The Effect of Tungstate and Ethanolamines Added in Tap Water on Corrosion Inhibition of Ductile Cast Iron Pipe for Nuclear Power Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Corrosive Environment
2.2. Corrosion Tests
3. Results
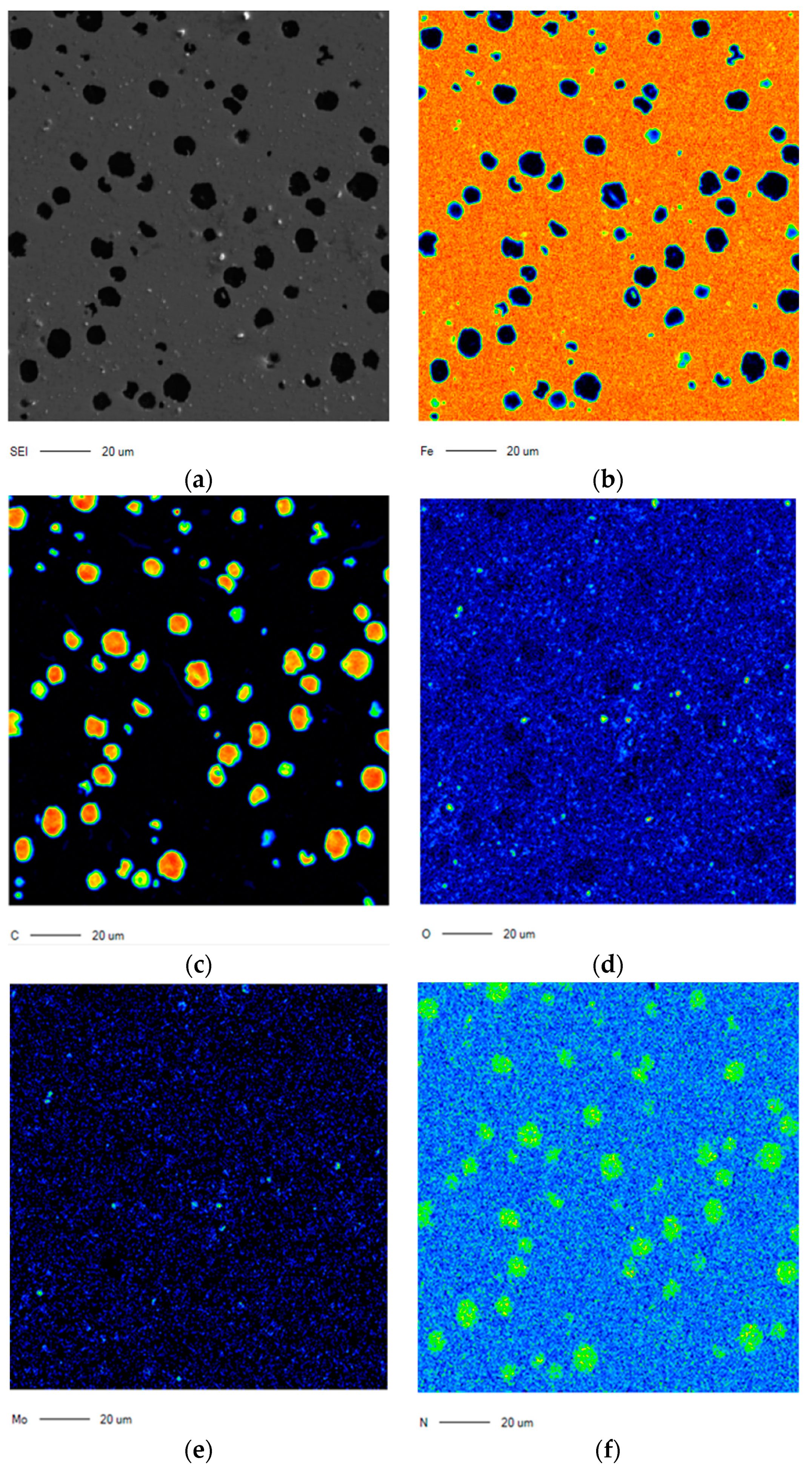
4. Discussion
5. Conclusions
- (1)
- In order to inhibit corrosion of ductile cast iron, a high concentration of tungstate is required when it is used alone, but a synergistic effect is observed when ethanolamines are co-added. Among three kinds of ethanolamines, monoethanolamine addition was very effective.
- (2)
- Immersion and electrochemical tests in tap water with 1 k ppm tungstate and 1 k ppm monoethanolamine showed that corrosion was completely suppressed. Polarization resistance (Rp) of the passive film formed in the test solution was high due to the formation of oxide film on the matrix of ductile cast iron, and the surface analysis of specimens revealed that oxygen and nitrogen induced from monoethanolamine were mainly detected at porous graphite sites. Therefore, the synergistic effect was due to that tungstate forms an oxide film that acts as a passivation layer by oxidizing the base metal surface, and at the same time, ethanolamine is adsorbed on the spheroidized graphite surface to enhance the corrosion inhibition of ductile cast iron.
Author Contributions
Funding
Conflicts of Interest
References
- McCafferty, E. Introduction to Corrosion Science; Springer: New York, NY, USA, 2010; p. 13. [Google Scholar] [CrossRef]
- Philip, A.S. Corrosion of Linings and Coatings: Cathodic and Inhibitor Protection and Corrosion Monitoring, Corrosion Engineering Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 55–57. [Google Scholar]
- Clark, B. Recommendations for an Effective Program. to Control. the Degradation of Buried and Underground Piping and Tanks (1016456, Revision 1); EPRI: Palo Alto, CA, USA, 2010. [Google Scholar]
- USNRC. Generic Aging Lessons Learned (GALL) Report, NUREG-1801, Rev. 2. In XI.M41 Buried and Underground Piping and Tanks; United States Nuclear Regulatory Commission: Rockville, ML, USA, 2010. [Google Scholar]
- USNRC. Inspection Procedure 62002, Inspection of Structures, Passive Components, and Civil. Engineering Features at Nuclear Power Plants; United States Nuclear Regulatory Commission: Rockville, ML, USA, 1996. [Google Scholar]
- KHNP. Final Safety Analysis Report for Korean Standard Nuclear Power Plants; Korea Hydro & Nuclear Power Co., Ltd.: Gyeongju-si, Korea, 1998. [Google Scholar]
- KHNP. Equipment Failure Report 06-Hanbit4-1: Hanbit #4 Firewater Supply Valve Downstream Buried Piping Leakage of Intake Area; Korea Hydro & Nuclear Power Co., Ltd.: Gyeongju-si, Korea, 2006. [Google Scholar]
- Sastri, V.S. Green Corrosion Inhibitors: Theory and Practice, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 216–222. [Google Scholar]
- Mohana, K.N.; Badiea, A.M. Effect of sodium nitrite-borax blend on the corrosion rate of low carbon steel in industrial water medium. Corros. Sci. 2008, 50, 2939–2947. [Google Scholar] [CrossRef]
- Cohen, M. Inhibition of steel Corrosion by sodium nitrite in water. J. Electrochem. Soc. 1948, 93, 26–39. [Google Scholar] [CrossRef]
- Pyke, R.; Cohen, M. Rate of Breakdown and mechanism of nitrite of steel corrosion. J. Electrochem. Soc. 1948, 93, 63–78. [Google Scholar] [CrossRef]
- Cohen, M.; Pyke, R.; Marier, P. The effect of oxygen on inhibition of corrosion by nitrite. J. Electrochem. Soc. 1949, 96, 254–261. [Google Scholar] [CrossRef]
- Matsuda, S.; Uhlig, H.H. Effect of pH, sulfates, and chlorides on behavior of sodium chromate and nitrite as passivators for steel. J. Electrochem. Soc. 1964, 111, 156–161. [Google Scholar] [CrossRef]
- Karim, S.; Mustafa, C.M.; Assaduzzaman, M.; Islam, M. Effect of nitrite ion on corrosion inhibition of mild steel in simulated cooling water. Chem. Eng. Res. Bull. 2010, 14, 87–91. [Google Scholar] [CrossRef]
- Abosrra, L.; Youseffi, M.; Ashour, A.F. Effectiveness of Calcium Nitrite in Retarding Corrosion of Steel in Concrete. Int. J. Concr. Struct. Mater. 2011, 5, 65–73. [Google Scholar] [CrossRef]
- Horng, Y.T.; Tsai, Y.L. An Investigation of Mild Steel with Nitrogen-containing Inhibitor in Hydrochloric Acid. Corros. Sci. Tech. 2003, 2, 233–237. [Google Scholar]
- Mehra, R.; Soni, A. Inhibition of corrosion of mild steel by nitrite, hydrogen phosphate and molybdate ions in aqueous solution of sodium chloride. Indian J. Eng. Mater. Sci. 2020, 9, 141–146. [Google Scholar]
- Kim, K.T.; Chang, H.Y.; Lim, B.T.; Park, H.B.; Kim, Y.S. New Mechanism on Synergistic Effect of Nitrite and Triethanolamine Addition on the Corrosion of Ductile Cast Iron. Adv. Mater. Sci. Eng. 2016, 2016, 4935602. [Google Scholar] [CrossRef]
- Okeniyi, J.O.; Popoola, A.P.I.; Loto, C.A.; Omotosho, O.A.; Okpala, S.O.; Ambrose, I.J. Effect of NaNO2 and C6H15NO3 Synergistic Admixtures on Steel-Rebar Corrosion in Concrete Immersed in Aggressive Environments. Adv. Mater. Sci. Eng. 2015, 2015, 540395. [Google Scholar] [CrossRef]
- Ridhwan, A.M.; Rahim, A.A.; Shah, A.M. Synergistic Effect of Halide Ions on the Corrosion Inhibition of Mild Steel in Hydrochloric Acid using Mangrove Tannin. Int. J. Electrochem. Sci. 2012, 7, 8091–8104. [Google Scholar]
- Vishnudevan, M. Synergistic Influence of Nitrite on Inhibition of Mild Steel Corrosion in Chloride Contaminated Alkaline Solution. Iran. J. Mater. Sci. Eng. 2012, 9, 17–27. [Google Scholar]
- Carrillo, I.; Valdez, B.; Zlatev, R.; Stoytcheva, M.; Carrillo, M.; Bäßler, R. Electrochemical Study of Oxyanions Effect on Galvanic Corrosion Inhibition. Int. J. Electrochem. Sci. 2012, 7, 8688–8701. [Google Scholar]
- Kim, K.T.; Kim, H.W.; Chang, H.Y.; Lim, B.T.; Park, H.B.; Kim, Y.S. Corrosion Inhibiting Mechanism of Nitrite Ion on the Passivation of Carbon Steel and Ductile Cast Iron for Nuclear Power Plants. Adv. Mater. Sci. Eng. 2015, 2015, 408138. [Google Scholar] [CrossRef]
- Kim, K.T.; Chang, H.Y.; Lim, B.T.; Park, H.B.; Kim, Y.S. Effect of Ethanolamines on Corrosion Inhibition of Ductile Cast Iron in Nitrite Containing Solutions. Corros. Sci. Tech. 2016, 15, 171–181. [Google Scholar] [CrossRef]
- Olsson, C.O.A.; Landolt, D. Passive films on stainless steels-chemistry structure and growth. Electrochim. Acta 2003, 48, 1093–1104. [Google Scholar] [CrossRef]
- Brookes, H.C.; Bayles, J.W.; Graham, F.J. Nucleation and growth of anodic films on stainless steel alloys. I. Influence of minor alloying elements and applied potential on passive film growth. J. Appl. Electrochem. 1990, 20, 223–230. [Google Scholar] [CrossRef]
- Ameer, M.; Fakry, A.; Heakal, F. Electrochemical behaviour of passive films on molybdenum-containing austenitic stainless steels in aqueous solutions. Electrochem. Acta. 2004, 50, 43–49. [Google Scholar] [CrossRef]
- Brooks, A.R.; Clayton, C.R.; Doss, K.; Lu, Y.C. On the Role of Cr in the Passivity of Stainless Steel. J. Electrochem. Soc. 1986, 133, 2459–2465. [Google Scholar] [CrossRef]
- Clayton, C.R.; Lu, Y.C. A bipolar model of the passivity of stainless steels-III. The mechanism of MoO42− formation and incorporation. Corros. Sci. 1989, 29, 881–898. [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, Y.S. A Study on Effects of N Addition on the Passivating Mechanism of Stainless Steels. J. Corr. Sci. Soc. Korea 1989, 18, 97–108. [Google Scholar]
- Lu, Y.C.; Clayton, C.R.; Brooks, A.R. A bipolar model of the passivity of stainless steels-II. The influence of aqueous molybdate. Corros. Sci. 1989, 29, 863–880. [Google Scholar] [CrossRef]
- Kim, K.T.; Chang, H.Y.; Lim, B.T.; Park, H.B.; Kim, Y.S. Synergistic Effect of Molybdate and Monoethanolamine on Corrosion Inhibition of Ductile Cast Iron in Tap Water. Corros. Sci. Tech. 2017, 16, 31–37. [Google Scholar] [CrossRef][Green Version]
- Bui, N.; Irhzo, A.; Dabosi, F.; Limou Zin-Marie, Y. On the Mechanism for improved passivation by additions of tungsten to austenitic stainless steels. Corrosion 1983, 39, 491–496. [Google Scholar] [CrossRef]
- Robertson, W.D. Molybdate and tungstate as corrosion inhibitors and the mechanism of inhibition. J. Electrochem. Soc. 1951, 98, 94–100. [Google Scholar] [CrossRef]
- Pryor, M.J.; Cohen, M. The inhibition of the corrosion of iron by some anodic inhibitors. J. Electrochem. Soc. 1953, 100, 203–215. [Google Scholar] [CrossRef]
- Abd El Kader, J.M.; El Warraky, A.A.; Abd El Aziz, A.M. Corrosion inhibition of mild steel by sodium tungstate in neutral solution Part 1: Behaviour in distilled water. Br. Corros. J. 1998, 33, 139–144. [Google Scholar] [CrossRef]
- Abd El Kader, J.M.; El Warraky, A.A.; Abd El Aziz, A.M. Corrosion inhibition of mild steel by sodium tungstate in neutral solution Part 2: Behaviour in NaCl and Na2SO4. Br. Corros. J. 1998, 33, 145–151. [Google Scholar] [CrossRef]
- Abd El Kader, J.M.; El Warraky, A.A.; Abd El Aziz, A.M. Corrosion inhibition of mild steel by sodium tungstate in neutral solution Part 3: Co-inhibitors and synergism. Br. Corros. J. 1998, 33, 152–157. [Google Scholar] [CrossRef]
- Celeste, R.A.; Vieira, V.A. Localized corrosion inhibition of stainless steel in pure water by oxyanions tungstate and molybdate. Electrochim. Acta 2004, 49, 2779–2785. [Google Scholar] [CrossRef]
- Qiang, S.; Xingqi, Q. Study on the complex of sodium tungstate and urotropine as inhibitors against stainless steel corrosion in the NaCl solution. Mater. Sci. Forum 2011, 689, 450–454. [Google Scholar] [CrossRef]
- Korean Agency for Technology and Standards. KS D4311, Ductile Iron Pipe; Korean Agency for Technology and Standards: Gyeonggi-do, Korea, 2010. [Google Scholar]
- ASTM. G31-2009, Standard Practice for Laboratory Immersion Corrosion Testing of Metals; ASTM International: West Conshohocken, PA, USA.
- ASTM. G5-2004, Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements; ASTM International: West Conshohocken, PA, USA.
- ASTM. G106-2004, Standard Practice for Verification of Algorithm and Equipment for Electrochemical Impedance Measurements; ASTM International: West Conshohocken, PA, USA.
- Macdonald, D.D. The Point Defect Model for the Passive State. Int. J. Electrochem. Sci. 1992, 139, 3434–3449. [Google Scholar] [CrossRef]
- Bastidas, D.M. Interpretation of Impedance Data for Porous Electrodes and Diffusion Processes. Corrosion 2007, 63, 515–521. [Google Scholar] [CrossRef]
- Ganash, A.A. Comparative Evaluation of Anticorrosive Properties of Mahaleb Seed Extract on Carbon Steel in Two Acidic Solutions. Materials 2019, 12, 3013. [Google Scholar] [CrossRef] [PubMed]
- Sherif, E.M.; Almajid, A.A.; Bairamov, A.K.; Al-zahrani, E. Corrosion of Monel-400 in Aerated Stagnant Arabian Gulf Seawater after Different Exposure Intervals. Int. J. Electrochem. Sci. 2011, 6, 5430–5444. [Google Scholar]
- Fajardo, S.; Llorente, I.; Jiménez, J.A.; Bastidas, J.M.; Bastidas, D.M. Effect of Mn additions on the corrosion behaviour of TWIP Fe-Mn-Al-Si austenitic steel in chloride solution. Corros. Sci. 2019, 154, 246–253. [Google Scholar] [CrossRef]
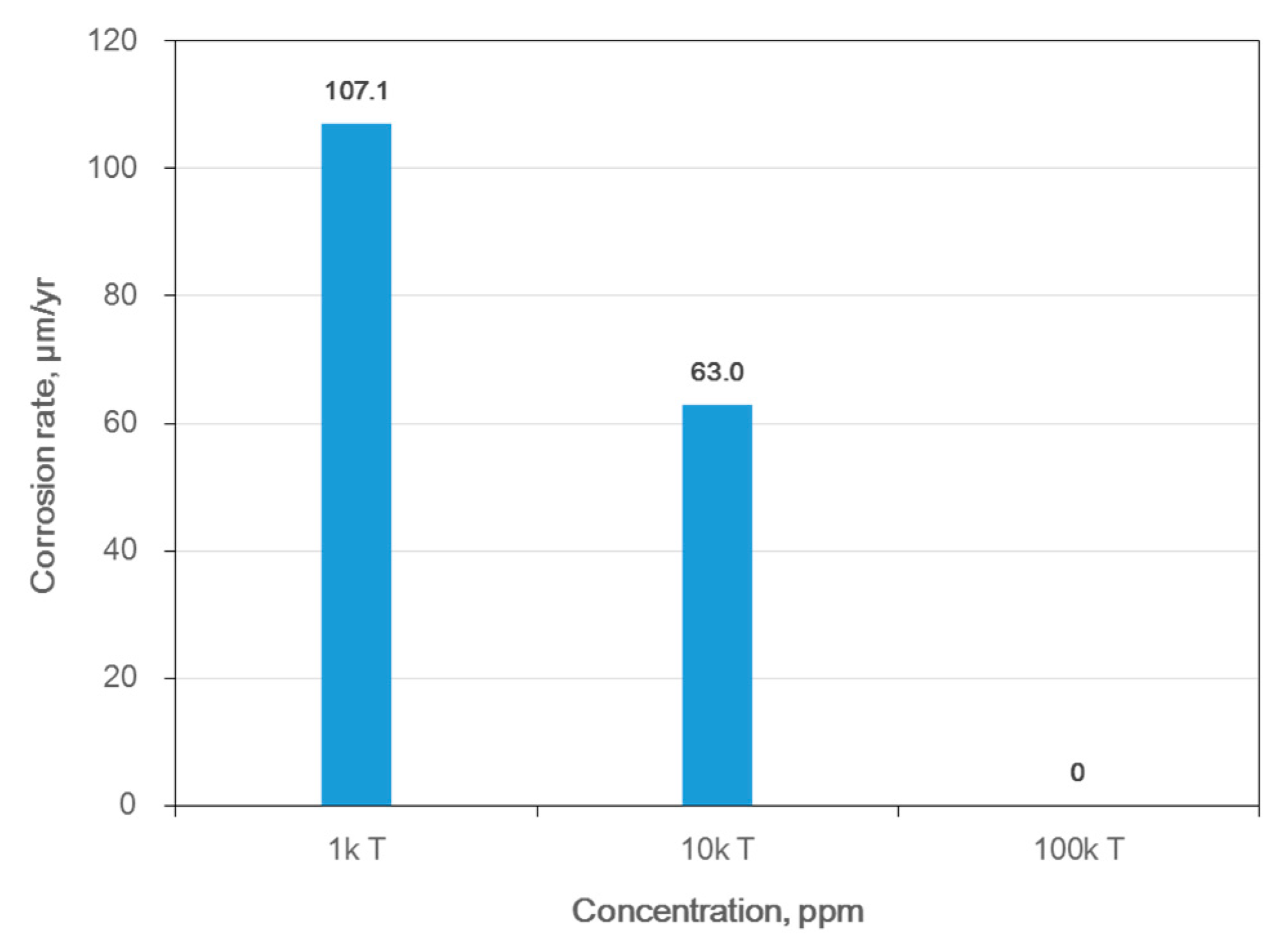
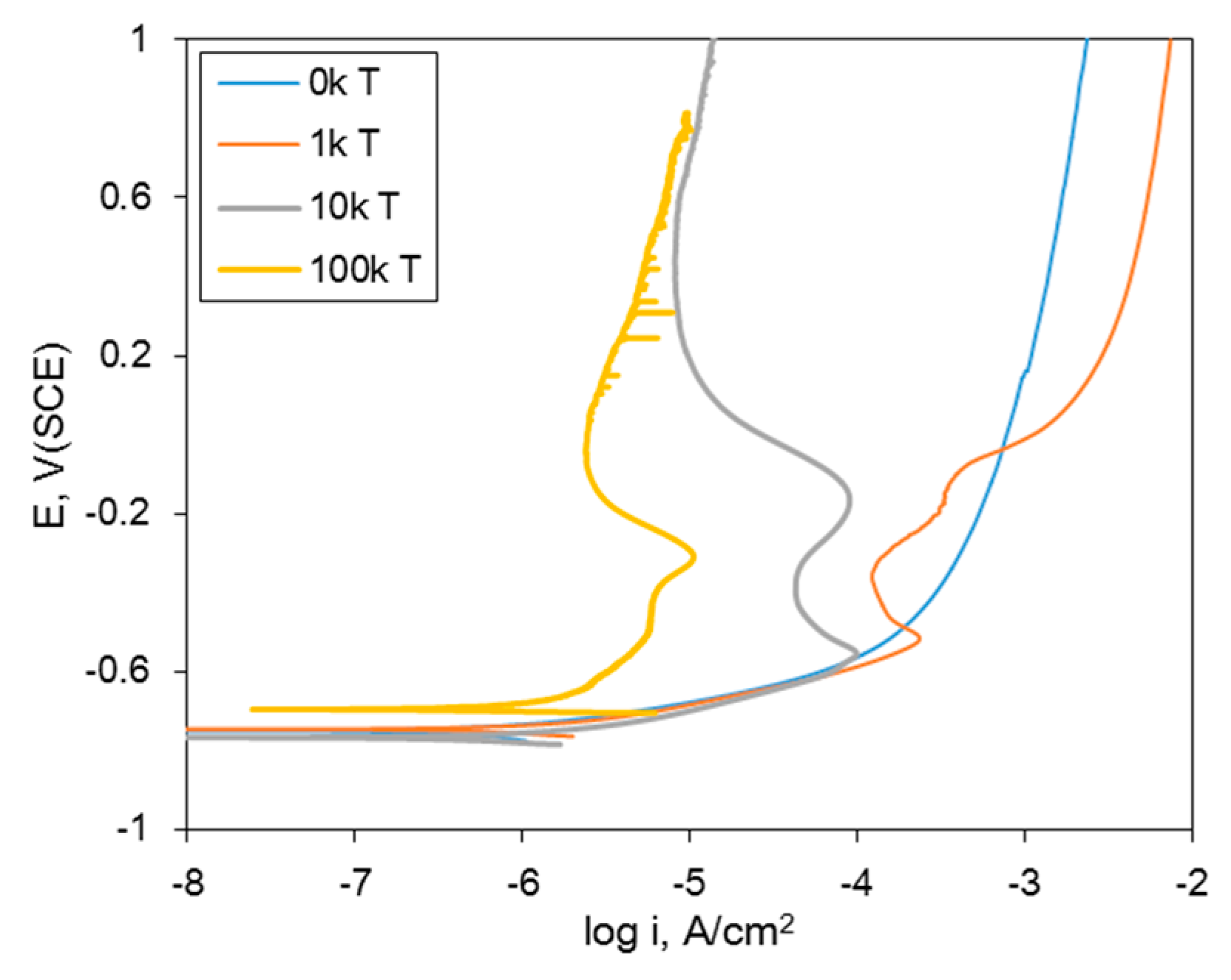
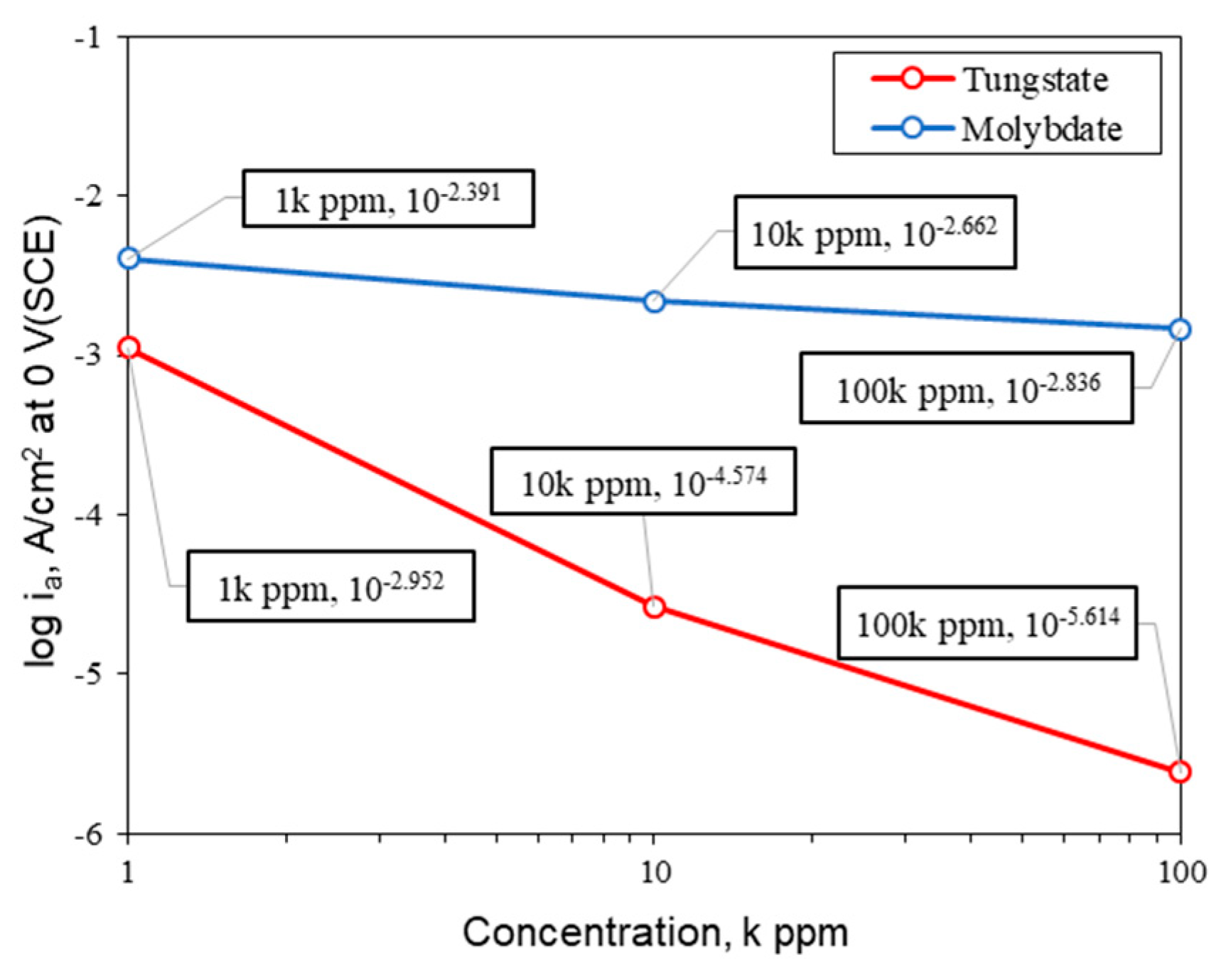



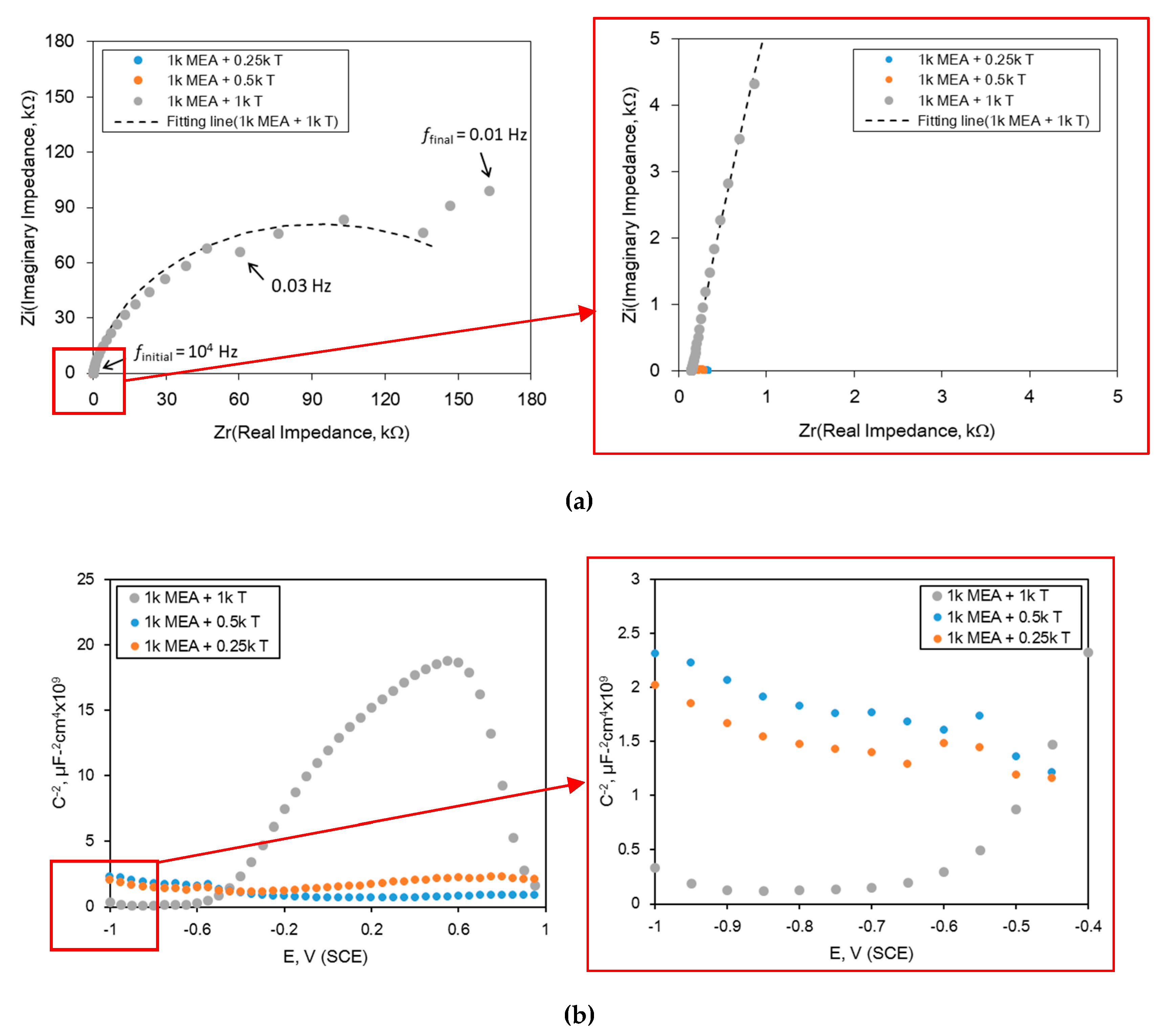




| Material | Elemental Composition, wt% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | Mn | P | S | Si | Cu | Mo | Ni | V | Fe | |
| Ductile Cast Iron (DCI) | 4.008 | 0.173 | 0.022 | 0.026 | 1.528 | 0.023 | 0.028 | 0.059 | 0.016 | balance |
| Tap Water | Quality Analysis | |||
|---|---|---|---|---|
| Residual Chlorine | Turbidity | pH | Bacteria | |
| Average | 0.25 ppm | 0.09 NTU | 7.1 | 0 CFU/mL |
| Corrosion Factors | 1 k TEA + 1 k T | 1 k DEA + 1 k T | 1 k MEA + 1 k T |
|---|---|---|---|
| Ecorr, V(SCE) | −0.588 | −0.671 | −0.409 |
| Etr, V(SCE) | 0.439 | 0.766 | 0.779 |
| ipassive at 0 V(SCE), A/cm2 | 9.73 × 10−6 | 4.11 × 10−6 | 2.70 × 10−6 |
| RS, Ω cm2 | Rfilm, Ω cm2 | Yfilm, S cm−2 snfilm | nfilm | Rct, Ω cm2 | Ydl, S cm−2 sndl | ndl | |
|---|---|---|---|---|---|---|---|
| 1 k MEA + 0.25 k T | 275.6 | 32.81 | 91.08 × 10−6 | 0.397 | 29.04 | 611.4 × 10−6 | 0.715 |
| 1 k MEA + 0.5 k T | 202.7 | 63.31 | 10.03 × 10−3 | 0.271 | 51.75 | 153.7 × 10−6 | 0.871 |
| 1 k MEA + 1 k T | 142.7 | 185.8 × 103 | 34.80 × 10−6 | 0.914 | 9.065 | 69.90 × 10−6 | 0.936 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, B.; Kim, K.; Chang, H.; Park, H.; Kim, Y. The Effect of Tungstate and Ethanolamines Added in Tap Water on Corrosion Inhibition of Ductile Cast Iron Pipe for Nuclear Power Plants. Metals 2020, 10, 1597. https://doi.org/10.3390/met10121597
Lim B, Kim K, Chang H, Park H, Kim Y. The Effect of Tungstate and Ethanolamines Added in Tap Water on Corrosion Inhibition of Ductile Cast Iron Pipe for Nuclear Power Plants. Metals. 2020; 10(12):1597. https://doi.org/10.3390/met10121597
Chicago/Turabian StyleLim, Butaek, Kitae Kim, Hyunyoung Chang, Heungbae Park, and Youngsik Kim. 2020. "The Effect of Tungstate and Ethanolamines Added in Tap Water on Corrosion Inhibition of Ductile Cast Iron Pipe for Nuclear Power Plants" Metals 10, no. 12: 1597. https://doi.org/10.3390/met10121597
APA StyleLim, B., Kim, K., Chang, H., Park, H., & Kim, Y. (2020). The Effect of Tungstate and Ethanolamines Added in Tap Water on Corrosion Inhibition of Ductile Cast Iron Pipe for Nuclear Power Plants. Metals, 10(12), 1597. https://doi.org/10.3390/met10121597






