Synthesis and Electrochemical Characterisation of Magnetite Coatings on Ti6Al4V-ELI
Abstract
:1. Introduction
2. Materials and Methods
2.1. Magnetite Coating Synthesis
2.2. Surface and Structural Characterisation
2.3. Electrochemical Characterization
3. Results and Discussion
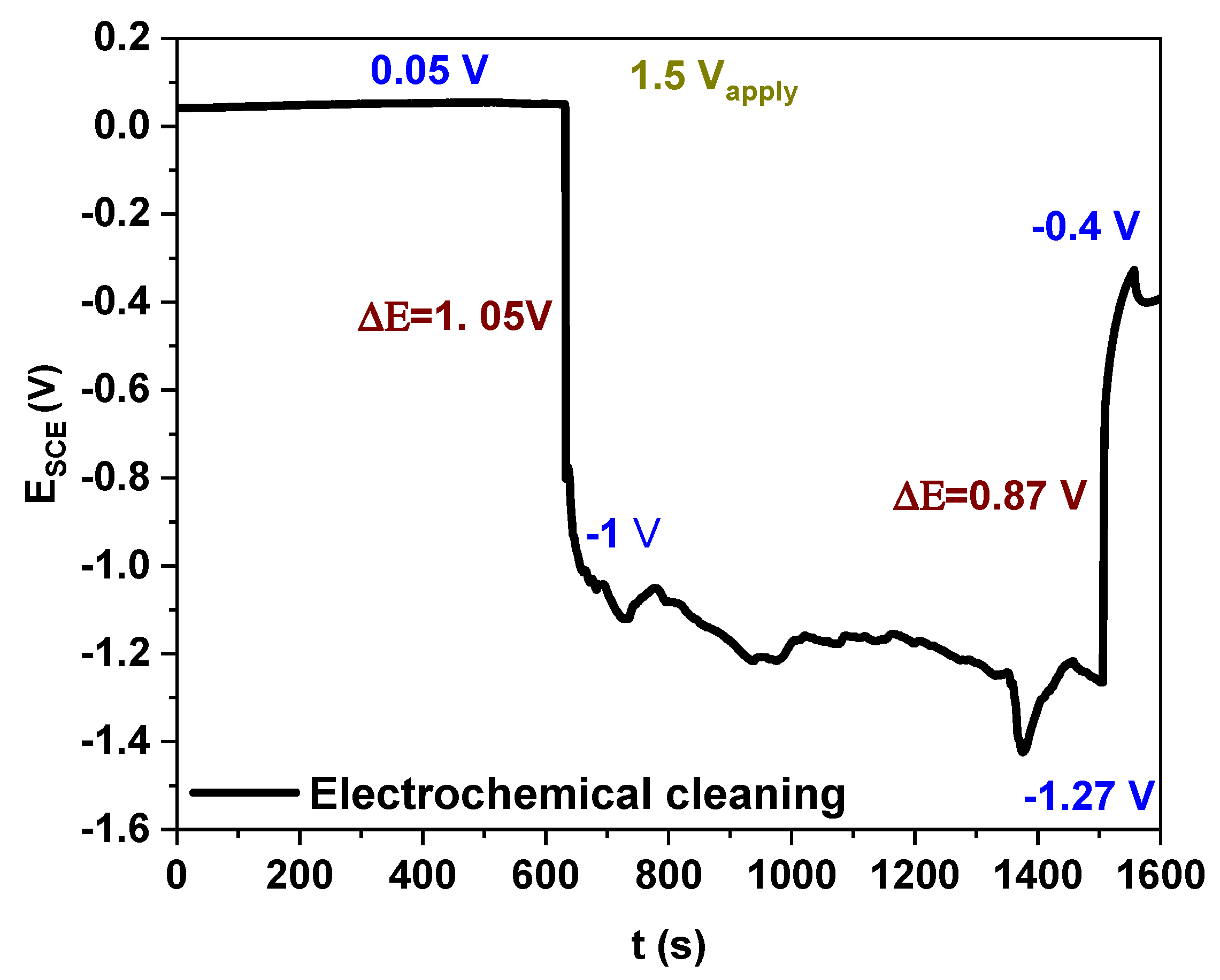
3.1. Magnetite Coatings Synthesis and Corrosion Potential Result
3.2. Surface and Structural Results
3.2.1. X-ray Diffraction (XRD)
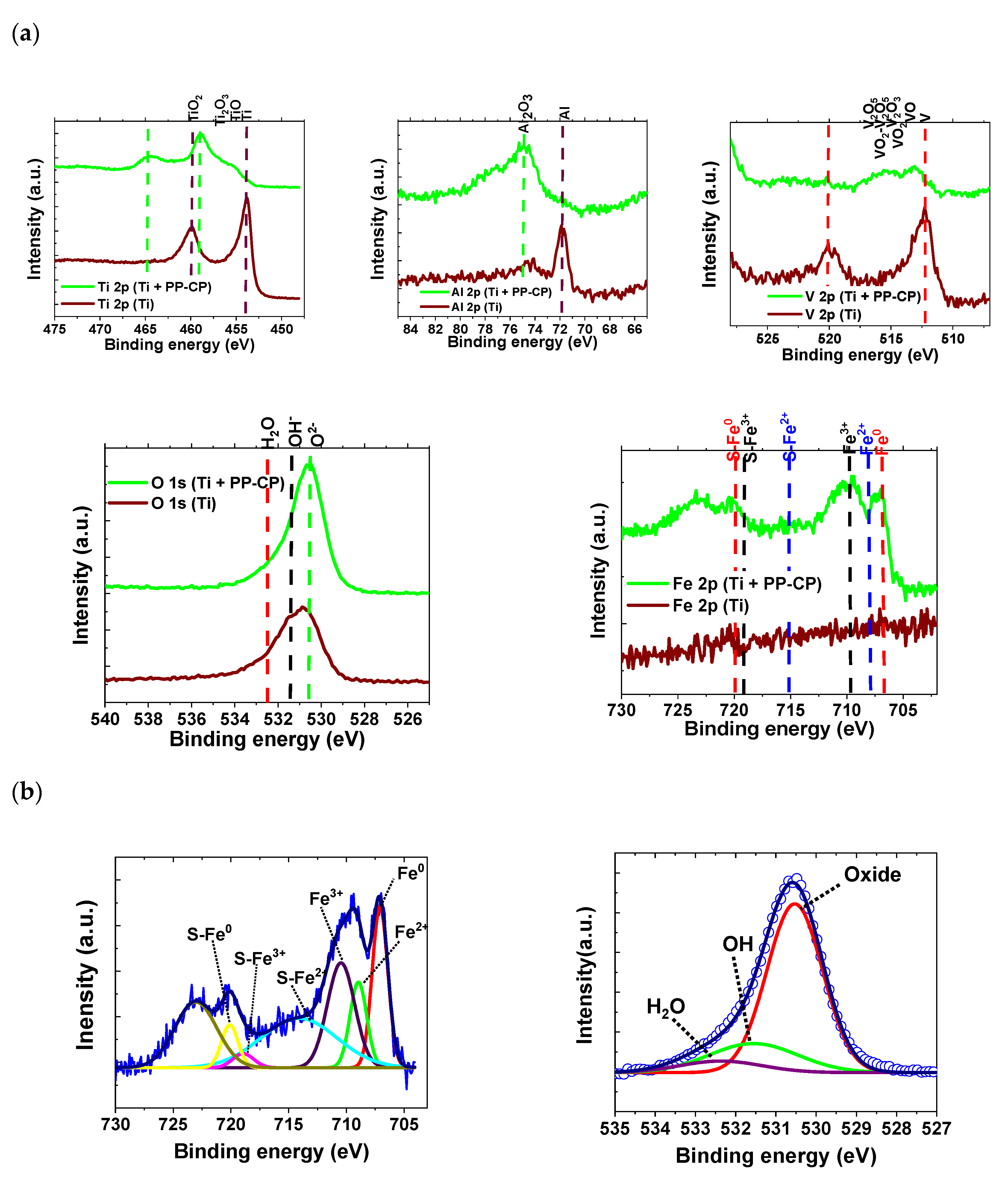
3.2.2. X-ray Photoelectron Spectroscopy (XPS)
3.2.3. Atomic Force Microscope (AFM)
3.2.4. Ellipsometry Results
3.3. Electrochemical Results
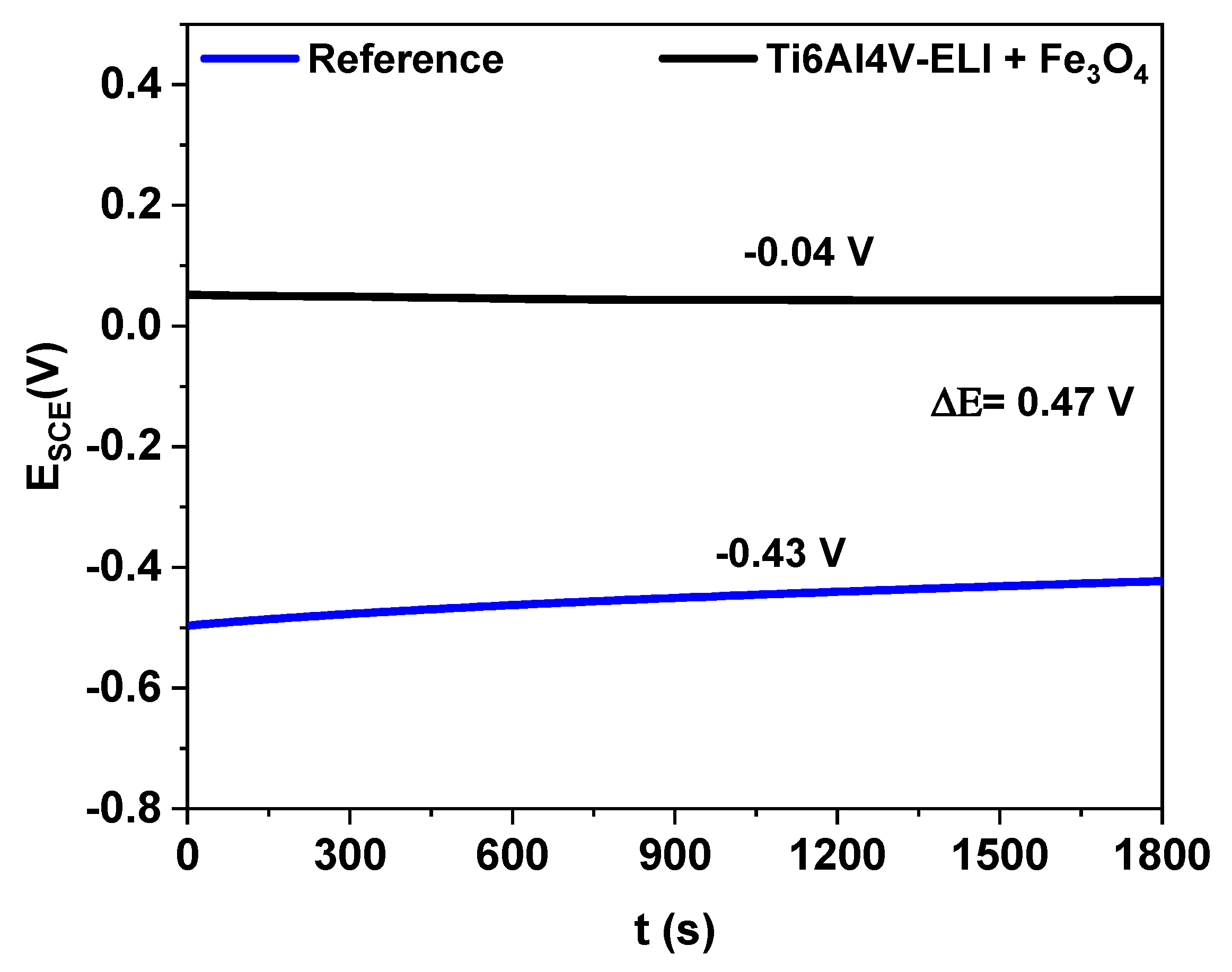
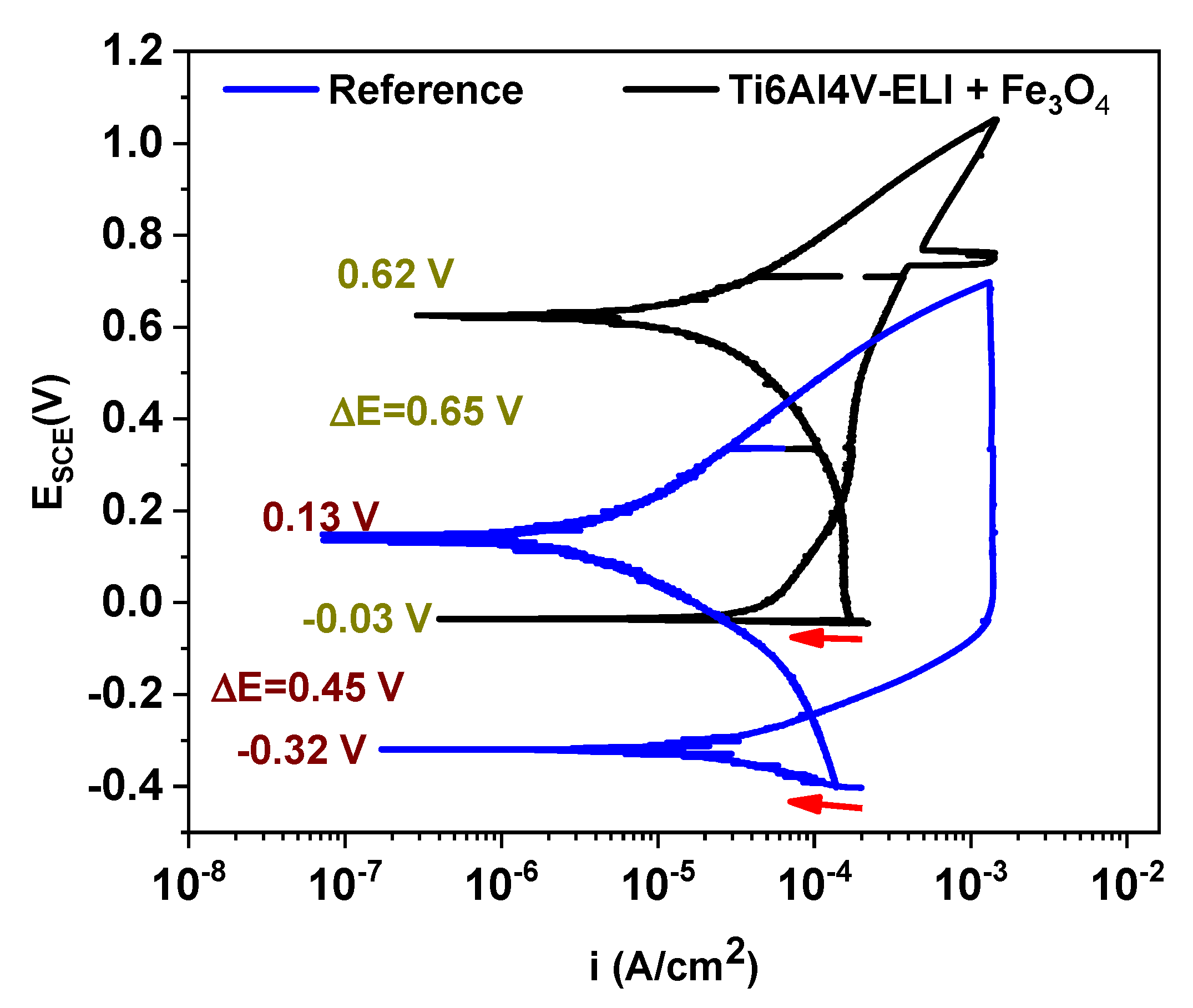
3.3.1. Corrosion Potential
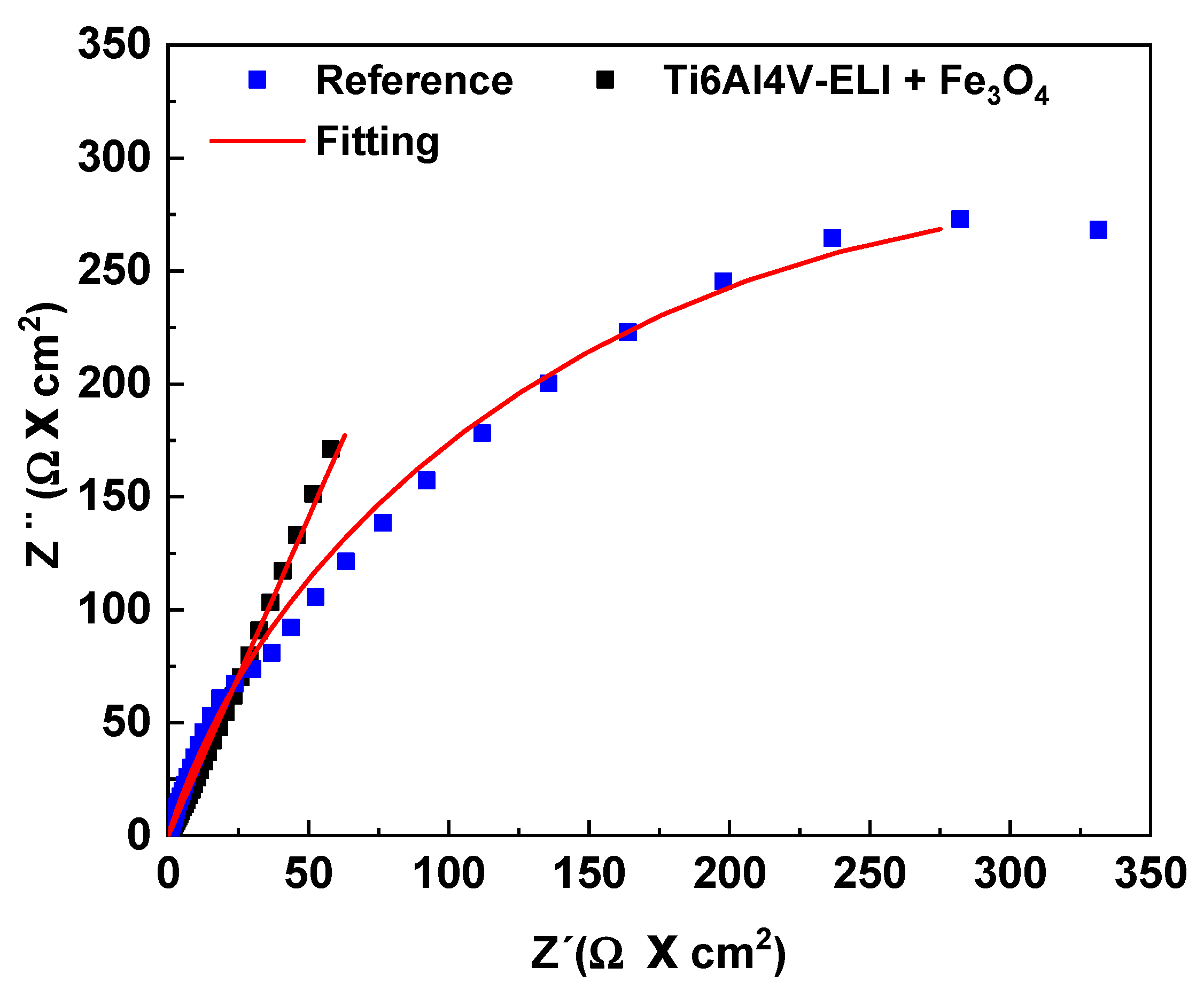
3.3.2. Electrochemical Impedance Spectroscopy (EIS)
3.3.3. Cyclic Polarisation Curves
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bitar, D.; Parvizi, J. Biological response to prosthetic debris. World J. Orthop. 2015, 6, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic Implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.J.; Anderson, S.; Stafford, T.; Glant, T.; Jacobs, J.J. Lymphocyte responses in patients with total hip arthroplasty. J. Orthop. Res. 2005, 23, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Sargeant, A.; Goswami, T. Hip implants: Paper V. Physiological effects. Mater. Des. 2006, 27, 287–307. [Google Scholar] [CrossRef]
- Vilardell, A.M.; Fredriksson, G.; Yadroitsev, I.; Krakhmalev, P. Fracture mechanisms in the as-built and stress-relieved laser powder bed fusion Ti6Al4V ELI alloy. Opt. Laser Technol. 2019, 109, 608–615. [Google Scholar] [CrossRef]
- Donachie, M.J. Titanium: A Technical Guide, 2nd ed.; ASM International: Materials Park, OH, USA, 2000. [Google Scholar]
- Moletsane, M.G.; Krakhmalev, P.; Kazantseva, N.; Du Plessis, A.; Yadroitsava, I.; Yadroitsev, I. Tensile Properties and microstructure of direct metal laser-sintered Ti6Al4V (ELI) alloy. S. Afr. J. Ind. Eng. 2016, 27, 110–121. [Google Scholar] [CrossRef] [Green Version]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Ye, X.; Cai, S.; Xu, G.; Dou, Y.; Hu, H.; Ye, X. Preparation and in vitro evaluation of mesoporous hydroxyapatite coated β-TCP porous scaffolds. Mater. Sci. Eng. C 2013, 33, 5001–5007. [Google Scholar] [CrossRef]
- Manilo, M.V.; Netreba, S.V.; Prokopenko, V.A.; Lebovka, N.I.; Barany, S. Overcharging of magnetite nanoparticles in electrolyte solutions. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 291–297. [Google Scholar] [CrossRef]
- Abe, S.; Ohnuma, S. Magnetite thin films containing a small amount of Ge. Appl. Phys. Express 2008, 1, 111304. [Google Scholar] [CrossRef]
- Alexe, M.; Ziese, M.; Hesse, D.; Esquinazi, P.; Yamauchi, K.; Fukushima, T.; Picozzi, S.; Gösele, U. Ferroelectric switching in multiferroic magnetite (Fe3O4) thin films. Adv. Mater. 2009, 21, 4452–4455. [Google Scholar] [CrossRef]
- Furubayashi, T. Magnetite films prepared by reactive evaporation. J. Magn. Magn. Mater. 2004, 272, E781–E783. [Google Scholar] [CrossRef]
- Chang, H.S.W.; Chiou, C.-C.; Chen, Y.-W.; Sheen, S.R. Synthesis, Characterization, and Magnetic Properties of Fe3O4Thin Films Prepared via a Sol–Gel Method. J. Solid State Chem. 1997, 128, 87–92. [Google Scholar] [CrossRef]
- Tang, N.J.; Zhong, W.; Jiang, H.Y.; Wu, X.L.; Liu, W.; Du, Y. Nanostructured magnetite (Fe3O4) thin films prepared by sol–gel method. J. Magn. Magn. Mater. 2004, 282, 92–95. [Google Scholar] [CrossRef]
- Chiba, M.; Morio, K.; Koizumi, Y. Microstructure and magnetic properties of iron oxide thin films by solid reaction. J. Magn. Magn. Mater. 2002, 239, 457–460. [Google Scholar] [CrossRef]
- Utkan, G.G.; Sayar, F.; Batat, P.; Ide, S.; Kriechbaum, M.; Pişkin, E. Synthesis and characterization of nanomagnetite particles and their polymer coated forms. J. Colloid Interface Sci. 2011, 353, 372–379. [Google Scholar] [CrossRef]
- Coral, D.F.; Mera, J.A. Una guía para el estudio de nanopartículas magnéticas de óxidos de hierro con aplicaciones biomédicas. Parte I. Ing. Cienc. 2017, 25, 229–249. [Google Scholar] [CrossRef] [Green Version]
- Lemine, O.M.; Omri, K.; Zhang, B.; El Mir, L.; Sajieddine, M.; Alyamani, A.; Bououdina, M. Sol–gel synthesis of 8 nm magnetite (Fe3O4) nanoparticles and their magnetic properties. Superlattices Microstruct. 2012, 52, 793–799. [Google Scholar] [CrossRef]
- Gazeau, F.; Bacri, J.C.; Gendron, F.; Perzynski, R.; Raikher, Y.L.; Stepanov, V.I.; Dubois, E. Magnetic resonance of ferrite nanoparticles: Evidence of surface effects. J. Magn. Magn. Mater. 1998, 186, 175–187. [Google Scholar] [CrossRef]
- Gilchrist, R.K.; Medal, R.; Shorey, W.D.; Hanselman, R.C.; Parrott, J.C.; Taylor, C.B. Selective Inductive Heating of Lymph Nodes. Ann. Surg. 1957, 146, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.V.; Prasad, M.J.N.V. Pulsed electrodeposition and hardness of microstructurally graded iron. Surf. Coat. Technol. 2018, 342, 121–128. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Holban, A.M.; Andronescu, E.; Mogoșanu, G.D.; Vasile, B.S.; Chifiriuc, M.C.; Lazăr, V.; Andrei, E.; Constantinescu, A.; Maniu, H. Anionic polymers and 10 nm Fe3O4@UA wound dressings support human foetal stem cells normal development and exhibit great antimicrobial properties. Int. J. Pharm. 2014, 463, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Anghel, I.; Grumezescu, A.M.; Holban, A.-M.; Ficai, A.; Anghel, A.G.; Chifiriuc, M.C. Biohybrid Nanostructured Iron Oxide Nanoparticles and Satureja hortensis to Prevent Fungal Biofilm Development. Int. J. Mol. Sci. 2013, 14, 18110–18123. [Google Scholar] [CrossRef] [PubMed]
- Anghel, I.; Holban, A.M.; Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Anghel, A.G.; Maganu, M.; Lazar, V.; Chifiriuc, M.-C. Modified wound dressing with phyto-nanostructured coating to prevent staphylococcal and pseudomonal biofilm development. Nanoscale Res. Lett. 2012, 7, 690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anghel, I.; Grumezescu, A.M.; Andronescu, E.; Anghel, A.G.; Ficai, A.; Saviuc, C.; Grumezescu, V.; Vasile, B.S.; Chifiriuc, M.C. Magnetite nanoparticles for functionalized textile dressing to prevent fungal biofilms development. Nanoscale Res. Lett. 2012, 7, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rădulescu, M.; Andronescu, E.; Holban, A.M.; Vasile, B.S.; Iordache, F.; Mogoantă, L.; Mogosanu, G.D.; Grumezescu, A.M.; Georgescu, M.; Chifiriuc, M.C. Antimicrobial Nanostructured Bioactive Coating Based on Fe3O4 and Patchouli Oil for Wound Dressing. Metals 2016, 6, 103. [Google Scholar] [CrossRef] [Green Version]
- Anjaneyulu, U.; Vijayalakshmi, U. Preparation and characterization of novel sol-gel derived hydroxyapatite/Fe3O4 composites coatings on Ti-6Al-4V for biomedical applications. Mater. Lett. 2017, 189, 118–121. [Google Scholar] [CrossRef]
- Jaafar, A.; Hecker, C.; Árki, P.; Joseph, Y. Sol-Gel Derived Hydroxyapatite Coatings for Titanium Implants: A Review. Bioengineering 2020, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Dufour, J.; Marrón, J.O.; Negro, C.; Latorre, R.; Formoso, A.; López-Mateos, F. Mechanism and kinetic control of the oxyprecipitation of sulphuric liquors from steel pickling. Chem. Eng. J. 1997, 68, 173–187. [Google Scholar] [CrossRef]
- Roonasi, P.; Holmgren, A. A study on the mechanism of magnetite formation based on iron isotope fractionation. In EPD Congress 2009: Proceedings of Sessions and Symposia Sponsored by the Extraction and Processing Division (EPD) of The Minerals, Metals & Materials Society (TMS); Held during TMS 2009 Annual Meeting & Exhibition, San Francisco, California, USA, February 15–19, 2009; Howard, S.M., Ed.; Minerals, Metals & Materials Society: Warrendale, PA, USA, 2009; pp. 829–836. [Google Scholar]
- Olowe, A.A.; Rezel, D.; Génin, J.M.R. Mechanism of formation of magnetite from ferrous hydroxide in aqueous corrosion processes. Hyperfine Interact. 1989, 46, 429–436. [Google Scholar] [CrossRef]
- Bullen, T.D.; White, A.F.; Childs, C.W.; Vivit, D.V.; Schulz, M.S. Demonstration of significant abiotic iron isotope fractionation in nature. Geology 2001, 29, 699–702. [Google Scholar] [CrossRef]
- Chávez-Díaz, M.P.; Escudero-Rincón, M.L.; Arce-Estrada, E.M.; Cabrera-Sierra, R. Osteoblast Cell Response on the Ti6Al4V Alloy Heat-Treated. Materials 2017, 10, 445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, R.; Wu, X.; Wang, X.; Ma, W.; Yuan, L.; Gao, L.; Huang, K.; Feng, S. Low-temperature hydrothermal fabrication of Fe3O4 nanostructured solar selective absorption films. Appl. Surf. Sci. 2018, 458, 629–637. [Google Scholar] [CrossRef]
- Wu, R.; Chen, X.-G.; Wei, J.Z.; Yang, Y.B.; Xia, Y.H.; Ma, X.B.; Yang, J.B. Interplay of shape and magnetocrystalline anisotropy in electrodeposited Fe3O4 films. J. Magn. Magn. Mater. 2014, 361, 107–111. [Google Scholar] [CrossRef]
- Patra, S.; Roy, E.; Madhuri, R.; Sharma, P.K. Fast and Selective Preconcentration of Europium from Wastewater and Coal Soil by Graphene Oxide/Silane@Fe3O4 Dendritic Nanostructure. Environ. Sci. Technol. 2015, 49, 6117–6126. [Google Scholar] [CrossRef]
- Milošev, I.; Metikoš-Huković, M.; Strehblow, H.-H. Passive film on orthopaedic TiAlV alloy formed in physiological solution investigated by X-ray photoelectron spectroscopy. Biomaterials 2000, 21, 2103–2113. [Google Scholar] [CrossRef]
- Milošev, I.; Kosec, T.; Strehblow, H.-H. XPS and EIS study of the passive film formed on orthopaedic Ti–6Al–7Nb alloy in Hank’s physiological solution. Electrochim. Acta 2008, 53, 3547–3558. [Google Scholar] [CrossRef]
- Lin, T.-C.; Seshadri, G.; Kelber, J.A. A consistent method for quantitative XPS peak analysis of thin oxide films on clean polycrystalline iron surfaces. Appl. Surf. Sci. 1997, 119, 83–92. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Bronstein, L.M.; Huang, X.; Retrum, J.; Schmucker, A.; Pink, M.; Stein, B.D.; Dragnea, B. Influence of Iron Oleate Complex Structure on Iron Oxide Nanoparticle Formation. Chem. Mater. 2007, 19, 3624–3632. [Google Scholar] [CrossRef]
- Fujii, T.; De Groot, F.M.F.; Sawatzky, G.A.; Voogt, F.C.; Hibma, T.; Okada, K. In Situ XPS analysis of various iron oxide films grown by NO2-assisted molecular-beam epitaxy. Phys. Rev. B 1999, 59, 3195–3202. [Google Scholar] [CrossRef] [Green Version]
- Fattah-Alhosseini, A.; Khan, H.Y.; Heidarpour, A. Comparison of anti-corrosive properties between hot alkaline nitrate blackening and hydrothermal blackening routes. J. Alloys Compd. 2016, 676, 474–480. [Google Scholar] [CrossRef]
- Montiel-García, A.; Bustamante, E.O.; Escudero-Rincón, M.; De La Cruz-Terrazas, E.C.; Torres-Huerta, A.M. Study of reinforcing steel corrosion behaviour treated by bluing and cerium chemical conversion treatments, part I: Conventional electrochemical techniques. Cem. Concr. Compos. 2018, 90, 202–217. [Google Scholar] [CrossRef]
- Onofre-Bustamente, E.; Domínguez-Crespo, M.A.; Genesca, J.; Rodriger-Gómez, F. Characteristics of blueing as an alternative chemical conversion treatment on carbon steel. Surf. Coat. Technol. 2007, 201, 4666–4676. [Google Scholar] [CrossRef]
- Martin, M.; Caballero, A.; Feliu, S. Propiedades protectoras de la capa de pavon en relacion con las condiciones de su obtención. Rev. Metall. 1972, 8, 179–183. [Google Scholar]
- Suresh, S.; Rangarajan, S.; Bera, S.; Krishnan, R.; Amirthapandian, S.; Sivakumar, M.; Velmurugan, S. Evaluation of corrosion resistance of nano nickel ferrite and magnetite double layer coatings on carbon steel. Thin Solid Films 2018, 645, 77–86. [Google Scholar] [CrossRef]
- Boukamp, B.A. Manual AC-Immittance Data Analysis System ‘Equivalent Circuit’, (Version 4.50); University of Twente: Enschede, The Netherlands, 1993. [Google Scholar]
- McDonald, J.R. Impedance Spectroscopy: Emphasizing Solid Materials and Systems; John Willey & Sons: New York, NY, USA, 1987. [Google Scholar]
- Lorenz, W.; Mansfeld, F. Determination of corrosion rates by electrochemical DC and AC methods. Corros. Sci. 1981, 21, 647–672. [Google Scholar] [CrossRef]
- Mosiałek, M.; Mordarski, G.; Nowak, P.; Simka, W.; Nawrat, G.; Hanke, M.; Socha, R.P.; Michalska, J. Phosphate–permanganate conversion coatings on the AZ81 magnesium alloy: SEM, EIS and XPS studies. Surf. Coat. Technol. 2011, 206, 51–62. [Google Scholar] [CrossRef]
- Jamesh, M.; Kumar, S.; Narayanan, T.S.N.S. Corrosion behavior of commercially pure Mg and ZM21 Mg alloy in Ringer’s solution—Long term evaluation by EIS. Corros. Sci. 2011, 53, 645–654. [Google Scholar] [CrossRef]


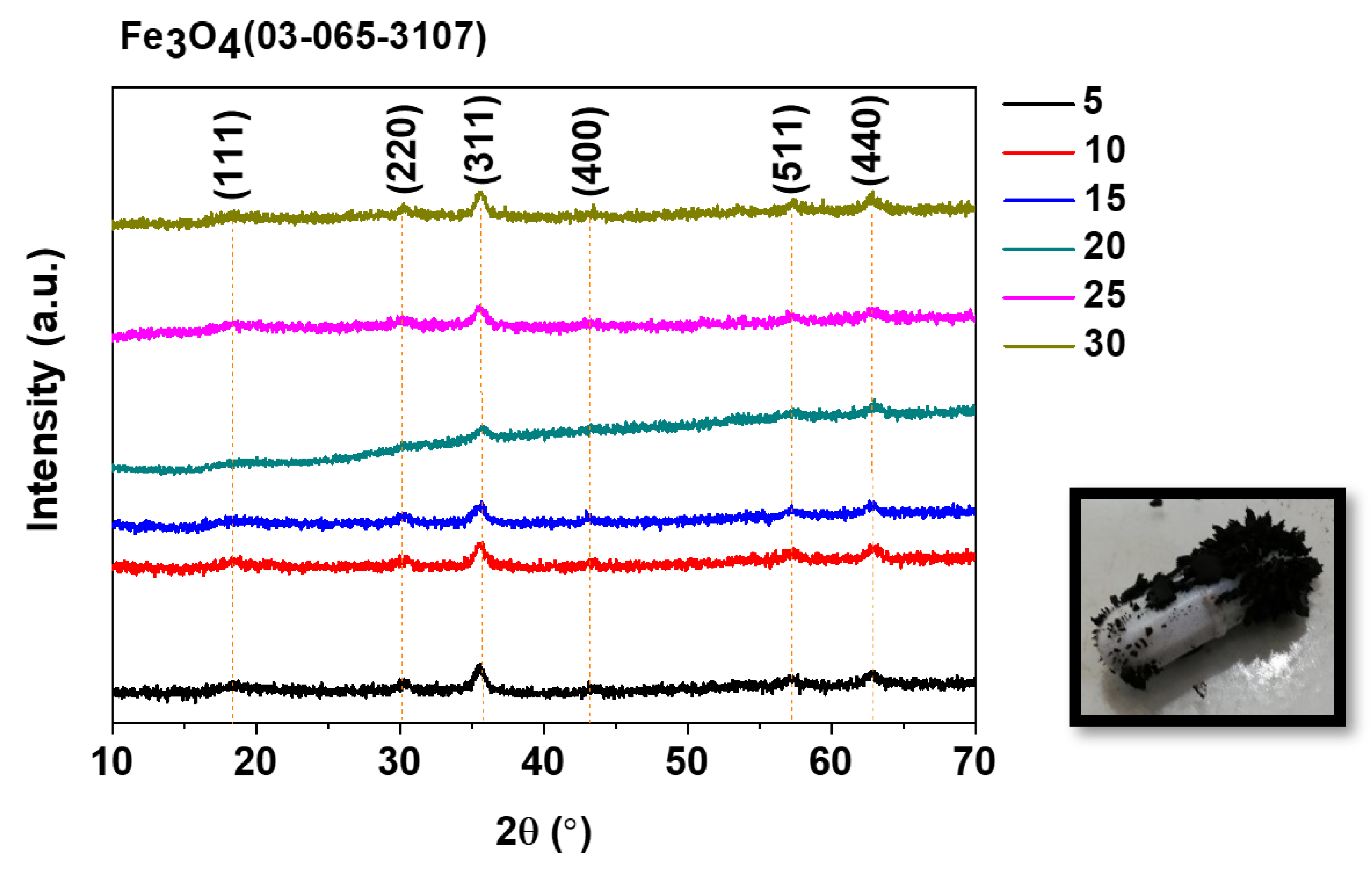

| 2θ | D (nm) t5 | D (nm) t10 | D (nm) t15 | D (nm) t20 | D (nm) t25 | D (nm) t30 |
|---|---|---|---|---|---|---|
| 35.5 | 9.86 | 9.64 | 8.7 | 7.98 | 9.17 | 9.4 |
| Fe 2p | Satellite | Satellites | O 1s | ||||||
|---|---|---|---|---|---|---|---|---|---|
| - | Fe0 | Fe2+ | Fe3+ | S-Fe0 | S-Fe2+ | S-Fe3+ | O2− | OH− | H2O |
| BE | 707.08 | 708.96 | 710.46 | 720.09 | 714.10 | 718.93 | 530.53 | 531.55 | 532.36 |
| FWHM | 1.58 | 1.71 | 2.78 | 1.69 | 7.71 | 2.16 | 1.63 | 2.58 | 2.37 |
| Area | 1.19 | 0.68 | 1.36 | 0.33 | 1.78 | 0.14 | 1.47 | 0.40 | 0.146 |
| Ti6Al4V-Eli + Fe3O4 | L1 (nm) | L2 (nm) | LT (nm) | X2 |
|---|---|---|---|---|
| Ti6Al4V-Eli + Fe3O4…middle | 209.8 | 71.4 | 281.2 | 14.6 |
| Ti6Al4V-Eli + Fe3O4…edge | 273.9 | 78.3 | 372.2 | 16.8 |
| Reference | Ti6Al4V-ELI+ Fe3O4 | |
|---|---|---|
| Ecorr (V) | −0.43 | 0.04 |
| Rs (Ω·cm2) | 44.33 | 42.86 |
| Rtc (Ω·cm2) | 711,070 | - |
| RFe3O4 (Ω·cm2) | - | 1 × 10+20 |
| CPE Fe3O4 (Ss) | 1.94 × 10−5 | - |
| CPE Fe3O4 (Ss) | - | 479 × 10−5 |
| ndl | 0.84 | - |
| nFe3O4 | - | 0.72 |
| Equivalent Circuit |  | 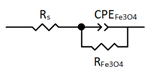 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montiel, A.; Bustamante, E.O.; Escudero, M.L. Synthesis and Electrochemical Characterisation of Magnetite Coatings on Ti6Al4V-ELI. Metals 2020, 10, 1640. https://doi.org/10.3390/met10121640
Montiel A, Bustamante EO, Escudero ML. Synthesis and Electrochemical Characterisation of Magnetite Coatings on Ti6Al4V-ELI. Metals. 2020; 10(12):1640. https://doi.org/10.3390/met10121640
Chicago/Turabian StyleMontiel, Adriana, Edgar Onofre Bustamante, and María Lorenza Escudero. 2020. "Synthesis and Electrochemical Characterisation of Magnetite Coatings on Ti6Al4V-ELI" Metals 10, no. 12: 1640. https://doi.org/10.3390/met10121640




