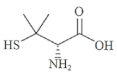
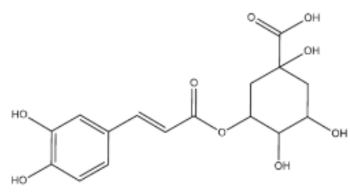
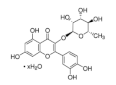
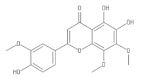
Abstract
Molecular dynamics (MD) simulation is a powerful tool to study the molecular level working mechanism of corrosion inhibitors in mitigating corrosion. In the past decades, MD simulation has emerged as an instrument to investigate the interactions at the interface between the inhibitor molecule and the metal surface. Combined with experimental measurement, theoretical examination from MD simulation delivers useful information on the adsorption ability and orientation of the molecule on the surface. It relates the microscopic characteristics to the macroscopic properties which enables researchers to develop high performance inhibitors. Although there has been vast growth in the number of studies that use molecular dynamic evaluation, there is still lack of comprehensive review specifically for corrosion inhibition of organic inhibitors on ferrous metal in acidic solution. Much uncertainty still exists on the approaches and steps in performing MD simulation for corrosion system. This paper reviews the basic principle of MD simulation along with methods, selection of parameters, expected result such as adsorption energy, binding energy and inhibitor orientation, and recent publications in corrosion inhibition studies.
1. Introduction
The use of computational method for studies of metal corrosion inhibition has progressively increased due to time, cost and environmental considerations as well as the inadequacy of conventional methods to provide notable insight on metal surface-inhibitor interaction. The performance of corrosion inhibitors in inhibiting metallic corrosion are conventionally evaluated using experimental procedures such as weight loss method, electrochemical impedance spectroscopy, potentiodynamic polarization and cyclic voltammetry. These methods are sufficient to obtain physical and electrochemical properties of the corrosion inhibitor, yet require the usage of chemicals and are time consuming. Computational studies of metal corrosion inhibition using molecular dynamic (MD) simulation provides significant insight into the mechanism and interaction between a metal surface and inhibitor molecules with minimum cost. While MD was first introduced in 1956 by Berni Alder, the use of MD simulation for metal corrosion inhibition application was never until reported 17 years ago in 2003 by Bartley et al., who studied copper corrosion inhibition using alkyl ester compounds [1,2,3]. Since then, the use of MD for simulating metal surface-inhibitor interaction had gained the attention of scientists and researchers all over the globe and had progressed vastly in the field of corrosion science.
Ferrous metals are iron-based metals such as mild steel, stainless steel and cast iron that account for 80% of all metallic materials [4]. These metals are commonly used for transportation, construction and manufacturing industries, owing to their high durability, great tensile strength and relatively low cost. The major drawback of ferrous metals especially low carbon steel is its low corrosion resistance [5,6]. The corrosion and oxidation resistance of steel can be improved by adding alloy elements such as nickel and chromium, yielding high alloy steel such as duplex stainless steel [7]. However, the price of stainless steel is almost four times higher (2314 USD/tonne) compared to mild steel (614 USD/tonne) as of April 2020 [8]. For most cases with economical concern, using lower-cost material with proper corrosion protection is preferable.
Corrosion inhibitors (CIs) have been used extensively to prevent corrosion of ferrous metals [9,10,11,12,13]. Inorganic CIs underlying mechanism are electron sharing and/or film-forming action. However, toxic inorganic CIs such as chromates and nitrites are banned due to health and environmental concern. Hence, the scientific community is devoting major efforts to develop organic CIs that are environmentally benign. The inhibition mechanism of organic CIs can be generally explained by the adsorption of the inhibitor compounds on the metal surface by displacing water molecules. This occurs physically and/or chemically, depending on the nature, chemical structure and electron distribution of the inhibitor molecules. Typical organic CIs contain heteroatoms such as nitrogen, oxygen, sulphur and phosphorus with lone pairs of electrons which act as the active sites of adsorption. The adsorbed inhibitors form a thin layer of molecules which shields the active sites of corrosion reaction [14]. The key to further understand the mechanism of the inhibition lies in knowing the atomic and molecular level information on the metal inhibitor interface. MD simulation is capable of identifying this information that can help us understand the detailed mechanism of the inhibition. Other than the corrosion field, MD simulation also offers numerous advantages in various sectors such as renewable energy generation, food technology, medicine, and pharmaceutical [15,16,17,18]. A broad list of reviews of MD simulation application for corrosion sector as well as other sectors had been published in the literature [14,19,20,21,22]. In this context, numerous works have shown that MD simulation is a promising method to obtain the molecular level information of a system. Thus, in this paper, the basic principle of MD simulation along with the methods, parameters and applications in corrosion inhibition are reviewed. This review also focuses on the selection of parameters such as the force field, time step and ensemble in performing an MD simulation for corrosion studies, which to the best of our knowledge, has not been discussed in any review article found in the literature. The scope of this review is limited to recent studies of ferrous metals corrosion inhibition using CIs in acidic solution.
2. Corrosion of Ferrous Metals in Acidic Solution
2.1. Corrosion Mechanism
Corrosion is the deterioration of a metal as a result of interactions or chemical reaction with the surrounding environment. It is an electrochemical reaction which occurs due to anodic and cathodic reaction. In the case of ferrous metal corrosion, the anodic reaction is the oxidation of iron that can be expressed as follows:
The generated electrons are consumed in cathodic reactions, which in an acidic environment is predominantly hydrogen evolution as shown in Equation (2) [23]. In an aerated condition where dissolved oxygen is present in the solution, a reaction in Equation (3) is also possible.
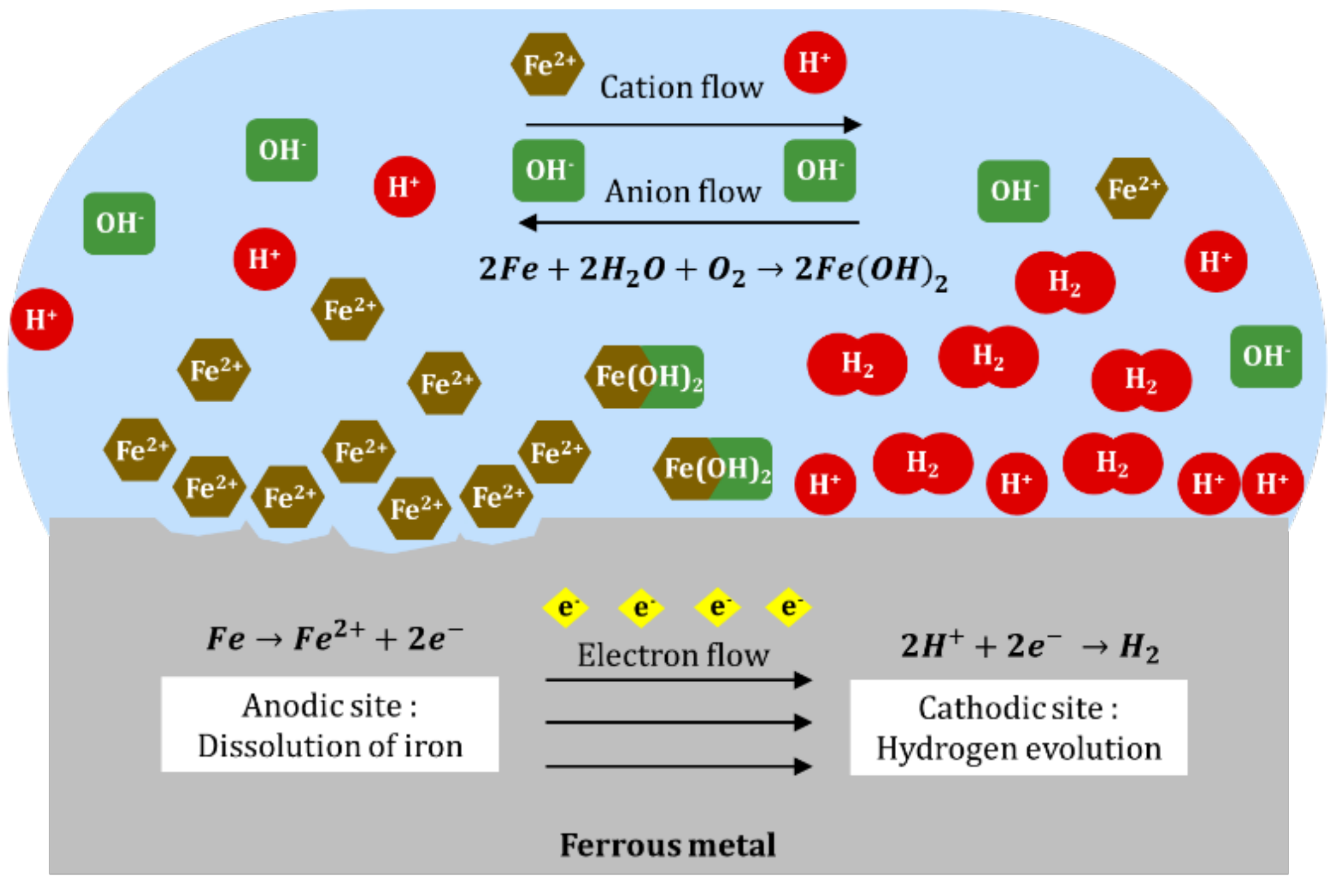
Along with anodic and cathodic reactions, electron flows through the metal from anodic site to cathodic site and an ionic flows through the solution in which cations (positively charged ions) move from anode to cathode, and vice versa [24]. As the cations and anions interact between the anode and cathode, a precipitate of insoluble ferrous hydroxide is likely to form (Equation (4)). In excess oxygen, ferrous hydroxide is rapidly oxidized to ferric hydroxide (Equation (5)). The rust that is usually found on the surface of metal is the dehydrated form of ferric hydroxide, which is ferric hydroxide (Equation (6)). Figure 1 illustrates the processes of a ferrous metal corrosion system in acidic solution:
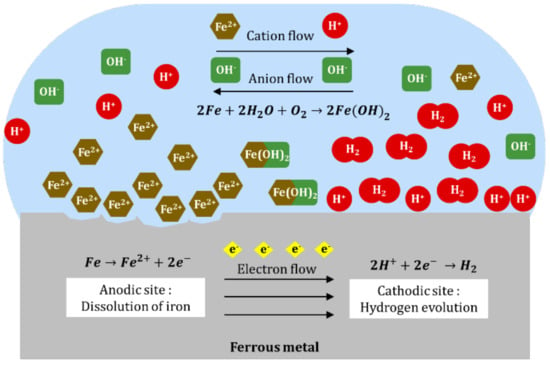
Figure 1.
Electrochemical process of ferrous metal corrosion in acidic solution.
2.2. Corrosion Inhibition of Ferrous Metal
A corrosion inhibitor (CI) is a substance that can reduce the corrosion rate of metal effectively. A wide range of CIs that include inorganic and organic (natural and synthetic) CIs has been discovered in the past decade [25]. Inorganic inhibitor compound is typically a compound that lacks carbon-hydrogen bonds. Zinc nitrate and sodium molybdate are examples of efficient inorganic inhibitors for steel and nickel respectively [26,27]. The understood mechanism of inorganic CIs is due to electron sharing and film-forming action [26,27]. On the other hand, the inhibition capability of organic CIs is believed to be due to the presence of heteroatoms such as oxygen, nitrogen, sulfur and phosphorus that exist as polar functional groups (hydroxyl, amine, methoxy, and carboxyl groups) in the compounds [14,25]. Generally, organic CIs inhibit corrosion by adsorbing physically and/or chemically on the interface of metal, forming several monolayers that block ions from reacting with the metal. The protective layer retards anodic, cathodic, or both reactions depending on the type of CI used. Nowadays, organic CI is preferred over inorganic CI due to sustainability, environmental, health and economical concerns [25].
In the perspective of ferrous metal corrosion inhibition in acidic solution, the inhibition starts as the heteroatoms in the structure of organic CI get protonated and adsorbed on the negatively charged iron surface due to electrostatic interaction [28]. This is known as physical adsorption or physisorption. At this stage, the inhibitor ions are not in direct contact with the iron atoms, but rather “bridged” by a layer of water molecules or negative ions from acid such as chloride from hydrochloric acid [29,30]. This kind of adsorption is relatively weak, reversible, nearly temperature-independent and has low adsorption energy (typically 20 kJ/mol) [31]. During the later stages of interaction, the neutral form of CI molecules begins to move towards the iron surface and start to share their free electron pairs (p electrons) from the heteroatoms and π electrons from the double and triple bonds, respectively, with the vacant d-orbital of the iron atoms [29]. This chemical interaction forms a strong chemical bond which is recognized as chemical adsorption or chemisorption. It is characterized as a slow, irreversible, temperature-dependent and has high adsorption energy (equal or more than 40 kJ/mol).
The adsorption strength is significantly affected by a plethora of variables related to the inhibitor such as the molecular weight, electron density, asymmetry, polarity, hydrophobicity and solubility. As the performance of a CI is significantly affected by the adsorption strength, it is crucial to take all parameters into consideration. Conventional experimental method alone is not capable of considering molecular level parameters into account. Hence, the use of a computational tool such as MD along with experimental approaches such as gravimetric analysis, electrochemical impedance spectroscopy, potentiodynamic polarization and cyclic voltammetry has therefore been of considerable benefit.
3. Molecular Dynamics Simulation of Ferrous Metal Corrosion Inhibition
3.1. Basics of Molecular Dynamics Simulation
Molecular dynamics (MD) simulation is a method used to compute the trajectory or transport properties of a macromolecular structure. In simpler words, it determines how atoms or molecules move within a specified time frame by conducting calculations computationally. The goal of MD simulation may vary depending on one’s interest, but typically it is conducted to obtain the equilibrium properties of the system [14]. In the context of a corrosion system, for instance, an inhibitor molecule surrounded with water molecules that mimic a corrosive solution positioned next to the iron atoms that represent the metal surface, the MD simulation is intended to reach the most stable state (lowest energy at equilibrium) of the inhibitor molecule. Based on the result, one can determine whether the inhibitor molecule prefer to be adsorbed or desorbed from the metal surface. Aside from that, the strength of the adsorption can also be obtained in terms of energy which can be used to screen inhibitor molecules before conducting a real experiment.
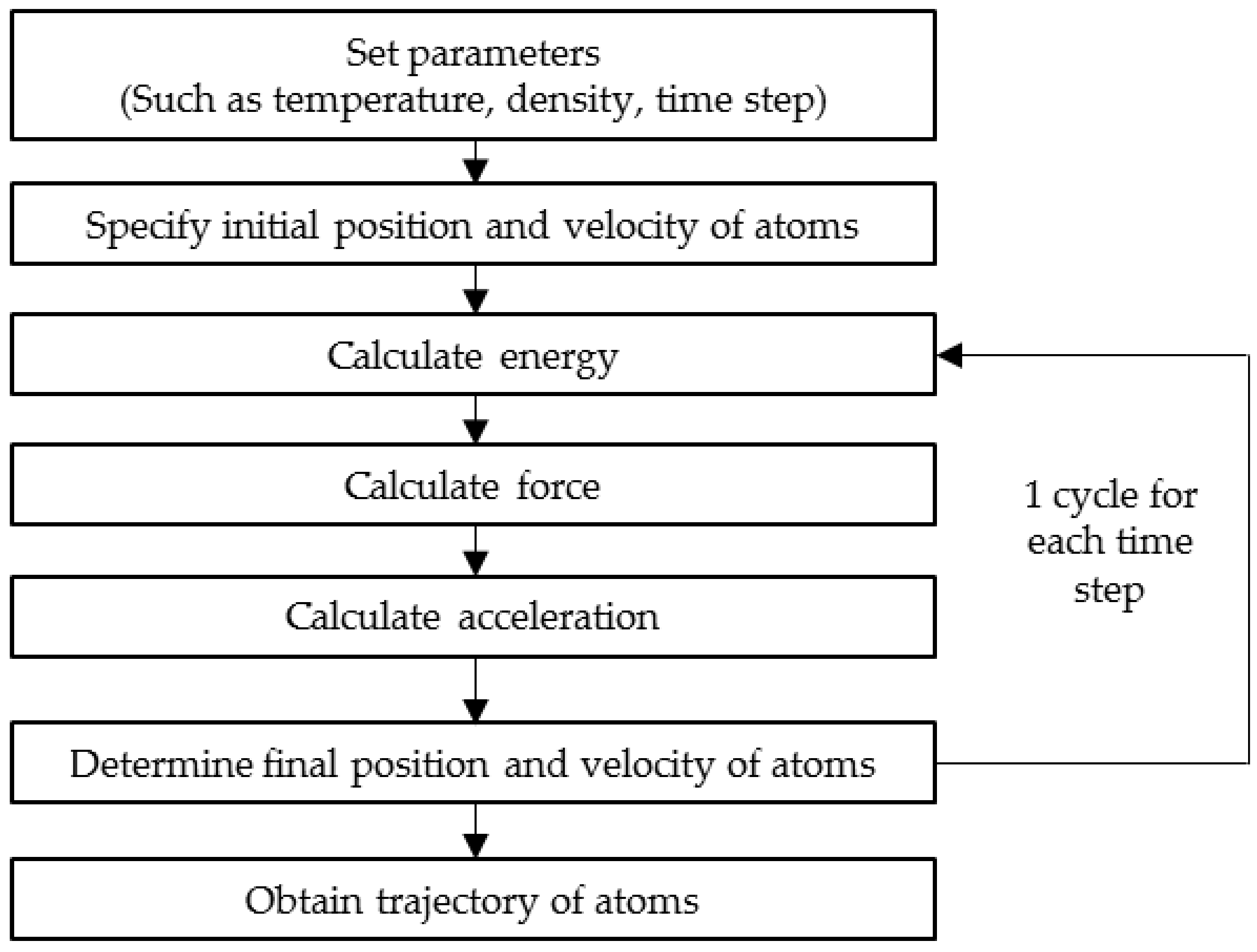
The underlying principle behind an MD simulation is simple and straightforward. Given the initial positions (coordinates) and velocity of all atoms in a system, the force acting on each atom by all other atoms in the system in terms of potential energy can be obtained. The potential energy that acts on an atom can be categorised into two; bonding potentials (bonds, angles and dihedrals) and non-bonding potentials (van der Waal and Columbic). Upon knowing the potential energy, the force can be derived from the energy function. This stage of computing the force is the utmost importance in getting a reliable and accurate simulation result [32]. Finally, Newton’s law of motion is integrated in order to calculate the acceleration of each atom, enabling the prediction of the new position of each atom as a function of time [33]. Again, the new potential energy, force and acceleration can be obtained from the updated position and velocity of each atom. As this step is repeated, it is possible to simulate the trajectory of each atom that describes each configuration of the atoms within a small time interval in real-time. The overall step in MD simulation is illustrated in Figure 2.
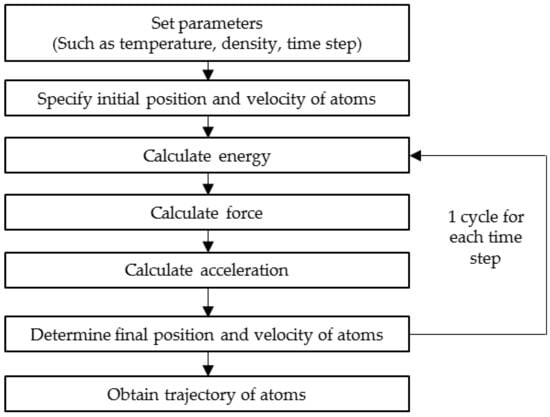
Figure 2.
Computational flow of molecular dynamics simulation.
3.2. Steps in Performing a Molecular Dynamics Simulation for Corrosion Studies
3.2.1. Construction of a Corrosion System
The first step of performing an MD simulation for corrosion system is constructing the components of the interaction model. A corrosion system typically consists of two components; an inhibitor molecule and a metal surface. Typically, the inhibitor molecule is sketched and its geometry is optimized to obtain an accurate geometry. In real condition, the inhibitor does not exist in isolated space of vacuum, but as an aqueous phase in the corrosive solution [32]. Besides, the degree of inhibitor adsorption is greatly influenced by the surroundings as an unbounded inhibitor is more vigorous in interacting with metal surface compared to inhibitor surrounded by solvent molecules [14]. Hence, to ensure that the simulation resembles actual scenario as close as possible, the simulation should be performed in the presence of water molecules and ions, typically ranging from 200 to 600 total molecules combined [34,35,36]. In addition, the proportion of water molecules to the ions should also roughly resemble the actual concentration of the solution. For instance, 1 M of hydrochloric acid (HCl) is composed of 55.5 mol of water molecules (H2O), 1 mol of hydronium ions (H3O+) and 1 mol of chloride ions (Cl−), which makes the ratio of water to hydrogen chloride is 55.5:1 or roughly 500:9. Thus, it can be deduced that the most suitable composition of 1M HCl aqueous phase is 500 H2O, 9 H3O+ and 9 Cl-. The aqueous phases used for several acidic solutions in recently reported studies are summarized in Table 1.

Table 1.
Aqueous phase of acidic solution in MD simulation.
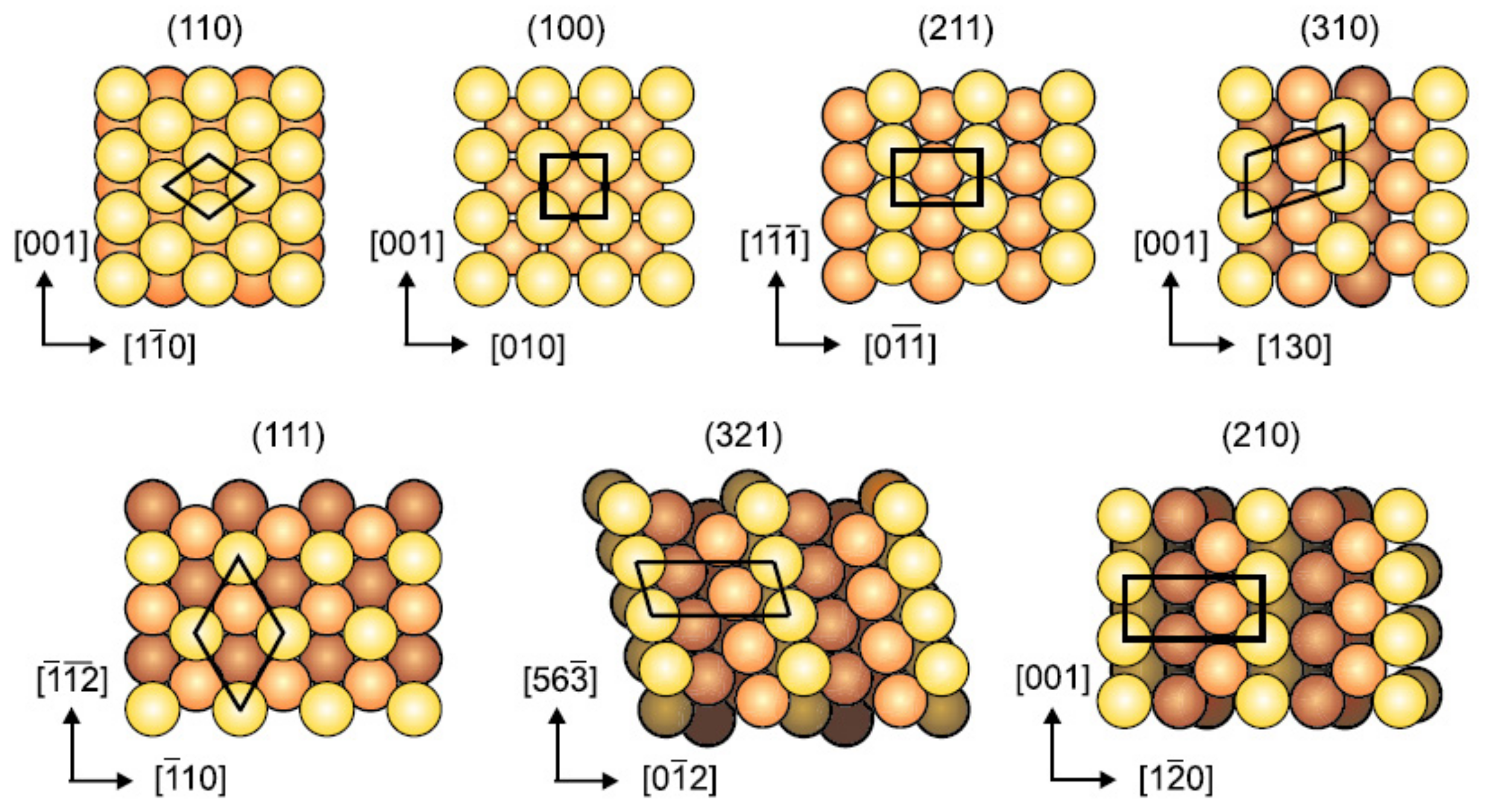
The second component resembles the metal surface which is composed of iron atoms in the case of ferrous metal. In normal temperature and pressure, iron exists as body-centred cubic (bcc) structure with (110), (100), (211), (311), (111), (321) and (210) as the most densely packed surfaces as depicted in Figure 3. Typically, a Fe(110) surface is used as the adsorption site for simulation of corrosion inhibitor adsorption because it contributes the largest area of the Fe crystal and is the most thermodynamically stable facet among the others [53,54,55,56,57,58]. Contrarily, Zhu et al. [59] demonstrated the use of Fe(100) surface as the adsorption site for 2-aminobenzimidazole derivative inhibitor. The order of surface energy of the iron surface is Fe(110) < Fe(100) < Fe(111), which makes Fe (110) to be the most stable, followed by Fe(100) and Fe(111) facets [60]. Regardless of the type of ferrous metal (carbon steel, alloy steel or stainless steel) studied experimentally, pure iron atoms instead of a mixture of iron with alloying elements are usually used for theoretical studies [11,38,61,62,63]. Table 2 shows the implementation of several Fe surfaces for different types and grade of ferrous metal in corrosion inhibition studies in acid solution.
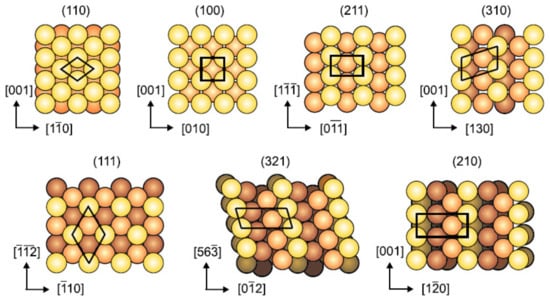
Figure 3.
Top view of most densely packed surfaces of bcc structure, reproduced from [53], with permission from Elsevier, 2007.

Table 2.
Fe surfaces used to resemble several types and grades of ferrous metals in MD simulation.
3.2.2. Specifying Boundary Condition
Simulating a bulk of system with an infinite number of molecules is computationally expensive. As the positions, velocities and forces of each atom need to be recomputed in every time step, the calculation time required for a large number of molecules (>10000) using average performance computers is infinite [69]. Constructing a smaller system in an enclosed simulation box is a less expensive option, but the forces acting on atoms near the “walls” of the box and atoms in the center differs significantly due to boundary effect. To solve this issue, periodic boundary condition (PBC) can be implemented to the system, which replicates the simulation box in all directions as images. These images behave exactly like the original simulation box and have the same number, position and momentum of atoms. PBC is always employed for the simulation of corrosion inhibitor adsorption onto the metal surface to avoid any arbitrary boundary effects [70,71,72,73,74].
3.2.3. Simulation Tool
To perform any computational simulation, it is necessary to use a tool or module that is usually embedded in the simulation software. Forcite or Forcite Plus is an advanced tool in Materials Studio Software (BIOVIA Materials Studio 2017, 17.1.0.48, San Diego, CA, USA), to compute classical simulation that utilizes molecular mechanics or molecular dynamics [75]. It can be used to calculate energy, optimize geometry or determine the trajectory of a system. Forcite module has been extensively used for a broad range of properties determination in countless types of system such as the interaction property of nanoform zinc oxide with Covid19, the mechanical properties of polyvinyl chloride/high-density polyethylene composite, the mechanism of Schiff base as an anticancer drug and a lot more [76,77,78]. On top of that, Forcite is the most frequently used module for corrosion inhibition studies using MD simulation reported in the literature [45,79,80,81,82].
3.2.4. Selection of Ensembles
An ensemble is a collection of microscopic states (position and velocity of atoms at a timestep) that have the same thermodynamic state such as energy, E, volume, V, temperature, T, pressure, P and number of particles, N. The most common ensembles for MD simulation are microcanonical ensemble (constant N,V,E), canonical ensemble (constant N,V,T) and isothermal-isobaric ensemble (constant N,P,T). Canonical ensemble or NVT ensemble is usually used for the determination of adsorption energy in a corrosion system [11,49,83,84,85]. With the implementation of this ensemble, the number of particles, volume and temperature of the system are conserved in every step of calculation or integration.
3.2.5. Choosing Time Step
MD simulation works by integrating Newtonian equation to compute the state of a system (velocity and position) by given the initial state of the system. Any mistake in specifying the numerical integrator will produce biased results and errors in calculation. Hence, it is crucial to select the proper time step to obtain a reliable result. As the integration in MD simulation computes the movement of atomic-scale system with chemical bonds, the scale of time step should match the scale of the fastest vibrational frequency of the chemical bonds which is on the order of fractions of femtosecond (fs). Ideally, the time step should be set as large as possible in order to simulate for a longer time span, with less integration step. The relationship of the time step with the integration step and length of the simulation is expressed as follows:
However, large time step will cause “exploding”; a phenomenon where the total energy increases rapidly with time, which causes atomic collision [86]. On the other hand, small time steps will cause unnecessary calculations which extend the calculation time. Therefore, the time step should be set optimum value, at least ten times lower than the highest frequency of the system [87]. For the simulation of compounds with hydrogen bonds (such as corrosion inhibitor molecules) that has the highest vibrational frequency among other chemical bonds, the ideal time step is 1–2 fs [39,88].
3.2.6. Selection of Force Field
A force field is a set of mathematical expression that is used to calculate the forces in terms of the potential energy of the interacting atoms in a simulation system. It is considered as the soul of MD as the selection of force field affects directly towards the accuracy of the simulation results [89]. The Condensed-phase Optimized Molecular Potentials for Atomistic Simulation Studies (COMPASS) force field is extensively used relative to other force fields for the simulation of corrosion inhibitor adsorption [11,90,91,92]. It is originally designed to describe forces for organic liquids and polymers, and then extended further for the application of organic compounds with H, C, N, O, S, P and metals [93]. Due to the broad coverage of COMPASS force field, it is a suitable force field to be used for a corrosion system, which typically contains organic compounds (inhibitor molecule) and metal atoms.
3.3. Parameters Derived from Molecular Dynamics Simulation of Corrosion Inhibition
3.3.1. Adsorption Energy
Adsorption energy or interaction energy resembles the amount of energy released or absorbed as 1 mole of adsorbate molecules is adsorbed on the adsorbent. The information of adsorption energy is crucial to understand the underlying mechanism of the adsorption. The main factors that affect the energy include the electronegativity, valence of adsorbate, and the coordination of active sites (adsorbent) [94]. In view of corrosion inhibition, the inhibitor molecule is regarded as the adsorbate, while the metal surface is referred as the adsorbent. It can be expressed as follows:
where Eads is the adsorption energy, Etotal is the total energy of the whole system, Emetal + solution is the energy of the metal and the aqueous phase and Einhibitor is the energy of the inhibitor molecule. A negative value of adsorption energy is an indication of an exothermic and spontaneous process. This means that the inhibitor is attracted towards the metal surface, causing adsorption of either physically, chemically or a combination of both. The greater the magnitude (more negative) of the energy released, the stronger the strength of adsorption, and hence the higher the corrosion inhibition efficiency [14].
3.3.2. Binding Energy
Binding energy is the energy required to separate a particle from a system. It can be viewed from several standpoints according to the distance and energy scale of the system of interest. Electron binding energy, atomic binding energy, bond dissociation energy, nuclear binding energy and gravitational binding energy are the types of binding energy for different systems. The adsorption of CIs on the metal surface is closely related to bond dissociation energy because the formation and dissociation of chemical bonds are ubiquitous in the process [94]. Hence in the perspective of corrosion inhibition, binding energy is the energy required to desorb the inhibitor molecule from the metal surface due to bond dissociation. It is regarded as the reciprocal of adsorption energy as expressed below:
where Ebind is the binding energy. The greater the binding energy (more positive), the stronger the attraction force between the inhibitor and the metal surface, and hence the higher the inhibition efficiency.
3.3.3. Inhibitor Molecule Orientation
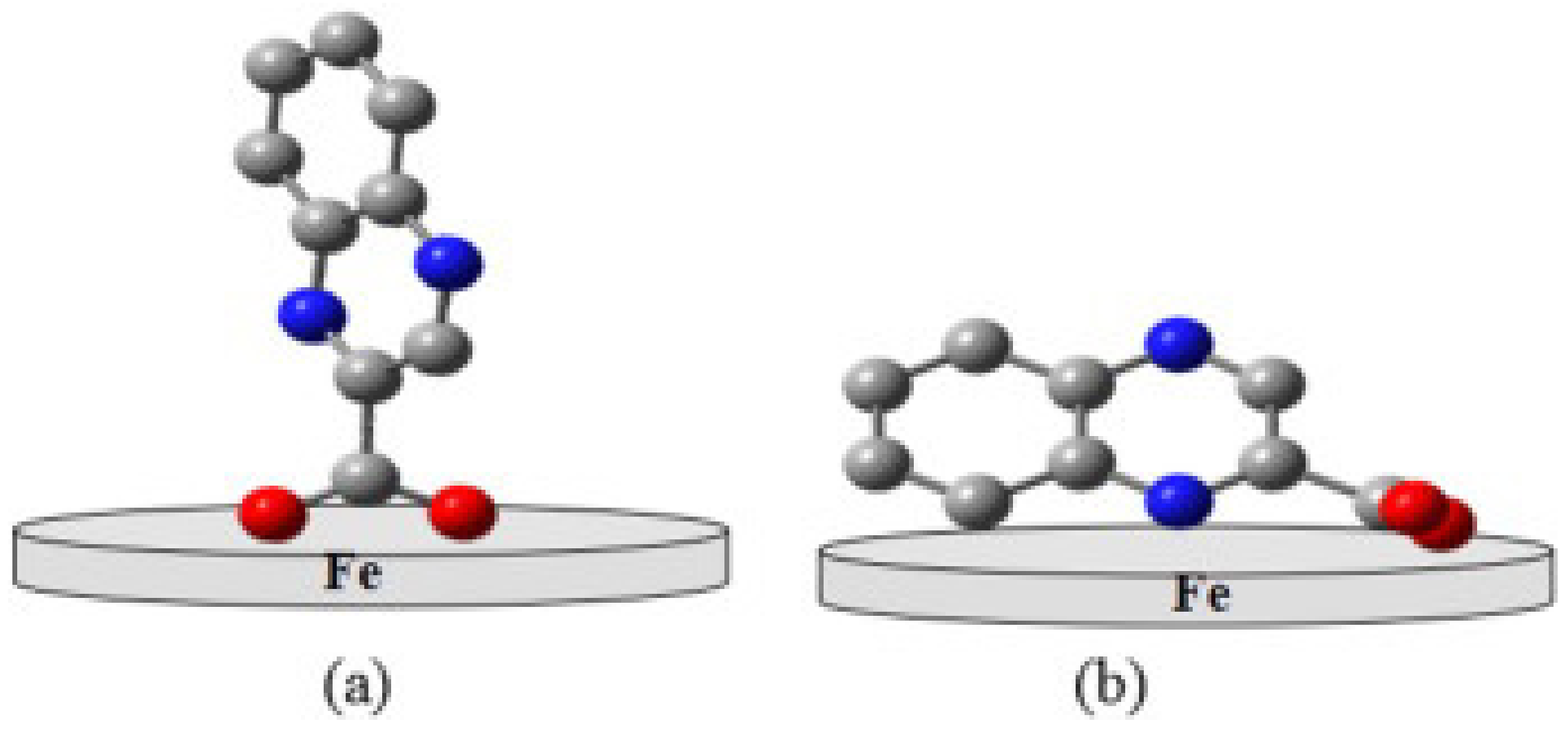
The adsorption configuration of the inhibitor molecule on the metal surface is an important finding from MD simulation. Upon several calculation iterations done by the software, the most stable configuration of inhibitor with lowest energy level will be obtained. The final configuration of adsorption depends on the chemical structure and electron density of the inhibitor molecule [95]. Figure 4 shows the example of different possible configuration of sodium 2-quinoxalinecarboxylate on an iron substrate [96]. Typically, horizontal, flat or planar orientation as depicted in Figure 4b is preferred for better corrosion inhibition performance because it can cover a larger surface area of metal and has higher binding energy [14].
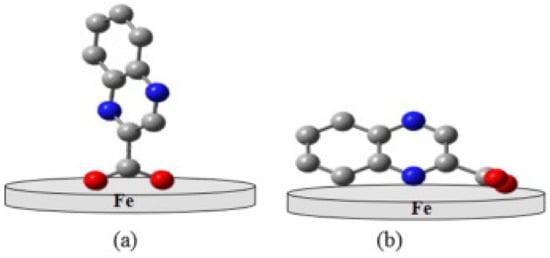
Figure 4.
Adsorption configuration of sodium 2-quinoxalinecarboxylate on iron substrate; (a) vertical and (b) horizontal orientation, reproduced from [96], with permission from Elsevier, 2016.
3.4. Application of Molecular Dynamics Simulation for Corrosion Inhibition Studies of Ferrous Metal in Acidic Solution
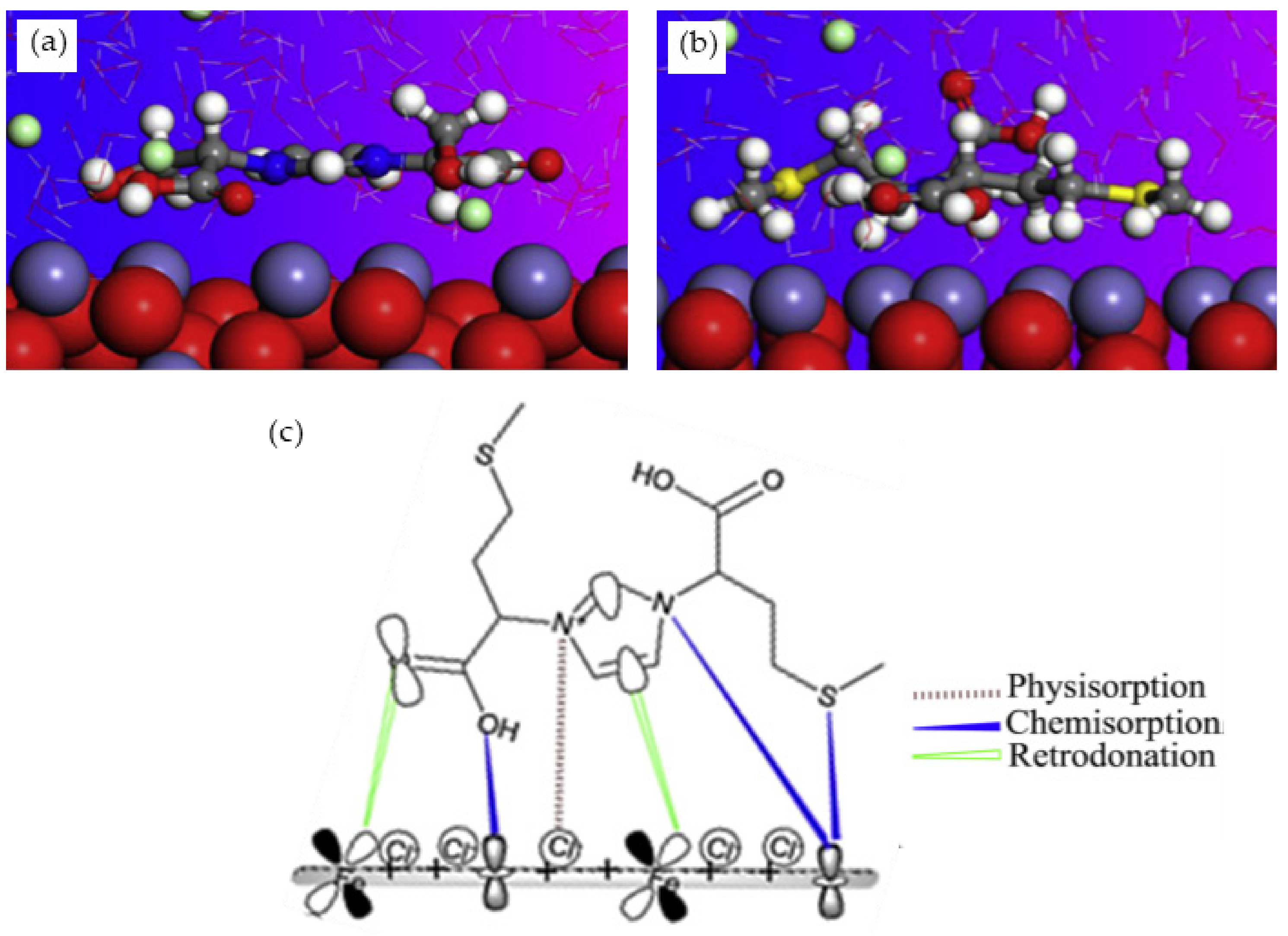
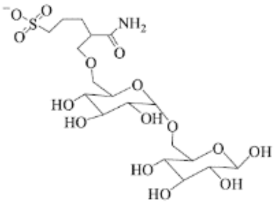
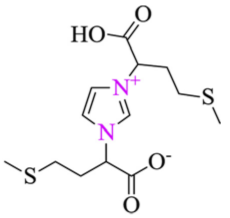
The corrosion inhibition of ferrous metals in acidic media has been extensively investigated due to its exceptional industrial use [97]. As it is crucial to understand the mechanism of corrosion inhibition, MD simulation is normally performed alongside experimental procedures. Haque et al. carried out an experimental and an MD simulation to elucidate the inhibition of mild steel corrosion in 1 M hydrochloric acid (HCl) by two synthesized amino acid derivatives; hydroxyl-containing zwitterion (H-zwitterion) and sulfur-containing zwitterion (S-zwitterion) [54]. Two types of adsorption sites that represent the metal surface was used in the simulation for comparison purposes; neutral Fe (110) and α-Fe2O3 (110). The MD simulation results revealed that both inhibitors were adsorbed in a planar position with both surfaces (Figure 5a,b). However, S-zwitterion provided a greater extend of adsorption than the H-zwitterion based on the interaction and binding energy. This suggests that the presence of sulfur atom promotes bonding with the iron atoms. The adsorption model of S-zwitterion is illustrated in Figure 5c. Aside from that, it was also found that there was no significant difference in adsorption behaviour for Fe (110) and α-Fe2O3 (110) surface.
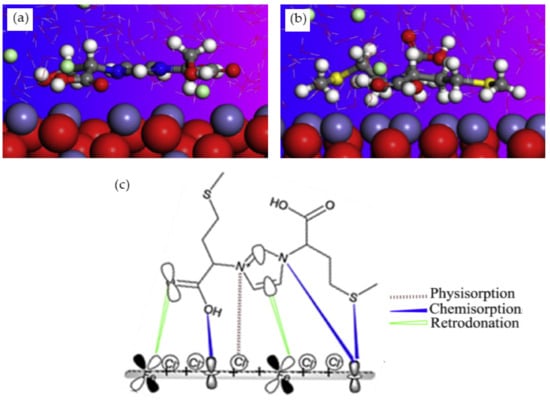
Figure 5.
Side view of (a) H-zwitterion; and (b) S-zwitterion adsorbed on α-Fe2O3 (110) surface; and (c) adsorption model of S-zwitterion, reproduced from [54], with permission from Elsevier, 2020.
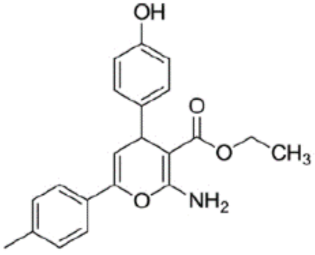
Saranya and coworkers described the adsorption of three synthesized pyran derivatives; ethyl 2-amino-4-(4-hydroxyphenyl)-6-(p-tolyl)-4H-pyran-3-carboxylate (HP), ethyl 2-amino-4-(4-methoxyphenyl)-6-(p-tolyl)-4H-pyran-3-carboxylate (MP) and ethyl 2-amino-4-(4-hydroxy-3,5-dimethoxyphenyl)-6-(p-tolyl)-4H-pyran-3-carboxylate (HDMP) on Fe (110) surface in 1 M H2SO4 [51]. It is interesting to note that only the p-tolyl-pyran skeleton of all molecules preferred to adsorb completely on the iron surface with flat orientation. This phenomena can be explained by the non-planar geometry of the inhibitor molecules, which caused only the most electron-rich site to be adsorbed on the metal surface. The strongest adsorption property on Fe (110) surface was manifested by HDMP followed by MP and HP molecule. The presence of oxygen atoms, π electrons and electron-donating groups in the molecular structure can be attributed as the driving force for adsorption.
The inhibition effect of pentaglycidyl ether pentabisphenol A of phosphorus (PGEPBAP) phosphorus polymer on carbon steel corrosion in 1 M HCl was investigated by Hsissou and coworkers [36]. According to the MD simulation result, it was revealed that the PGEPBAP molecule was adsorbed in a parallel position to the metal surface. The molecule surface with phosphorus and oxygen heteroatoms was oriented towards the iron atom. 1478 kcal/mol of adsorption energy was obtained from Monte Carlo simulation, but there was no quantification of interaction or binding energy reported from MD simulation.
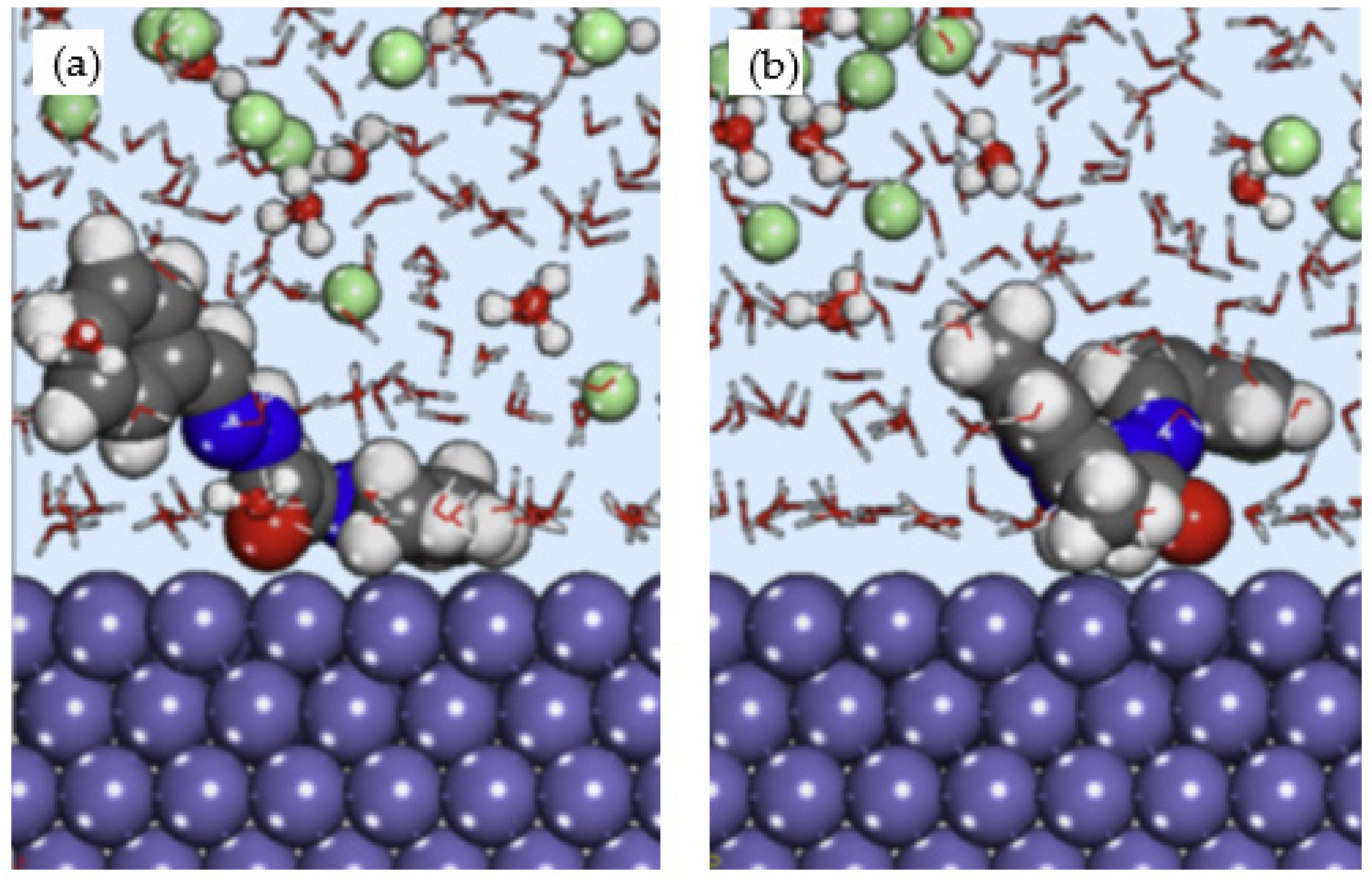
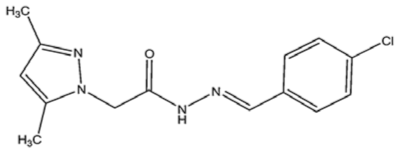
El Arrouji et al. performed an experimental, Monte Carlo simulation and MD simulation to study the corrosion inhibition of two pyrazole derivatives which are (E)-N′-benzylidene-2-(3,5-dimethyl-1H-pyrazol-1-yl) acetohydrazide (DPP) and (E)-N′-(4-chlorobenzylidene)-2-(3,5-dimethyl-1H-pyrazol-1-yl)acetohydra zide (4-CP) on steel in 1 M HCl [41]. The Monte Carlo simulation suggested that 4-CP inhibitor exhibited stronger adsorption on Fe (110) surface with −109.18 kcal/mol adsorption energy compared to DPP inhibitor with −103.38 kcal/mol adsorption energy, respectively. This finding was in agreement with the experimental result, which showed that the inhibition efficiency of 4-CP is relatively higher than DPP inhibitor. The lowest energy configuration of the inhibitor and Fe (110) surface obtained from MD simulation is shown in Figure 6. The site of the inhibitor molecules with oxygen and nitrogen heteroatoms were oriented towards the metal surface, but it is apparent that the orientation of the overall molecule was not completely parallel. This is probably due to the arrangement of heteroatoms, which is more concentrated at one side of the molecule instead of evenly distributed throughout the structure. No energy value was computed from the MD simulation.
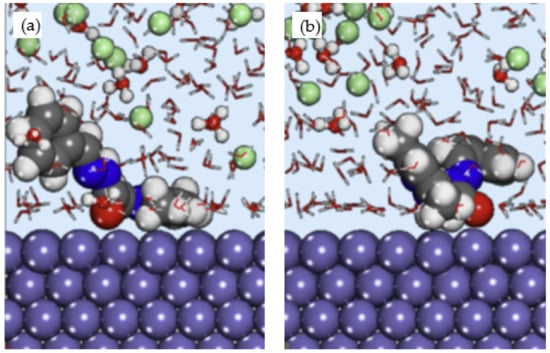
Figure 6.
Side view of (a) DPP; and (b) 4-CP adsorbed on Fe (110) in HCl, reproduced from [41], with permission from Elsevier, 2020.
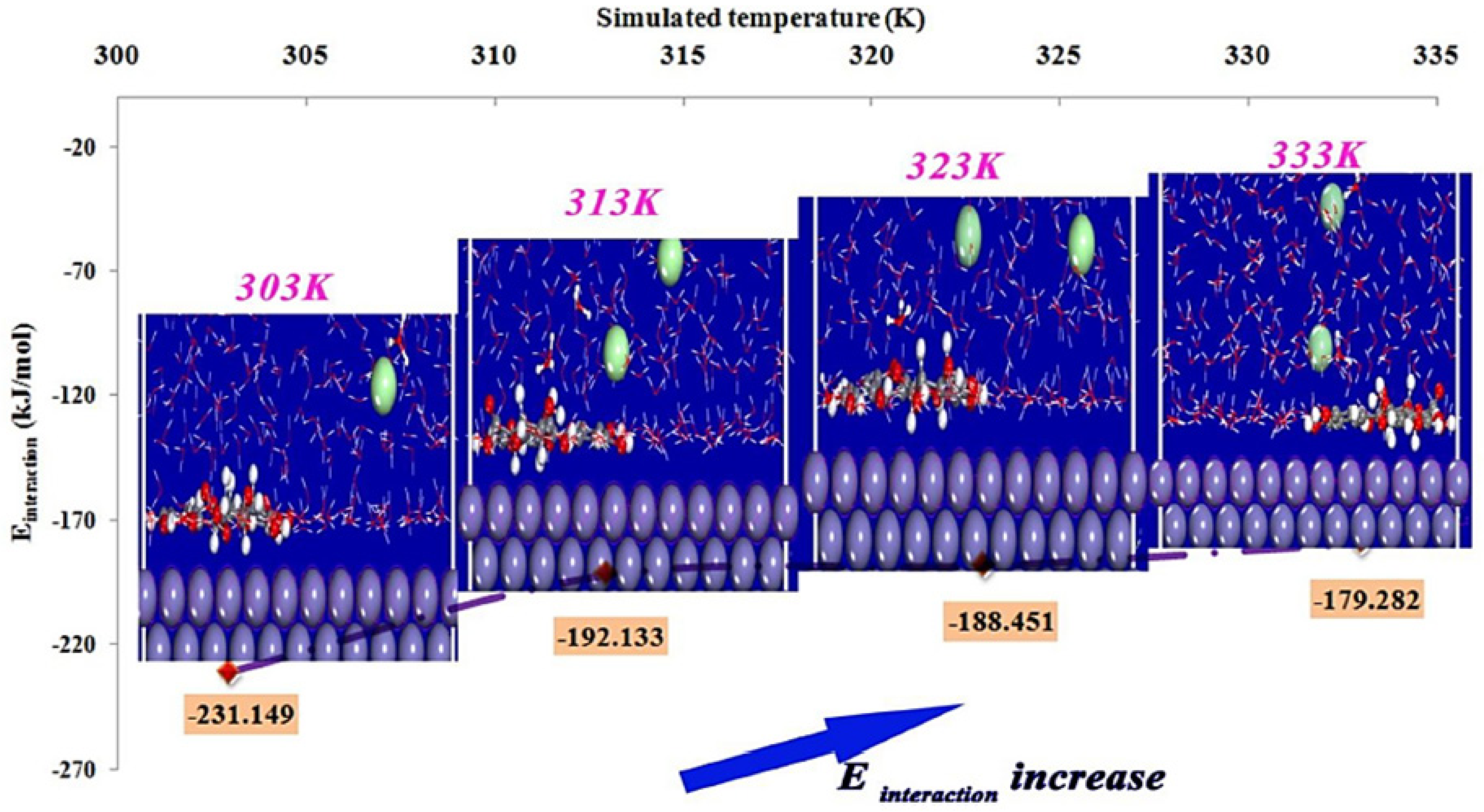
An MD simulation study on the adsorption of quercetin-3-glucuronide, a major compound of Lavandula mairei (LM) ethanolic extract, on mild steel in 1 M HCl was reported by Berrissoul et al. [98]. The final configurations of the inhibitor and their respective interaction energy after equilibrium at different simulated temperatures (303, 313, 323 and 333 K) are reflected in Figure 7. It is apparent that the LM inhibitor was adsorbed in planar arrangement to the Fe (110) surface, which is due to the aromatic rings and oxygen atoms that are present in the inhibitor molecule. As the temperature was increased, it was found that the interaction energy of the LM inhibitor-Fe (110) system was becoming less negative. This indicates that the adsorption strength is weaker at elevated temperature, hence the LM inhibitor should be intended for lower temperature application to anticipate high performance.
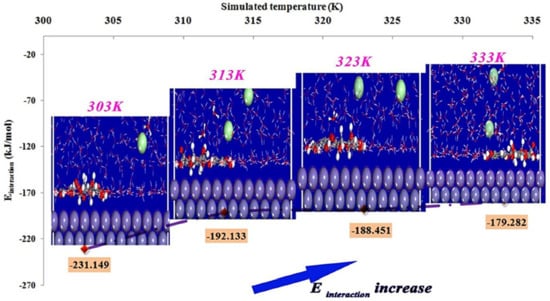
Figure 7.
Side view of adsorbed LM molecule on Fe (110) and its interaction energy at different temperatures reproduced from [98], with permission from Elsevier, 2020.
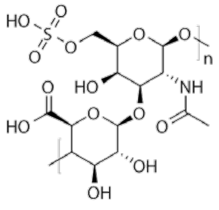
Zhang et al. investigated the inhibition of mild steel corrosion in 15% HCl using the extract of aloe gel, which is composed of polysaccharides. For MD simulation purpose, four main monomers of the polysaccharide; glucose, mannose, galactose and fructose were used to resemble the extract. The final positions of the inhibitors were completely parallel to the Fe (110) surface, thanks to the planar geometry of the monomers. Based on the computed binding energy, fructose exhibited the strongest adsorption strength (598.35 kJ/mol), followed by mannose (593.96 kJ/mol), galactose (587.47 KJ/mol) and glucose (578.22 kJ/mol).
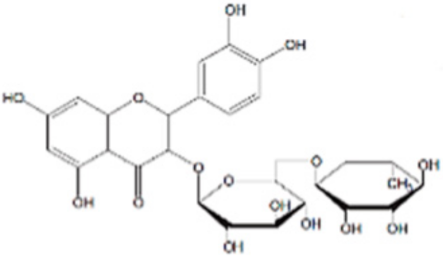
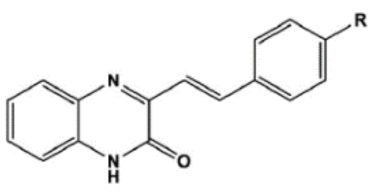
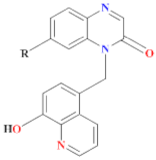
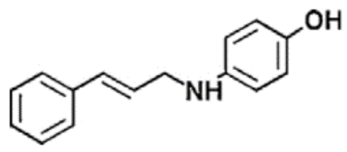
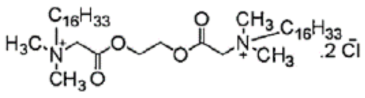
The chemical structures, MD simulation parameters, and the results of the MD of several investigated organic corrosion inhibitors for various types of ferrous metal in acidic solution are summarized in Table 3. Generally, most studies had focused on the corrosion inhibition of mild steel, which is probably due to the extensive use in the industry relative to other ferrous metal and its poor resistance towards acid corrosion. With regard to the acidic media, the majority of the reviewed literature investigated corrosion inhibition in HCl solution, followed by H2SO4. It seemed like there was less than a handful of studies that performed MD simulation in other acidic media such as perchlorate acid and ethanoic acid. In terms of the simulation result, after a detailed review, it can be inferred that most inhibitors were adsorbed on the metal surface in planar or parallel orientation. This configuration is preferred as the molecule can cover a larger surface of the metal and displace more water molecules and other corrosive species.

Table 3.
Name and chemical structure of corrosion inhibitors for ferrous metals in acid solution, MD simulation parameters and MD results.
4. Future Prospects
MD simulations have been used to obtain molecular-level information in order to understand the mechanism of corrosion inhibition. The expected results of MD simulation in corrosion studies can be categorized into two: (1) the configuration of the inhibitor molecule upon adsorption on the metal surface and (2) the strength of adsorption based on the computed energy values (adsorption and binding energy). The main challenges for upcoming circumstances are to further explore the ability of MD simulation to provide value-added results that is able to describe the corrosion inhibition mechanism in more detail. For instance, the simulation of more than one inhibitor molecule can be performed with the aid of high performance computers, which is possible to simulate the adsorption isotherm (such as Langmuir, Freundlich and Temkin) theoretically. Possibly, the electrochemical potential of the corrosion system can be modelled with the integration of MD simulation with other computational methods.
With respect to the current technology of MD simulation, the scope of reported corrosion inhibition studies which concentrated on the effect of the inhibitor molecular structure and different metal substrate towards inhibition performance is only the tip of the iceberg. Other feasibly conducted studies such as the effect of different types of aqueous phase and flow condition (stagnant or agitated) towards the adsorption strength have yet to be reported elsewhere. In addition, the presence of impurities in the actual metal (such as carbon in mild steel) could also affect the performance of corrosion inhibitor to an extent. More broadly, future research should consider the alloying elements of the metal instead of only using pure metal atoms in the simulation model. Apparently, the use of mixtures of atoms in MD simulation model; such as iron and carbon that resembles steel, is well established in other field especially in material science. Hence, it is possible to construct similar system for corrosion application as well. It is also worth to mention that the metal surface roughness is another parameter that can be taken into account in an MD simulation model of inhibitor adsorption. Quite a number of studies related to this parameter in other fields are available in the literature, yet none was found in the field of corrosion. Since the surface roughness is a significant parameter in the experimental approach, adding this parameter into consideration will produce results that are applicable to real cases.
It is encouraging to mention that the use of radial distribution function (RDF) in MD in the field of corrosion science has been emerging over the past years. RDF provides the information that can be used to determine the type of adsorption of the inhibitor on the metal atoms; either physically or chemically. It is anticipated that more corrosion inhibitor adsorption simulation that utilizes RDF are to be published in the future.
5. Conclusions
In this review, MD simulation is described in terms of its basic principles, computational flow and expected outcome on the inhibition mechanism of ferrous metal in acidic solution. Steps in performing MD simulation such as the construction of a corrosion system, and selection of the boundary condition, simulation tool, ensembles, time step and force field are emphasized in the hope to provide readers better understanding in the theoretical approach of corrosion studies.
Aside from that, some recent works on interactions between a corrosion inhibitor and ferrous metal surface in acid solution studied using MD simulation have been collected and summarized in this review. It can be concluded that most inhibitors are adsorbed on the metal surface in a planar or parallel orientation, which larger surface coverage of the metal, and hence better performance. The vital parameters quantified from the MD simulation such as binding energy and adsorption energy are useful to determine the degree of adsorption between the inhibitor and metal atoms. The insights gained from this review may be of assistance to researchers in developing new and improved corrosion inhibitors. Finally, the challenges and future trends of MD simulation in corrosion science are also obviously highlighted in the final section. Considerably more work will need to be done to overcome the challenges and to boost the potential of MD simulation in the field of corrosion science.
Author Contributions
Writing—original draft preparation, N.I.N.H.; writing—review and editing, S.S.; supervision, S.S., Y.A.Y., N.K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universiti Putra Malaysia, Putra Grant IPS, grant number 9657500.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bartley, J.; Huynh, N.; Bottle, S.E.; Flitt, H.; Notoya, T.; Schweinsberg, D.P. Computer Simulation of the Corrosion Inhibition of Copper in Acidic Solution by Alkyl Esters of 5-Carboxybenzotriazole. Corros. Sci. 2003, 45, 81–96. [Google Scholar] [CrossRef]
- Battimelli, G.; Ciccotti, G. Berni Alder and the Pioneering Times of Molecular Simulation. Eur. Phys. J. H 2018, 43, 303–335. [Google Scholar] [CrossRef]
- Alder, B.; Wainwright, T.E. Molecular Dynamics by Electronic Computers. In International Symposium on Transport Processes in Statistical Mechanics; Prigogine, I., Ed.; John Wiley International: New York, NY, USA, 1957. [Google Scholar]
- Fourati, N.; Blel, N.; Lattach, Y.; Ktari, N.; Zerrouki, C. Chemical and Biological Sensors from Conducting and Semiconducting Polymers. Ref. Modul. Mater. Sci. Mater. Eng. 2016. [Google Scholar] [CrossRef]
- Ewis, D.; Talkhan, A.G.; Benamor, A.; Qiblawey, H.; Nasser, M.; Ba-Abbad, M.M.; El-Naas, M. Corrosion Behavior of API-X120 Carbon Steel Alloy in a GTL F-T Process Water Environment at Low COD Concentration. Metals 2020, 10, 707. [Google Scholar] [CrossRef]
- Ron, T.; Levy, G.K.; Dolev, O.; Leon, A.; Shirizly, A.; Aghion, E. Environmental Behavior of Low Carbon Steel Produced by a Wire Arc Additive Manufacturing Process. Metals 2019, 9, 888. [Google Scholar] [CrossRef]
- Ha, H.Y.; Lee, T.H.; Kim, S.D.; Jang, J.H.; Moon, J. Improvement of the Corrosion Resistance by Addition of Ni in Lean Duplex Stainless Steels. Metals 2020, 10, 891. [Google Scholar] [CrossRef]
- MEPS International Ltd. Global Composite Steel Price and Index; MEPS International Ltd.: Sheffield, UK, 2020. [Google Scholar]
- Alar, V.; Stojanović, I.; Mezdić, D. A Comparative Study of Green Inhibitors for Galvanized Steel in Aqueous Solutions. Metals 2020, 10, 448. [Google Scholar] [CrossRef]
- Lgaz, H.; Masroor, S.; Chafiq, M.; Damej, M.; Brahmia, A.; Salghi, R.; Benmessaoud, M.; Ali, I.H.; Alghamdi, M.M.; Chaouiki, A.; et al. Evaluation of 2-Mercaptobenzimidazole Derivatives as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid. Metals 2020, 10, 357. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Chauhan, D.S.; Quraishi, M.A.; Lgaz, H.; Chung, I.M. Comprehensive Investigation of Steel Corrosion Inhibition at Macro/Micro Level by Ecofriendly Green Corrosion Inhibitor in 15% HCl Medium. J. Colloid Interface Sci. 2020, 560, 225–236. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, D.; Chen, H.; Guo, S.; Yang, Q.; Chen, L.; Zhao, H.; Wang, L. A High-Efficiency Corrosion Inhibitor of N-Doped Citric Acid-Based Carbon Dots for Mild Steel in Hydrochloric Acid Environment. J. Hazard. Mater. 2020, 381, 121019. [Google Scholar] [CrossRef] [PubMed]
- Mouaden, K.E.; Quraishi, M.A.; Quraishi, M.A.; Bazzi, L. Thiocarbohydrazide-crosslinked chitosan as a bioinspired corrosion inhibitor for protection of stainless steel in 3.5% NaCl. Sustain. Chem. Pharm. 2020, 15, 100213. [Google Scholar] [CrossRef]
- Verma, C.; Lgaz, H.; Verma, D.K.; Ebenso, E.E.; Bahadur, I.; Quraishi, M.A. Molecular Dynamics and Monte Carlo Simulations as Powerful Tools for Study of Interfacial Adsorption Behavior of Corrosion Inhibitors in Aqueous Phase: A Review. J. Mol. Liq. 2018, 260, 99–120. [Google Scholar] [CrossRef]
- Ni, H.; Wu, J.; Sun, Z.; Lu, G.; Yu, J. Molecular Simulation of the Structure and Physical Properties of Alkali Nitrate Salts for Thermal Energy Storage. Renew. Energy 2019, 136, 955–967. [Google Scholar] [CrossRef]
- Ni, M.; Pan, J.; Hu, X.; Gong, D.; Zhang, G. Inhibitory Effect of Corosolic Acid on α-Glucosidase: Kinetics, Interaction Mechanism, and Molecular Simulation. J. Sci. Food Agric. 2019, 99, 5881–5889. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhang, H.; Bian, X.; Li, J.; Li, J.; Zhang, H. Insight into the Binding of ACE-Inhibitory Peptides to Angiotensin-Converting Enzyme: A Molecular Simulation. Mol. Simul. 2019, 45, 215–222. [Google Scholar] [CrossRef]
- Ganesan, P.; Ramalingam, R. Investigation of Structural Stability and Functionality of Homodimeric Gramicidin towards Peptide-Based Drug: A Molecular Simulation Approach. J. Cell. Biochem. 2019, 120, 4903–4911. [Google Scholar] [CrossRef]
- Hossain, S.; Kabedev, A.; Parrow, A.; Bergström, C.A.S.; Larsson, P. Molecular Simulation as a Computational Pharmaceutics Tool to Predict Drug Solubility, Solubilization Processes and Partitioning. Eur. J. Pharm. Biopharm. 2019, 137, 46–55. [Google Scholar] [CrossRef]
- Ganazzoli, F.; Raffaini, G. Dendrimer Dynamics: A Review of Analytical Theories and Molecular Simulation Methods. Polymers 2020, 12, 1387. [Google Scholar] [CrossRef]
- Hu, J.-P.; Wu, Z.-X.; Xie, T.; Liu, X.-Y.; Yan, X.; Sun, X.; Liu, W.; Liang, L.; He, G.; Gan, Y.; et al. Applications of Molecular Simulation in the Discovery of Antituberculosis Drugs: A Review. Protein Pept. Lett. 2019, 26, 648–663. [Google Scholar] [CrossRef]
- Feng, D.; Feng, Y.; Qiu, L.; Li, P.; Zang, Y.; Zou, H.; Yu, Z.; Zhang, X. Review on Nanoporous Composite Phase Change Materials: Fabrication, Characterization, Enhancement and Molecular Simulation. Renew. Sustain. Energy Rev. 2019, 109, 578–605. [Google Scholar] [CrossRef]
- Pedeferri, P. Uniform Corrosion in Acidic and Aerated Solutions. Transp. Metal-Oxide-Semicond. Struct. 2018, 145–167. [Google Scholar] [CrossRef]
- Lazzari, L. Basic Principles. In European Federation of Corrosion (EFC) Series; Woodhead Publishing: Cambridge, UK, 2017; pp. 1–23. [Google Scholar] [CrossRef]
- Popoola, L.T. Organic Green Corrosion Inhibitors (OGCIs): A Critical Review. Corros. Rev. 2019, 37, 71–102. [Google Scholar] [CrossRef]
- Rassouli, L.; Naderi, R.; Mahdavian, M. Study of the Active Corrosion Protection Properties of Epoxy Ester Coating with Zeolite Nanoparticles Doped with Organic and Inorganic Inhibitors. J. Taiwan Inst. Chem. Eng. 2018, 85, 207–220. [Google Scholar] [CrossRef]
- Noorbakhsh Nezhad, A.H.; Davoodi, A.; Mohammadi Zahrani, E.; Arefinia, R. The Effects of an Inorganic Corrosion Inhibitor on the Electrochemical Behavior of Superhydrophobic Micro-Nano Structured Ni Films in 3.5% NaCl Solution. Surf. Coat. Technol. 2020, 395, 125946. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Mazumder, M.A.J.; Quraishi, M.A.; Ansari, K.R. Chitosan-Cinnamaldehyde Schiff Base: A Bioinspired Macromolecule as Corrosion Inhibitor for Oil and Gas Industry. Int. J. Biol. Macromol. 2020, 158, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Popov, B.N. Chapter 14—Corrosion Inhibitors. In Corrosion Engineering: Principles and Solved Problems; Elsevier: Oxford, UK, 2015. [Google Scholar]
- Feng, L.; Zhang, S.; Qiang, Y.; Xu, Y.; Guo, L.; Madkour, L.H.; Chen, S. Experimental and Theoretical Investigation of Thiazolyl Blue as a Corrosion Inhibitor for Copper in Neutral Sodium Chloride Solution. Materials 2018, 11, 1042. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, C. Corrosion Inhibitors. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Elsevier: Oxford, UK, 2018; pp. 164–171. [Google Scholar] [CrossRef]
- Paquet, E.; Viktor, H.L. Molecular Dynamics, Monte Carlo Simulations, and Langevin Dynamics: A Computational Review. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Allen, M.P. Introduction to Molecular Dynamics Simulation Introduction. Comput. Soft Matter Synth. Polym. Proteins 2004, 23, 1–28. [Google Scholar]
- Fouda, A.S.; Ismail, M.A.; Elewady, G.Y.; Abousalem, A.S. Evaluation of 4-Amidinophenyl-2,2′-Bithiophene and Its Aza-Analogue as Novel Corrosion Inhibitors for CS in Acidic Media: Experimental and Theoretical Study. J. Mol. Liq. 2017, 240, 372–388. [Google Scholar] [CrossRef]
- Salarvand, Z.; Amirnasr, M.; Talebian, M.; Raeissi, K.; Meghdadi, S. Enhanced Corrosion Resistance of Mild Steel in 1 M HCl Solution by Trace Amount of 2-Phenyl-Benzothiazole Derivatives: Experimental, Quantum Chemical Calculations and Molecular Dynamics (MD) Simulation Studies. Corros. Sci. 2017, 114, 133–145. [Google Scholar] [CrossRef]
- Hsissou, R.; Abbout, S.; Seghiri, R.; Rehioui, M.; Berisha, A.; Erramli, H.; Assouag, M.; Elharfi, A. Evaluation of Corrosion Inhibition Performance of Phosphorus Polymer for Carbon Steel in [1 M] HCl: Computational Studies (DFT, MC and MD Simulations). J. Mater. Res. Technol. 2020, 9, 2691–2703. [Google Scholar] [CrossRef]
- Thanh, L.T.; Vu, N.S.H.; Binh, P.M.Q.; Dao, V.A.; Thu, V.T.H.; Van Hien, P.; Panaitescu, C.; Nam, N.D. Combined Experimental and Computational Studies on Corrosion Inhibition of Houttuynia Cordata Leaf Extract for Steel in HCl Medium. J. Mol. Liq. 2020, 315, 113787. [Google Scholar] [CrossRef]
- Asfia, M.P.; Rezaei, M.; Bahlakeh, G. Corrosion Prevention of AISI 304 Stainless Steel in Hydrochloric Acid Medium Using Garlic Extract as a Green Corrosion Inhibitor: Electrochemical and Theoretical Studies. J. Mol. Liq. 2020, 315, 113679. [Google Scholar] [CrossRef]
- Qiang, Y.; Guo, L.; Li, H.; Lan, X. Fabrication of Environmentally Friendly Losartan Potassium Film for Corrosion Inhibition of Mild Steel in HCl Medium. Chem. Eng. J. 2021, 406, 126863. [Google Scholar] [CrossRef]
- Chaouiki, A.; Chafiq, M.; Rbaa, M.; Salghi, R.; Lakhrissi, B.; Ali, I.H.; Bashir, S.; Chung, I.M. Comprehensive Assessment of Corrosion Inhibition Mechanisms of Novel Benzimidazole Compounds for Mild Steel in HCl: An Experimental and Theoretical Investigation. J. Mol. Liq. 2020, 114383. [Google Scholar] [CrossRef]
- El Arrouji, S.; Karrouchi, K.; Berisha, A.; Ismaily, K.; Warad, I.; Rais, Z.; Radi, S.; Taleb, M.; Ansar, M.; Zarrouk, A. New Pyrazole Derivatives as Effective Corrosion Inhibitors on Steel-Electrolyte Interface in 1 M HCl: Electrochemical, Surface Morphological (SEM) and Computational Analysis. Colloids Surf. A 2020, 604, 125325. [Google Scholar] [CrossRef]
- El Faydy, M.; Benhiba, F.; Berisha, A.; Kerroum, Y.; Jama, C.; Lakhrissi, B.; Guenbour, A.; Warad, I.; Zarrouk, A. An Experimental-Coupled Empirical Investigation on the Corrosion Inhibitory Action of 7-Alkyl-8-Hydroxyquinolines on C35E Steel in HCl Electrolyte. J. Mol. Liq. 2020, 317, 113973. [Google Scholar] [CrossRef]
- El Aoufir, Y.; Zehra, S.; Lgaz, H.; Chaouiki, A.; Serrar, H.; Kaya, S.; Salghi, R.; Abdelraheem, S.K.; Boukhris, S.; Guenbour, A.; et al. Evaluation of Inhibitive and Adsorption Behavior of Thiazole-4-Carboxylates on Mild Steel Corrosion in HCl. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125351. [Google Scholar] [CrossRef]
- Bedair, M.A.; Soliman, S.A.; Bakr, M.F.; Gad, E.S.; Lgaz, H.; Chung, I.M.; Salama, M.; Alqahtany, F.Z. Benzidine-Based Schiff Base Compounds for Employing as Corrosion Inhibitors for Carbon Steel in 1.0 M HCl Aqueous Media by Chemical, Electrochemical and Computational Methods. J. Mol. Liq. 2020, 317, 114015. [Google Scholar] [CrossRef]
- Ech-chihbi, E.; Nahle, A.; Salim, R.; Benhiba, F.; Moussaif, A.; El-hajjaji, F.; Oudda, H.; Guenbour, A.; Taleb, M.; Warad, I. Zarro. Computational, MD Simulation, SEM/EDX and Experimental Studies for Understanding Adsorption of Benzimidazole Derivatives as Corrosion Inhibitors in 1.0 M HCl Solution. 2020, 844, 155842. [Google Scholar] [CrossRef]
- Ituen, E.; Mkpenie, V.; Ekemini, E.; Eduok, S.; Yuanhua, L.; Akaranta, O. Inhibition of Acid and Bio-Corrosion of Pipeline Steel Using Tabersonine: Experimental, DFT and Molecular Dynamics Simulations Approaches. J. Bio-Tribo-Corrosion 2020, 6, 1–16. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Chen, B.; Zhang, L. Novel Surfactants as Green Corrosion Inhibitors for Mild Steel in 15% HCl: Experimental and Theoretical Studies. Chem. Eng. J. 2020, 402, 126219. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Ituen, E.; Guo, L.; Abdul Wahab, M.; Quraishi, M.A.; Kong, X.; Lin, Y. A New Series of Synthesized Compounds as Corrosion Mitigator for Storage Tanks: Detailed Electrochemical and Theoretical Investigations. Constr. Build. Mater. 2020, 259, 120421. [Google Scholar] [CrossRef]
- Guo, L.; Tan, J.; Kaya, S.; Leng, S.; Li, Q.; Zhang, F. Multidimensional Insights into the Corrosion Inhibition of 3,3-Dithiodipropionic Acid on Q235 Steel in H2SO4 Medium: A Combined Experimental and in Silico Investigation. J. Colloid Interface Sci. 2020, 570, 116–124. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, R.; Tan, B.; Li, W.; Liu, H.; Wu, S. Locust Bean Gum as a Green and Novel Corrosion Inhibitor for Q235 Steel in 0.5 M H2SO4 Medium. J. Mol. Liq. 2020, 310, 113239. [Google Scholar] [CrossRef]
- Saranya, J.; Benhiba, F.; Anusuya, N.; Subbiah, R.; Zarrouk, A.; Chitra, S. Experimental and Computational Approaches on the Pyran Derivatives for Acid Corrosion. Colloids Surf. A 2020, 603, 125231. [Google Scholar] [CrossRef]
- Berisha, A. Experimental, Monte Carlo and Molecular Dynamic Study on Corrosion Inhibition of Mild Steel by Pyridine Derivatives in Aqueous Perchloric Acid. Electrochem 2020, 1, 13. [Google Scholar] [CrossRef]
- Błoński, P.; Kiejna, A. Structural, Electronic, and Magnetic Properties of Bcc Iron Surfaces. Surf. Sci. 2007. [Google Scholar] [CrossRef]
- Haque, J.; Srivastava, V.; Quraishi, M.A.; Singh, D.; Lgaz, H.; Chung, I. Polar Group Substituted Imidazolium Zwitterions as Eco-Friendly Corrosion Inhibitors for Mild Steel in Acid Solution. Corros. Sci. 2020, 172, 108665. [Google Scholar] [CrossRef]
- Bajpai, D.; Murmu, M.; Banerjee, P.; Ahmad, M. Palmitic Acid Based Environmentally Benign Corrosion Inhibiting Formulation Useful during Acid Cleansing Process in MSF Desalination Plants. Desalination 2019, 472, 114128. [Google Scholar] [CrossRef]
- Sulaiman, K.O.; Onawole, A.T.; Faye, O.; Shuaib, D.T. Understanding the Corrosion Inhibition of Mild Steel by Selected Green Compounds Using Chemical Quantum Based Assessments and Molecular Dynamics Simulations. J. Mol. Liq. 2019, 279, 342–350. [Google Scholar] [CrossRef]
- Vengatesh, G.; Sundaravadivelu, M. Non-Toxic Bisacodyl as an Effective Corrosion Inhibitor for Mild Steel in 1 M HCl: Thermodynamic, Electrochemical, SEM, EDX, AFM, FT-IR, DFT and Molecular Dynamics Simulation Studies. J. Mol. Liq. 2019, 287, 110906. [Google Scholar] [CrossRef]
- Xu, L.; Kirvassilis, D.; Bai, Y.; Mavrikakis, M. Atomic and Molecular Adsorption on Fe(110). Surf. Sci. 2018, 667, 54–65. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, X.; Li, X.; Wang, J.; Hu, Z.; Ma, X. 2-Aminobenzimidazole Derivative with Surface Activity as Corrosion Inhibitor of Carbon Steel in HCl: Experimental and Theoretical Study. J. Mol. Liq. 2020, 297, 111720. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Quraishi, M.A.; Lin, Y. Investigation of Corrosion Inhibitors Adsorption on Metals Using Density Functional Theory and Molecular Dynamics Simulation. In Corrosion Inhibitors; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Douche, D.; Elmsellem, H.; Anouar, E.H.; Guo, L.; Hafez, B.; Tüzün, B.; El Louzi, A.; Bougrin, K.; Karrouchi, K.; Himmi, B. Anti-Corrosion Performance of 8-Hydroxyquinoline Derivatives for Mild Steel in Acidic Medium: Gravimetric, Electrochemical, DFT and Molecular Dynamics Simulation Investigations. J. Mol. Liq. 2020, 308, 113042. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, B.; Liang, X. Corrosion Inhibition Performance of Coconut Leaf Extract as a Green Corrosion Inhibitor for X65 Steel in Hydrochloric Acid Solution. Int. J. Electrochem. Sci. 2020. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Zhou, C.; Lu, H. 1-Phenyl-1H-Tetrazole-5-Thiol as Corrosion Inhibitor for Q235 Steel in 1 M HCl Medium: Combined Experimental and Theoretical Researches. Int. J. Electrochem. Sci. 2020, 2499–2510. [Google Scholar] [CrossRef]
- Pal, S.; Ji, G.; Lgaz, H.; Chung, I.; Prakash, R. Lemon Seeds as Green Coating Material for Mitigation of Mild Steel Corrosion in Acid Media: Molecular Dynamics Simulations, Quantum Chemical Calculations and Electrochemical Studies. J. Mol. Liq. 2020, 316, 113797. [Google Scholar] [CrossRef]
- Farhadian, A.; Rahimi, A.; Safaei, N.; Shaabani, A.; Abdouss, M. A Theoretical and Experimental Study of Castor Oil-Based Inhibitor for Corrosion Inhibition of Mild Steel in Acidic Medium at Elevated Temperatures. Corros. Sci. 2020, 175, 108871. [Google Scholar] [CrossRef]
- Khamaysa, O.M.A.; Selatnia, I.; Zeghache, H.; Lgaz, H.; Sid, A.; Chung, I.M.; Benahmed, M.; Gherraf, N.; Mosset, P. Enhanced Corrosion Inhibition of Carbon Steel in HCl Solution by a Newly Synthesized Hydrazone Derivative: Mechanism Exploration from Electrochemical, XPS, and Computational Studies. J. Mol. Liq. 2020, 315, 113805. [Google Scholar] [CrossRef]
- Ouksel, L.; Bourzami, R.; Chafaa, S.; Chafai, N. Solvent and Catalyst-Free Synthesis, Corrosion Protection, Thermodynamic, MDS and DFT Calculation of Two Environmentally Friendly Inhibitors: Bis-Phosphonic Acids. J. Mol. Struct. 2020, 1222, 128813. [Google Scholar] [CrossRef]
- Gao, C.; Zhao, X.; Liu, K.; Dong, X.; Wang, S.; Kong, F. Construction of Eco-Friendly Corrosion Inhibitor Lignin Derivative with Excellent Corrosion-Resistant Behavior in Hydrochloric Acid Solution. Mater. Corros. 2020, 71, 1903–1912. [Google Scholar] [CrossRef]
- Kurapati, Y. A Molecular Dynamics Study to Understand Behavior of Corrosion Inhibitors in Bulk Aqueous Phase and Near Metal-Water Interface. Ph.D. Thesis, Ohio University, Athens, OH, USA, 2018. [Google Scholar]
- John, S.; Joseph, A.; Sajini, T.; James Jose, A. Corrosion Inhibition Properties of 1,2,4-Hetrocyclic Systems: Electrochemical, Theoretical and Monte Carlo Simulation Studies. Egypt. J. Pet. 2017, 26, 721–732. [Google Scholar] [CrossRef]
- El Ibrahimi, B.; Jmiai, A.; Bazzi, L.; El Issami, S. Amino Acids and Their Derivatives as Corrosion Inhibitors for Metals and Alloys. Arab. J. Chem. 2020, 13, 740–771. [Google Scholar] [CrossRef]
- Nishikawa, K.; Akiyama, H.; Yagishita, K.; Washizu, H. Molecular Dynamics Analysis of Adsorption Process of Anti-Copper-Corrosion Additives to the Copper Surface. arXiv 2018, arXiv:1812.10647. [Google Scholar]
- Yan, T.; Zhang, S.; Feng, L.; Qiang, Y.; Lu, L.; Fu, D.; Wen, Y.; Chen, J.; Li, W.; Tan, B. Investigation of Imidazole Derivatives as Corrosion Inhibitors of Copper in Sulfuric Acid: Combination of Experimental and Theoretical Researches. J. Taiwan Inst. Chem. Eng. 2020, 106, 118–129. [Google Scholar] [CrossRef]
- Tan, J.; Guo, L.; Wu, D.; Duan, X.; Leng, S.; Obot, I.B.; Kaya, S. Electrochemical and Computational Investigations on the Corrosion Inhibition of X65 Steel by 2-Phenylbenzimidazole in H2SO4 Solution. Int. J. Electrochem. Sci. 2020, 8837–8848. [Google Scholar] [CrossRef]
- Materials Studio Forcite Plus Datasheet; Dassault Systèmes, 2014.
- Djefaflia, R.; Lerari, D.; Bachari, K. Covid-19 and Nanoform Zinc Oxide Interaction: A Forcite Module of Materials Studio Software Study. Clay Res. 2019, 38, 43. [Google Scholar] [CrossRef]
- Hadi Al Hasan, N. Molecular Dynamic Simulation of the Density and Mechanical Properties of Polyvinyl Chloride(PVC)/High Density Polyethylene (HDPE) Composites Based on Materials Studio. J. Phys. Conf. Ser. 2019, 1294, 052062. [Google Scholar] [CrossRef]
- Eshaghi Malekshah, R.; Fahimirad, B.; Aallaei, M.; Khaleghian, A. Synthesis and Toxicity Assessment of Fe3O4 NPs Grafted by ∼ NH2-Schiff Base as Anticancer Drug: Modeling and Proposed Molecular Mechanism through Docking and Molecular Dynamic Simulation. Drug Deliv. 2020, 27, 1201–1217. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, Q.; Wang, Y.; Tang, J.; Wang, Y. A Study on Inhibition Performance of Mercaptoalcohols As Corrosion Inhibitors by First Principle and Molecular Dynamics Simulation. Russ. J. Phys. Chem. A 2020, 94, 1877–1886. [Google Scholar] [CrossRef]
- Rbaa, M.; Benhiba, F.; Dohare, P.; Lakhrissi, L.; Touir, R.; Lakhrissi, B.; Zarrouk, A.; Lakhrissi, Y. Synthesis of New Epoxy Glucose Derivatives as a Non-Toxic Corrosion Inhibitors for Carbon Steel in Molar HCl: Experimental, DFT and MD Simulation. Chem. Data Collect. 2020, 27, 100394. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Quraishi, M.A.; Kaya, S.; Guo, L. Aminoantipyrine Derivatives as a Novel Eco-Friendly Corrosion Inhibitors for P110 Steel in Simulating Acidizing Environment: Experimental and Computational Studies. J. Nat. Gas Sci. Eng. 2020, 83, 103547. [Google Scholar] [CrossRef]
- Rbaa, M.; Dohare, P.; Berisha, A.; Dagdag, O.; Lakhrissi, L.; Galai, M.; Lakhrissi, B.; Touhami, M.E.; Warad, I.; Zarrouk, A. New Epoxy Sugar Based Glucose Derivatives as Eco Friendly Corrosion Inhibitors for the Carbon Steel in 1.0 M HCl: Experimental and Theoretical Investigations. J. Alloys Compd. 2020, 833, 154949. [Google Scholar] [CrossRef]
- El-Hajjaji, F.; Merimi, I.; El Ouasif, L.; El Ghoul, M.; Achour, R.; Hammouti, B.; Belghiti, M.E.; Chauhan, D.S.; Quraishi, M.A. 1-Octyl-2-(Octylthio)-1H-Benzimidazole as a New and Effective Corrosion Inhibitor for Carbon Steel in 1 M HCl. Port. Electrochim. Acta 2019, 37, 131–145. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Sorour, A.A.; Saha, S.K.; Banerjee, P. Triazole-Modified Chitosan: A Biomacromolecule as a New Environmentally Benign Corrosion Inhibitor for Carbon Steel in a Hydrochloric Acid Solution. RSC Adv. 2019, 9, 14990–15003. [Google Scholar] [CrossRef]
- Farahati, R.; Ghaffarinejad, A.; Mousavi-Khoshdel, S.M.; Rezania, J.; Behzadi, H.; Shockravi, A. Synthesis and Potential Applications of Some Thiazoles as Corrosion Inhibitor of Copper in 1 M HCl: Experimental and Theoretical Studies. Prog. Org. Coat. 2019, 132, 417–428. [Google Scholar] [CrossRef]
- Kim, S. Issues on the Choice of a Proper Time Step in Molecular Dynamics. Phys. Procedia 2014, 53, 60–62. [Google Scholar] [CrossRef]
- Schneider, R.; Sharma, A.R.; Rai, A. Introduction to Molecular Dynamics. In Computational Many-Particle Physics; Springer: Berlin/Heidelberg, Germany, 2008; pp. 3–40. [Google Scholar] [CrossRef]
- Farahati, R.; Mousavi-khoshdel, S.M.; Ghaffarinejad, A.; Behzadi, H. Progress in Organic Coatings Experimental and Computational Study of Penicillamine Drug and Cysteine as Water-Soluble Green Corrosion Inhibitors of Mild Steel. Prog. Org. Coatings 2020, 142, 105567. [Google Scholar] [CrossRef]
- Wang, X.; Tang, C.; Wang, Q.; Li, X.; Hao, J. Selection of Optimal Polymerization Degree and Force Field in the Molecular Dynamics Simulation of Insulating Paper Cellulose. Energies 2017, 10, 1377. [Google Scholar] [CrossRef]
- Manssouri, M.; Znini, M.; Lakbaibi, Z.; Ansari, A.; El Ouadi, Y. Experimental and Computational Studies of Perillaldehyde Isolated from Ammodaucus Leucotrichus Essential Oil as a Green Corrosion Inhibitor for Mild Steel in 1.0 M HCl. Chem. Pap. 2020. [Google Scholar] [CrossRef]
- Ituen, E.; Mkpenie, V.; Dan, E. Surface Protection of Steel in Oil Well Acidizing Fluids Using L-Theanine-Based Corrosion Inhibitor Formulations: Experimental and Theoretical Evaluation. Surf. Interfaces 2019, 16, 29–42. [Google Scholar] [CrossRef]
- Obot, I.B.; Haruna, K.; Saleh, T.A. Atomistic Simulation: A Unique and Powerful Computational Tool for Corrosion Inhibition Research. Arab. J. Sci. Eng. 2019, 44, 1–32. [Google Scholar] [CrossRef]
- Akkermans, R.L.C.; Spenley, N.A.; Robertson, S.H. COMPASS III: Automated Fitting Workflows and Extension to Ionic Liquids. Mol. Simul. 2020. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Li, B.; Liu, S.P.; Liu, X.; Jiang, Q. Determining the Adsorption Energies of Small Molecules with the Intrinsic Properties of Adsorbates and Substrates. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Han, P.; He, Y.; Chen, C.; Yu, H.; Liu, F.; Yang, H.; Ma, Y.; Zheng, Y. Study on Synergistic Mechanism of Inhibitor Mixture Based on Electron Transfer Behavior. Sci. Rep. 2016, 6, 33252. [Google Scholar] [CrossRef]
- Huo, S.J.; He, J.M.; Chen, L.H.; Fang, J.H. Adsorption Configuration of Sodium 2-Quinoxalinecarboxylate on Iron Substrate: Investigation by in Situ SERS, XPS and Theoretical Calculation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 156, 123–130. [Google Scholar] [CrossRef]
- Oyekunle, D.T.; Agboola, O.; Ayeni, A.O. Corrosion Inhibitors as Building Evidence for Mild Steel: A Review. J. Phys. Conf. Ser. 2019, 1378. [Google Scholar] [CrossRef]
- Berrissoul, A.; Ouarhach, A.; Benhiba, F.; Romane, A.; Zarrouk, A.; Guenbour, A.; Dikici, B.; Dafali, A. Evaluation of Lavandula Mairei Extract as Green Inhibitor for Mild Steel Corrosion in 1 M HCl Solution. Experimental and Theoretical Approach. J. Mol. Liq. 2020, 313, 113493. [Google Scholar] [CrossRef]
- Boughoues, Y.; Benamira, M. RSC Advances Experimental and Theoretical Investigations of Four Amine Derivatives as e Ff Ective Corrosion Inhibitors for Mild Steel in HCl Medium. RSC Adv. 2020, 10, 24145–24158. [Google Scholar] [CrossRef]
- Satpati, S.; Saha, S.K.; Suhasaria, A.; Banerjee, P.; Sukul, D. Adsorption and Anti-Corrosion Characteristics of Vanillin Schiff Bases on Mild Steel in 1 M HCl: Experimental and Theoretical Study. RSC Adv. 2020, 10, 9258–9273. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Li, N.; Xie, X. Inhibition Effect of Bamboo Leaves Extract on Cold Rolled Steel in Cl3CCOOH Solution. J. Mater. Res. Technol. 2017, 6, 158–170. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Lin, T.; Xie, X.; Du, G. Cassava Starch Ternary Graft Copolymer as a Corrosion Inhibitor for Steel in HCl Solution. J. Mater. Res. Technol. 2020, 9, 2196–2207. [Google Scholar] [CrossRef]
- Laabaissi, T.; Benhiba, F.; Missioui, M.; Roui, Z.; Rbaa, M.; Oudda, H.; Ramli, Y.; Guenbour, A.; Warad, I.; Zarrouk, A. Coupling of Chemical, Electrochemical and Theoretical Approach to Study the Corrosion Inhibition of Mild Steel by New Quinoxaline Compounds in 1 M HCl. Heliyon 2020, 6, e03939. [Google Scholar] [CrossRef] [PubMed]
- Chaouiki, A.; Lgaz, H.; Salghi, R.; Chafiq, M.; Gaonkar, S.L.; Bhat, K.S.; Oudda, H.; Ali, I.H.; Chung, I. Inhibitory Effect of a New Isoniazid Derivative as an Effective Inhibitor for Mild Steel Corrosion in 1.0 M HCl: Combined Experimental and Computational Study. Res. Chem. Intermed. 2020, 46, 2919–2950. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Zhang, Y.; Wang, L.; Wu, Y.; Li, H. 9-Substituted Acridines as Effective Corrosion Inhibitors for Mild Steel: Electrochemical, Surface Morphology, and Computational Studies. New J. Chem. 2020, 44, 6464–6474. [Google Scholar] [CrossRef]
- Suhasaria, A.; Murmu, M.; Satpati, S.; Banerjee, P.; Sukul, D. Bis-Benzothiazoles as Ef Fi Cient Corrosion Inhibitors for Mild Steel in Aqueous HCl: Molecular Structure-Reactivity Correlation Study. J. Mol. Liq. 2020, 313, 113537. [Google Scholar] [CrossRef]
- Chugh, B.; Singh, A.K.; Thakur, S.; Pani, B.; Lgaz, H.; Chung, I.; Jha, R.; Ebenso, E.E. Comparative Investigation of Corrosion-Mitigating Behavior of Thiadiazole-Derived Bis-Schi Ff Bases for Mild Steel in Acid Medium: Experimental, Theoretical, and Surface Study. ACS Omega 2020, 5, 13503–13520. [Google Scholar] [CrossRef]
- Hrimla, M.; Bahsis, L.; Boutouil, A.; Laamari, M.R.; Stiriba, S. A Combined Computational and Experimental Study on the Mild Steel Corrosion Inhibition in Hydrochloric Acid by New Multifunctional Phosphonic Acid Containing 1, 2, 3-Triazoles. J. Adhes. Sci. Technol. 2020, 34, 1741–1773. [Google Scholar] [CrossRef]
- El-hajjaji, F.; Ech-chihbi, E.; Rezki, N.; Benhiba, F.; Taleb, M.; Singh, D.; Quraishi, M.A. Electrochemical and Theoretical Insights on the Adsorption and Corrosion Inhibition of Novel Pyridinium-Derived Ionic Liquids for Mild Steel in 1 M HCl. J. Mol. Liq. 2020, 314, 113737. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, Y.; Chen, L.; Wang, L.; Wu, Y.; Li, H. Aloe Polysaccharide as an Eco-Friendly Corrosion Inhibitor for Mild Steel in Simulated Acidic Oil Fi Eld Water: Experimental and Theoretical Approaches. J. Mol. Liq. 2020, 307, 112950. [Google Scholar] [CrossRef]
- Chaouiki, A.; Chafiq, M.; Rbaa, M.; Lgaz, H.; Salghi, R.; Lakhrissi, B.; Ali, I.H.; Masroor, S.; Cho, Y. New 8-Hydroxyquinoline-Bearing Quinoxaline Derivatives as Effective Corrosion Inhibitors for Mild Steel in HCl: Electrochemical and Computational Investigations. Coatings 2020, 10, 811. [Google Scholar] [CrossRef]
- Boughoues, Y.; Benamira, M.; Messaadia, L.; Ribouh, N. Adsorption and Corrosion Inhibition Performance of Some Environmental Friendly Organic Inhibitors for Mild Steel in HCl Solution via Experimental and Theoretical Study. Colloids Surf. A 2020, 593, 124610. [Google Scholar] [CrossRef]
- Aslam, R.; Mobin, M.; Aslam, J.; Lgaz, H.; Chung, I.M. Inhibitory Effect of Sodium Carboxymethylcellulose and Synergistic Biodegradable Gemini Surfactants as Effective Inhibitors for MS Corrosion in 1 M HCl. J. Mater. Res. Technol. 2019, 8, 4521–4533. [Google Scholar] [CrossRef]
- Basik, M.; Mobin, M. Chondroitin Sulfate as Potent Green Corrosion Inhibitor for Mild Steel in 1 M HCl. J. Mol. Struct. 2020, 1214, 128231. [Google Scholar] [CrossRef]
- Shahmoradi, A.R.; Talebibahmanbigloo, N.; Javidparvar, A.A.; Bahlakeh, G.; Ramezanzadeh, B. Studying the Adsorption/ Inhibition Impact of the Cellulose and Lignin Compounds Extracted from Agricultural Waste on the Mild Steel Corrosion in HCl Solution. J. Mol. Liq. 2020, 304, 112751. [Google Scholar] [CrossRef]
- Berrissoul, A.; Loukili, E.; Mechbal, N.; Benhiba, F.; Guenbour, A.; Dikici, B.; Zarrouk, A.; Dafali, A. Anticorrosion Effect of a Green Sustainable Inhibitor on Mild Steel in Hydrochloric Acid. J. Colloid Interface Sci. 2020, 580, 740–752. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Applying Detailed Molecular/ Atomic Level Simulation Studies and Electrochemical Explorations of the Green Inhibiting Molecules Adsorption at the Interface of the Acid Solution-Steel Substrate. J. Mol. Liq. 2020, 299, 112220. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Potential Role of a Novel Green Eco-Friendly Inhibitor in Corrosion Inhibition of Mild Steel in HCl Solution: Detailed Macro/Micro-Scale Experimental and Computational Explorations. Constr. Build. Mater. 2020, 245, 118464. [Google Scholar] [CrossRef]
- Goyal, M.; Vashist, H.; Kumar, S.; Bahadur, I. Acid Corrosion Inhibition of Ferrous and Non-Ferrous Metal by Nature Friendly Ethoxycarbonylmethyltriphenylphosphonium Bromide (ECMTPB): Experimental and MD Simulation Evaluation. J. Mol. Liq. 2020, 315, 113705. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).