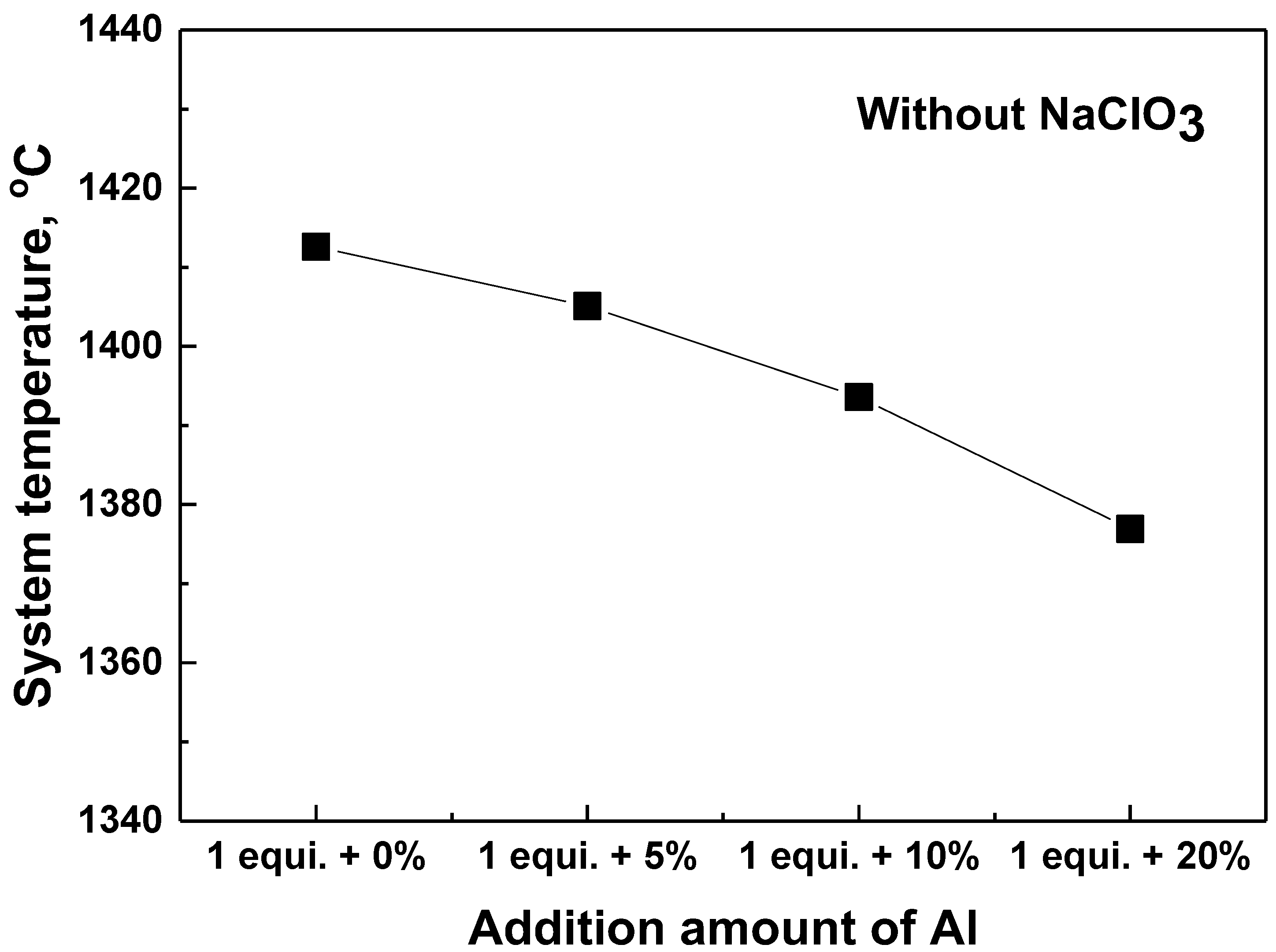
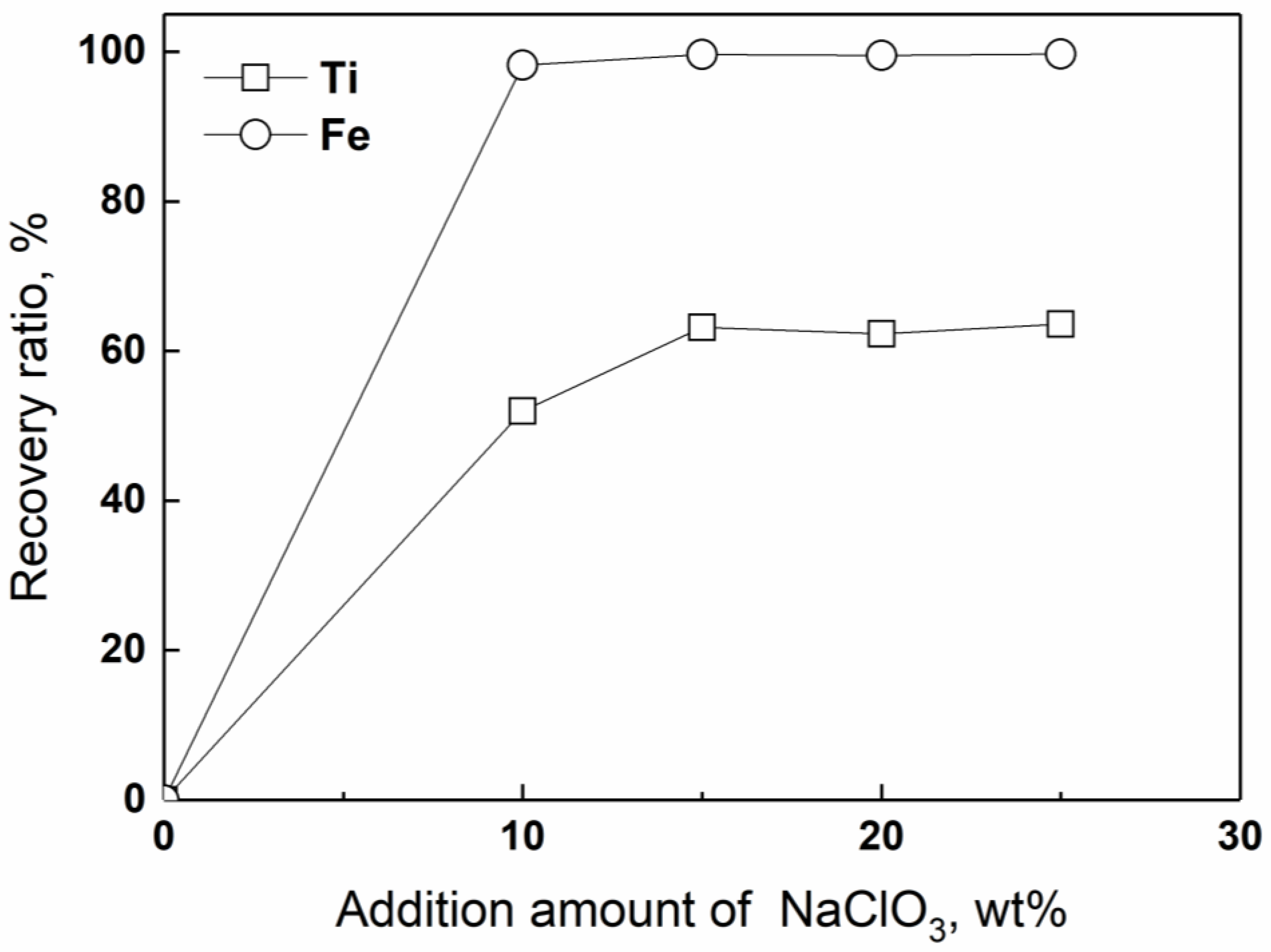
4.1. Effect of the Exothermal Agent
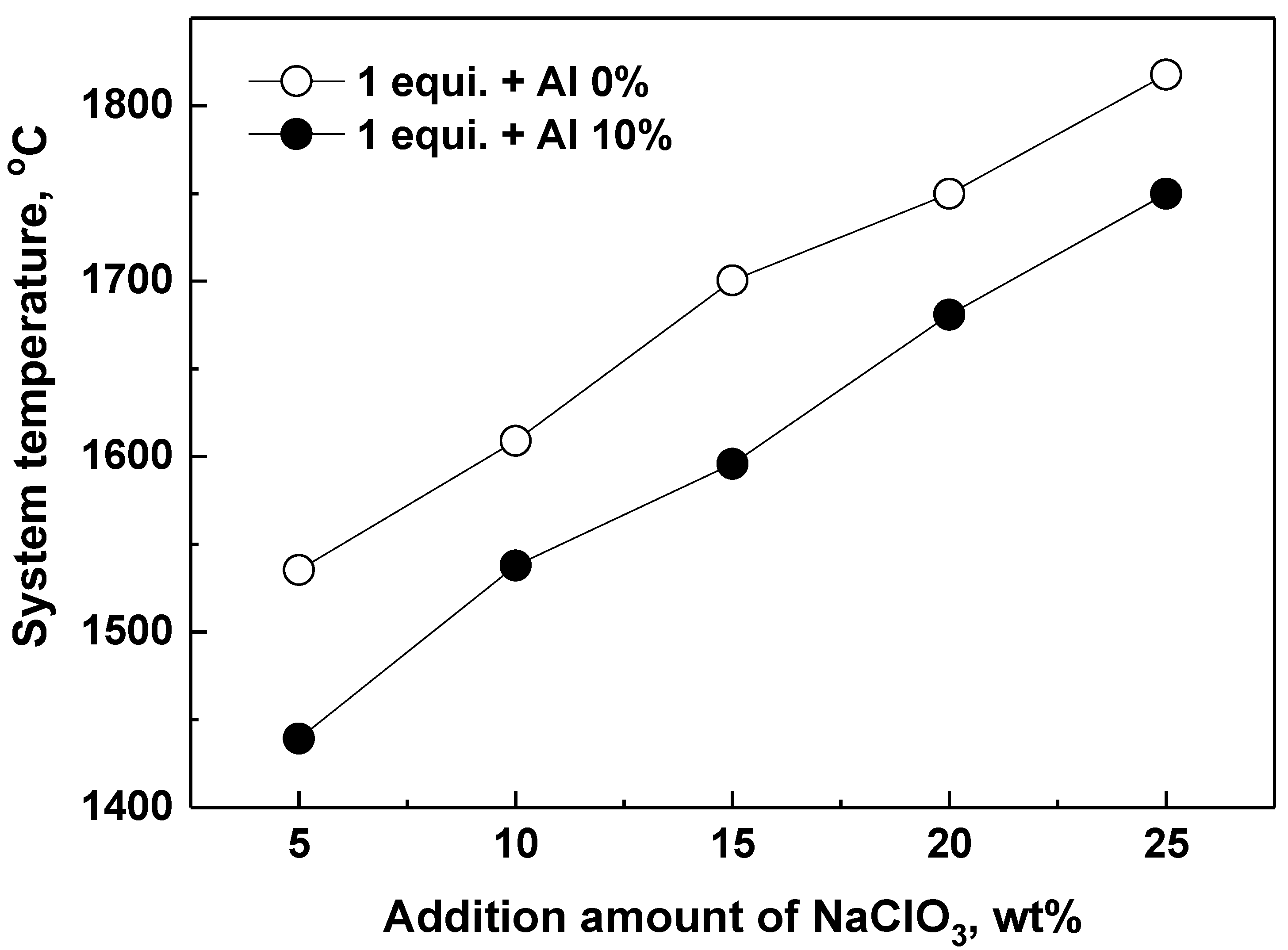
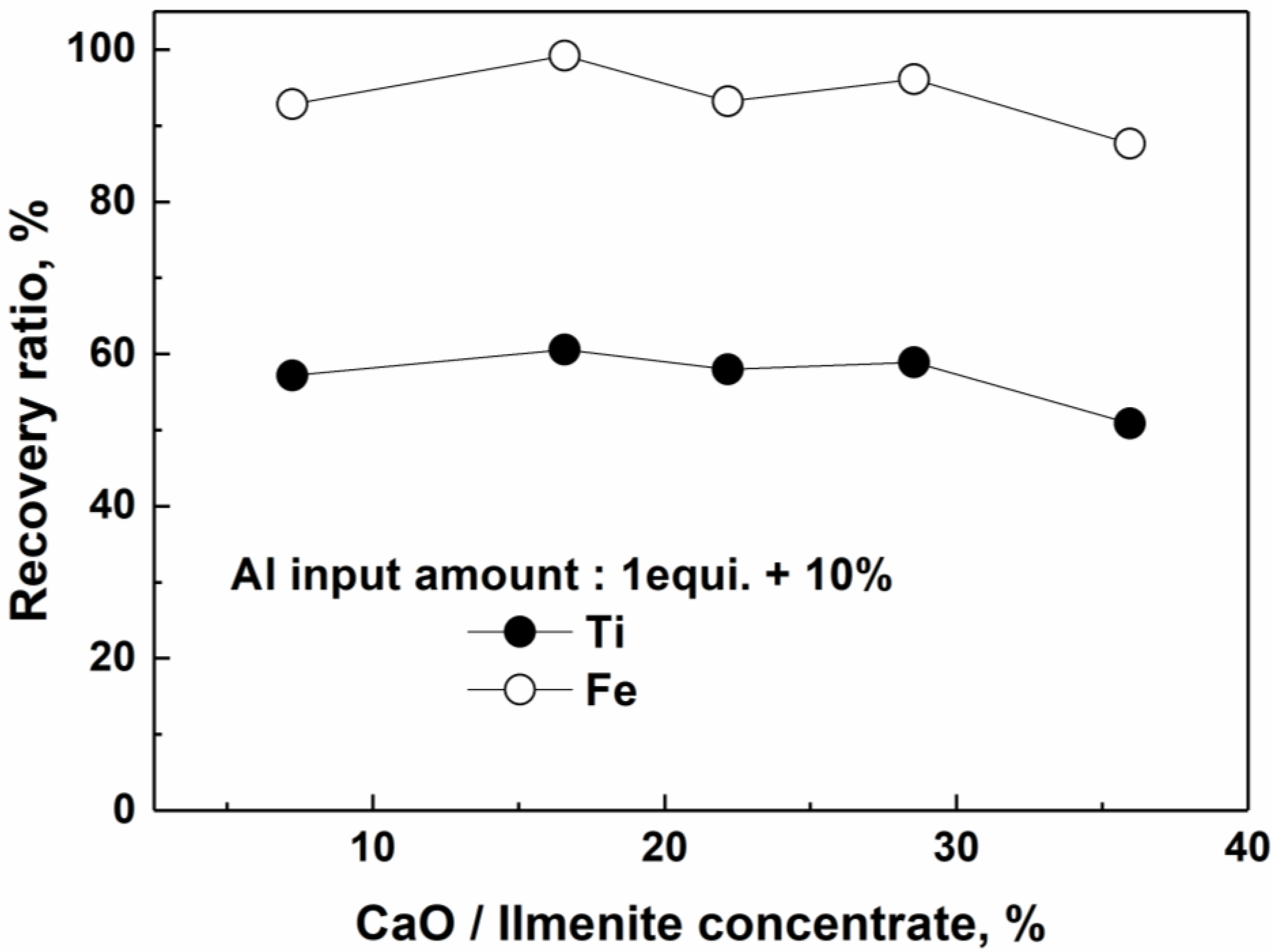
4.2. Effect of the Slag-Forming Agent
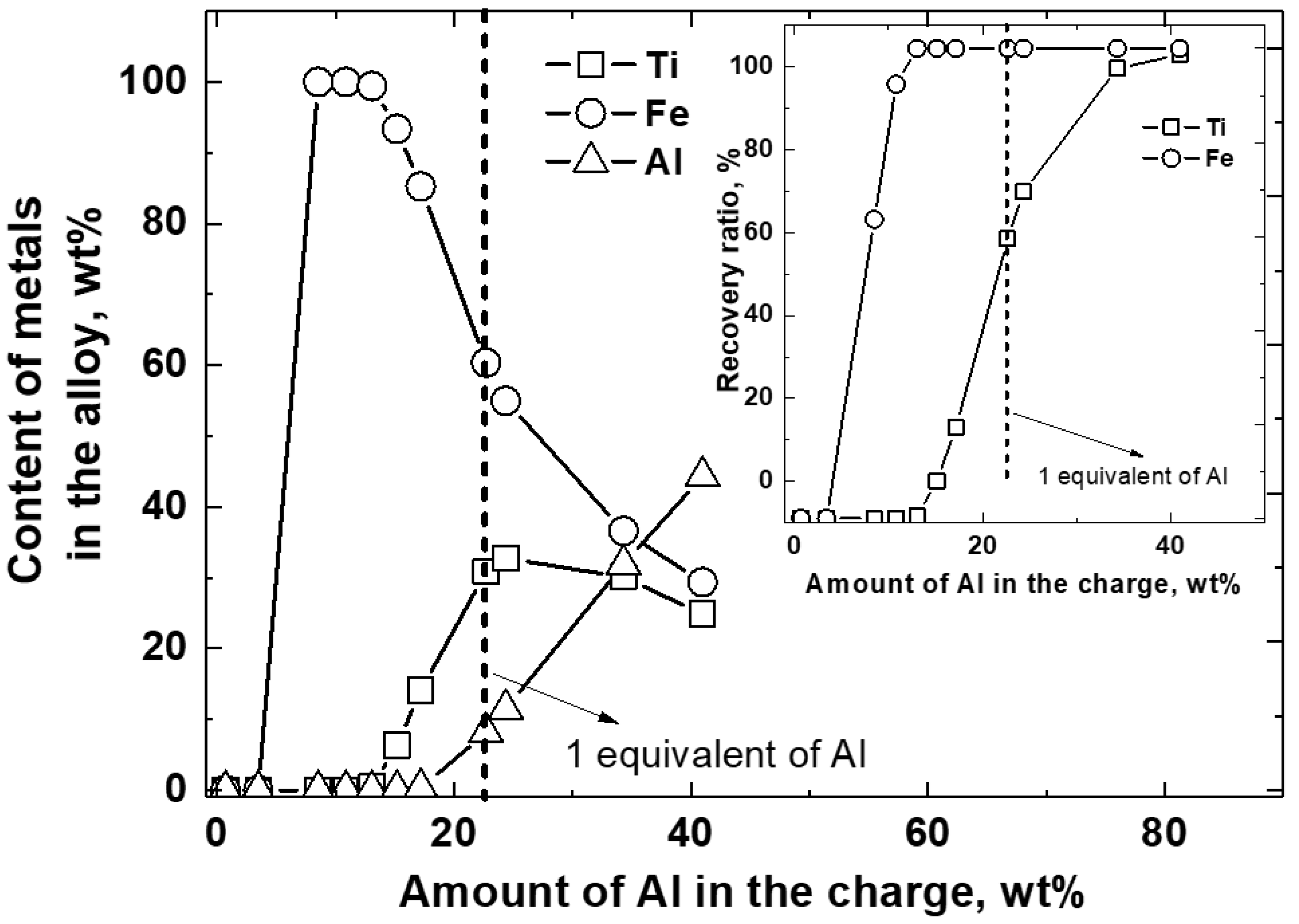
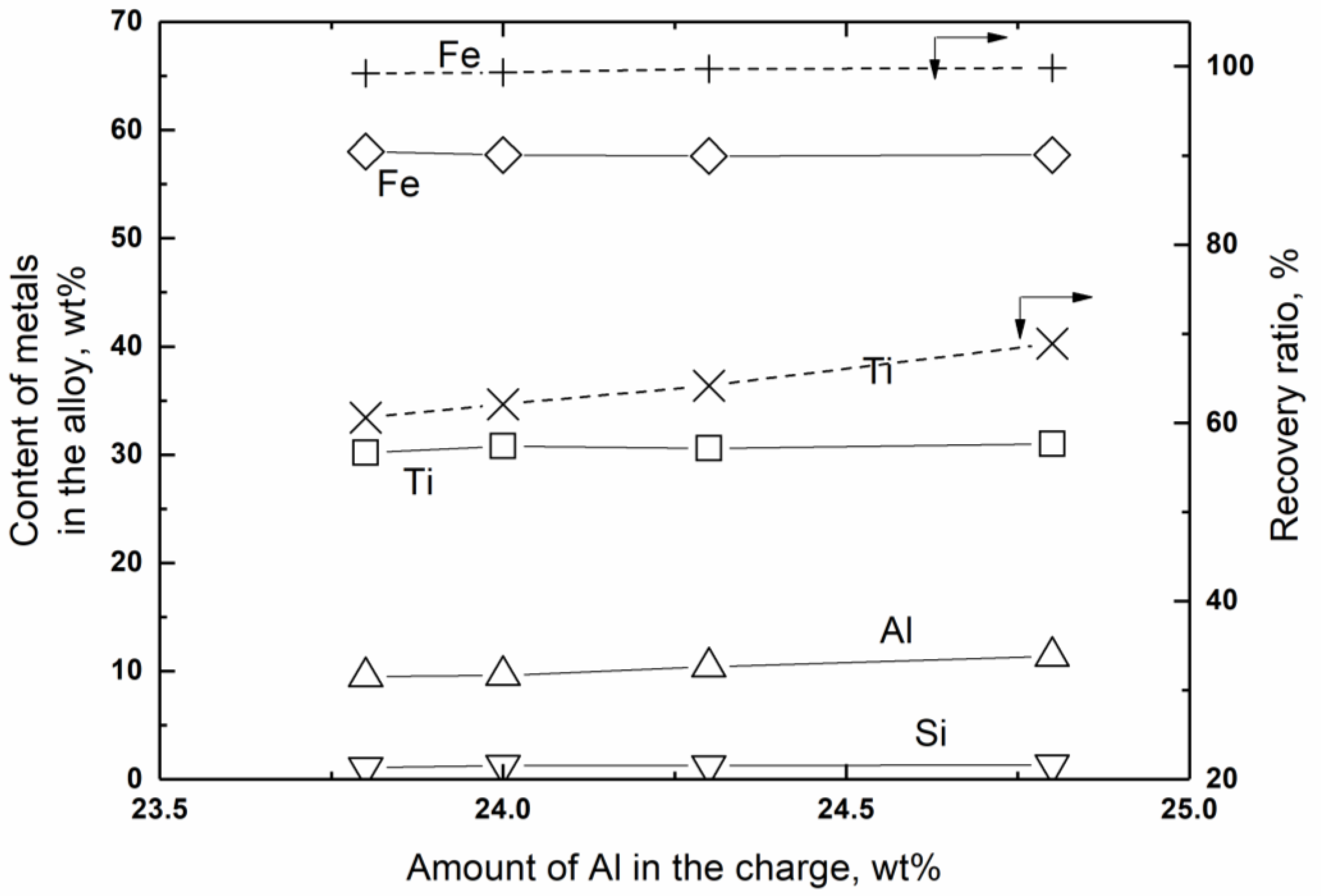
4.3. Effect of the Reducing Agent
5. Conclusions
- NaClO3: 20 wt % relative to the weight of TiO2 contained in the FeTiO3 concentrate.
- Recycled Al powder: Between 23.8 wt % (one equivalent) and 24.8 wt % (one equivalent + 6%) relative to the total charge amount.
- CaO: 16.6 wt % relative to the charge amount of the FeTiO3 concentrate.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Azizov, S.T.; Kachin, A.R.; Loryan, V.E.; Borovinskaya, I.P.; Mnatsakanyan, A.S. Aluminothermic SHS of ferrotitanium from ilmenite: Influence of Al and KClO4 content of green composition. Int. J. Self Propag. High Temp. Synth. 2014, 23, 161–164. [Google Scholar] [CrossRef]
- Chumarev, V.M.; Dubrovskii, A.Y.; Pazdnikov, I.P.; Shurygin, Y.Y.; Sel’menskikh, N.I. Technological possibilities of manufacturing high-grade ferrotitanium from crude ore. Russ. Metall. 2009, 2008, 459–463. [Google Scholar] [CrossRef]
- Zixian, G.; Gongjin, C.; He, Y.; Xiangxin, X.; Jongchol, R. Preparation of Ferrotitanium Using Ilmenite with Different Reduction Degrees. Metals 2019, 9, 662. [Google Scholar]
- Qi, C.-C.; Hua, Y.-X.; Chen, K.-H.; Jie, Y.-F.; Zhou, Z.-R.; Ru, J.-J.; Xiong, L.; Gong, K. Preparation of ferrotitanium alloy from ilmenite by electrochemical reduction in chloride molten salts. JOM 2015, 68, 668–674. [Google Scholar] [CrossRef]
- Pourabdoli, M.; Raygan, S.; Abdizadeh, H.; Hanaei, K. Production of high titania slag by Electro-Slag Crucible Melting (ESCM) process. Int. J. Miner. Process 2006, 78, 175–180. [Google Scholar] [CrossRef]
- Pourabdoli, M.; Raygan, S.; Abdizadeh, H.; Hanaei, K. A new process for the production of ferrotitanium from titania slag. Can. Metall. Q. 2007, 46, 17–23. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, T.S.; Heo, J.H.; Park, H.S.; Park, J.H. Influence of temperature on reaction mechanism of ilmenite ore smelting for titanium production. Metall. Mater. Trans. B 2019, 50, 1830–1840. [Google Scholar] [CrossRef]
- Razavi, S.S.; Soltanieh, M. The investigation of the aluminothermic reduction of dissolved ilmenite in molten cryolite. Can. Metall. Q. 2015, 54, 460–466. [Google Scholar] [CrossRef]
- Suzuki, K. An Introduction to the extraction, melting and casting technologies of titanium alloys. Met. Mater. Int. 2001, 7, 587–604. [Google Scholar] [CrossRef]
- Panigrahi, M.; Shibata, E.; Iizuka, A.; Nakamura, T. Production of Fe-Ti alloy from mixed ilmenite and titanium dioxide by direct electrochemical reduction in molten calcium chloride. Electrochim. Acta 2013, 93, 143–151. [Google Scholar] [CrossRef]
- Kang, J.; Moon, H.; Kim, M.-S.; Okabe, T.H. Production of High-grade Titanium Dioxide from Titanium Ore Using Titanium Scrap and Iron Chloride Waste. Met. Mater. Int. 2019, 25, 257–267. [Google Scholar] [CrossRef]
- Habashi, F. Handbook of Extractive Metallurgy; Wiley VCH Company: Weinheim, Germany, 1997; Volume 1, pp. 1129–1180. [Google Scholar]
- Ray, H.S.; Sridhar, R.; Abraham, K.P. Extraction of Nonferrous Metals; Affiliated East–West Press: New Delhi, India, 1996; pp. 445–484. [Google Scholar]
- Barbier, D.; Huang, M.; Bouaziz, O. A novel eutectic Fe-15 wt.% Ti alloy with an ultrafine lamellar structure for high temperature applications. Intermetallics 2013, 35, 41–44. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, H.W. Study for corrosion characteristics of ferritic stainless steel weld metal with respect to added contents of Ti and Nb. Met. Mater. Int. 2014, 20, 329–335. [Google Scholar] [CrossRef]
- Sokolov, V.M.; Babyuk, V.D.; Zhydkov, Y.A.; Skok, Y.Y. Aluminothermic studies of a liquid partial reduced ilmenite. Miner. Eng. 2008, 21, 143–149. [Google Scholar] [CrossRef]
- Yu, W.T.; Li, J.; Shi, C.-B.; Zhu, Q.-T. Effect of Titanium on the Microstructure and Mechanical Properties of High-Carbon Martensitic Stainless Steel 8Cr13MoV. Metals 2016, 6, 193. [Google Scholar] [CrossRef] [Green Version]
- Ha, M.H.; (Woltech Korea Co., Ltd., Gyeongju, Gyeongsangbukdo, Korea). Private Communication. 2019. [Google Scholar]
- Li, Z.N.; Wei, F.A.; La, P.Q.; Ma, F.L. Enhanced mechanical properties of 316L stainless steel prepared by aluminothermic reaction subjected to multiple warm rolling. Met. Mater. Int. 2018, 24, 633–643. [Google Scholar] [CrossRef]
- Piao, R.; Yang, S.; Ma, L.; Wang, T. Vacuum Electromagnetic Levitation Melting of Ti–Al Based Alloy Prepared by Aluminothermic Reduction of Acid Soluble Ti Bearing Slag. Met. Mater. Int. 2020, 26, 130–142. [Google Scholar] [CrossRef]
- Nersisyan, H.H.; Won, H.I.; Won, C.W. The laws of silicon ingot formation by combustion reaction. Met. Mater. Int. 2007, 13, 379–384. [Google Scholar] [CrossRef]
- Maeda, M.; Yahata, T.; Mitugi, K.; Ikeda, T. Aluminothermic reduction of titanium oxide. Mater. Trans. JIM 1993, 34, 599–603. [Google Scholar] [CrossRef] [Green Version]
- Misra, S.B.; Kamble, A.; Yadav, S.; Ranganathan, S. Influence of charge segregation on specific aluminium consumption in production of ferro-titanium. Can. Metall. Q. 2014, 54, 101–109. [Google Scholar] [CrossRef]
- Babyuk, V.; Friedrich, B.; Sokolov, V. Investigations of Liquid Phase Aluminothermic Reduction of Ilmenite. World Metall. 2007, 60, 255–261. [Google Scholar]
- Allibert, M.; Eisenhuttenleute, V.D. Slag Atlas, 2nd ed.; Verlag Stahleisen GmbH: Dusseldorf, Germany, 1995; p. 39. [Google Scholar]



| Gas | Chloride | Oxide | Oxide | Metal Phase |
|---|---|---|---|---|
| Al (g) | AlCl3 | Al2O3 | FeTi2O5 | Al |
| AlCl (g) | Al2Cl6 | Al2O3·TiO2 | Fe2TiO4 | Fe |
| AlCl2 (g) | AlClO | CaAl2O4 | NaFeO2 | FeTi |
| Al2O3 (g) | AlOCl | CaAl12O19 | Na8Fe2O7 | Fe2Ti |
| CaAl2Cl8 (g) | CaCl2 | Ca2Al2O5 | NaO2 | Na |
| CaCl (g) | CaOCl2 | CaFe3O5 | Na2O | Ti |
| CaCl2 (g) | FeCl2 | CaFe5O7 | Na2O2 | TiAl |
| CaO (g) | FeCl3 | CaO | Na2O·Al2O3 | TiAl3 |
| Cl (g) | FeOCl | CaO·Al2O3 | Na2O·Fe2O3 | - |
| Cl2 (g) | NaAlCl4 | CaO·2Al2O3 | Na2O·TiO2 | - |
| Fe (g) | Na2AlCl6 | CaO·6Al2O3 | Na2O·2TiO2 | - |
| FeAlCl6 (g) | Na3AlCl6 | 2CaO·Al2O3 | Na2O·3TiO2 | - |
| FeCl (g) | NaCl | 3CaO·Al2O3 | 4Na2O·5TiO2 | - |
| FeCl2 (g) | NaClO2 | 12CaO·7Al2O3 | Na2Ti6O13 | - |
| FeO (g) | NaClO3 | 4CaO·Al2O3·Fe2O3 | TiO | - |
| FeOCl (g) | NaClO4 | CaO·Fe2O3 | TiO2 | - |
| NaCl (g) | TiCl2 | 2CaO·Fe2O3 | Ti2O3 | - |
| Na2Cl2 (g) | TiClO | CaO·TiO2 | Ti3O2 | - |
| NaO (g) | - | 3CaO·2TiO2 | Ti4O7 | - |
| Na2O (g) | - | 4CaO·3TiO2 | Ti5O9 | - |
| Na2O2 (g) | - | Ca3Ti2O7 | Ti6O11 | - |
| O2 (g) | - | FeAl2O4 | Ti7O13 | - |
| Ti (g) | - | FeNaO2 | Ti8O15 | - |
| TiCl (g) | - | FeO | Ti9O17 | - |
| TiCl2 (g) | - | Fe2O3 | Ti10O19 | - |
| TiClO (g) | - | Fe3O4 | Ti20O39 | - |
| TiCl2O (g) | - | FeO·TiO2 | - | - |
| TiO (g) | - | 2FeO·TiO2 | - | - |
| TiO2 (g) | - | FeTiO3 | - | - |
| Al2O3 | CaO | MgO | MnO | Na2O | P2O5 | FeTiO3 | Fe2O3 | SiO2 |
|---|---|---|---|---|---|---|---|---|
| 0.95 | 0.21 | 2.14 | 0.56 | 0.02 | 0.04 | 87.31 | 4.49 | 1.03 |
| Sample No. | NaClO3 (g) | FeTiO3 Concentrate (g) | Recycled Al (g) | CaO (g) |
|---|---|---|---|---|
| 1 | - | 3770.5 | 1 equivalent = 1300.0 | 929.5 |
| 2 | 10 wt % relative to the TiO2 component in the charged FeTiO3 concentrate = 169.9 | 3565.8 | 1 equivalent = 1315.6 | 948.8 |
| 3 | 15 wt % relative to the TiO2 component in the charged FeTiO3 concentrate = 248.1 | 3471.5 | 1 equivalent = 1322.7 | 957.6 |
| 4 | 20 wt % relative to the TiO2 component in the charged FeTiO3 concentrate = 322.3 | 3382.1 | 1 equivalent = 1329.5 | 966.0 |
| 5 | 25 wt % relative to the TiO2 component in the charged FeTiO3 concentrate = 392.8 | 3297.2 | 1 equivalent = 1336 | 974.0 |
| Sample No. | FeTiO3 Concentrate (g) | Recycled Al (g) | CaO (g) | NaClO3 (g) |
|---|---|---|---|---|
| 1 | 3844.1 | 1 equivalent = 1511.1 | 278.4 | 366.3 |
| 2 | 3627.3 | 1 equivalent = 1425.9 | 601.1 | 345.7 |
| 3 | 3508.6 | 1 equivalent = 1379.3 | 777.8 | 334.4 |
| 4 | 3382.1 | 1 equivalent = 1329.5 | 966.0 | 322.3 |
| 5 | 3247.0 | 1 equivalent = 1276.4 | 1167.1 | 309.4 |
| Sample No. | Composition of FeTi Alloy Phase (wt %) | Charge Amount | Produced Alloy Weight | ||||
|---|---|---|---|---|---|---|---|
| Ti | Al | Fe | Si | Recycled Al | FeTiO3 Concentrate/CaO/NaClO3 | ||
| 1 | 30.2 | 9.5 | 58.0 | 1.1 | 23.8 wt % relative to total charge amount (1 equivalent + 0% = 1425.9 g) | 3627.3 g/601.1 g/345.7 g | 1917 g |
| 2 | 30.8 | 9.6 | 57.7 | 1.3 | 24.0 wt % relative to total charge amount (1 equivalent + 1.5% = 1447.3 g) | 2001 g | |
| 3 | 30.6 | 10.4 | 57.6 | 1.3 | 24.3 wt % relative to total charge amount (1 equivalent + 3% = 1468.7 g) | 2073 g | |
| 4 | 30.5 | 11.2 | 56.9 | 1.2 | 24.8 wt % relative to total charge amount (1 equivalent + 6% = 1511.5 g) | 2191 g | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-H.; Chang, H.; Ryu, T.; Nam, C.-W.; Kim, B.-S. Investigating the Aluminothermic Process for Producing Ferrotitanium Alloy from Ilmenite Concentrate. Metals 2020, 10, 1493. https://doi.org/10.3390/met10111493
Choi J-H, Chang H, Ryu T, Nam C-W, Kim B-S. Investigating the Aluminothermic Process for Producing Ferrotitanium Alloy from Ilmenite Concentrate. Metals. 2020; 10(11):1493. https://doi.org/10.3390/met10111493
Chicago/Turabian StyleChoi, Ji-Hyuk, Hankwon Chang, Taegong Ryu, Chul-Woo Nam, and Byung-Su Kim. 2020. "Investigating the Aluminothermic Process for Producing Ferrotitanium Alloy from Ilmenite Concentrate" Metals 10, no. 11: 1493. https://doi.org/10.3390/met10111493






