Abstract
In the present study, the mechanism of improving HAZ toughness of steel plate with Mg deoxidation after the simulated welding with the heat input of 400 kJ/cm was investigated through in situ observation, characterization with SEM-EDS and TEM-EDS, and thermodynamic calculation. It was found that intragranular acicular ferrite (IAF) and polygonal ferrite (PF) contributed to the improvements of HAZ toughness in steels with Mg deoxidation. With the increase of Mg content in steel, the oxide in micron size inclusion was firstly changed to MgO-Ti2O3, then to MgO with the further increase of Mg content in steel. The formation of nanoscale TiN particles was promoted more obviously with the higher Mg content in the steel. The growth rates of austenite grains at the high-temperature stage (1400~1250 °C) during the HAZ thermal cycle of steels with conventional Al deoxidation and Mg deoxidation containing 0.0027 and 0.0099 wt% Mg were 10.55, 0.89, 0.01 μm/s, respectively. It was indicated that nanoscale TiN particles formed in steel with Mg deoxidation were effective to inhibit the growth of austenite grain. The excellent HAZ toughness of steel plates after welding with a heat input of 400 kJ/cm could be obtained by control of the Mg content in steel to selectively promote the formation of IAF or retard the growth of austenite grain.
1. Introduction
In recent years, there is an increasing demand for steel plates with excellent weldability after high-heat-input welding used in the areas of shipbuilding, architectural construction, offshore structures, multipurpose gas carriers, and pipeline fields [1]. During the thermal cycle of high-heat-input welding, the base metal near the fusion line is heated to 1400 °C or higher for a considerable time. In addition, it undergoes a rather low cooling rate in the subsequent temperature zone of phase transformation. Thus, its microstructure becomes coarse and then forms a coarse-grained heat-affected zone (CGHAZ), which is a local brittle zone resulting in the deterioration in its toughness.
Nowadays, oxide metallurgy technology has been considered as an effective method to improve the heat-affected zone (HAZ) toughness of steel plates after high-heat-input welding [2,3,4]. The oxide metallurgy technology is to make use of oxide particles or other kinds of inclusions and precipitate particles as the nucleation sites of intragranular acicular ferrite (IAF), or for pinning the growth of austenite grain in HAZ of steel plate during the welding process. It is widely accepted that the single-phase inclusions such as the single oxide (Al2O3, MnO, and SiO2) and the isolated sulfide (MnS) are difficult to nucleate IAF [5]. However, the complex inclusions (oxy-sulfides) have been termed as effective nucleants for IAF [6]. The Ti2O3 inclusions play a key role in inducing the nucleation of IAF, resulting from the formation of Mn-depleted zone (MDZ) near the interface between inclusion and steel matrix [7,8,9,10]. However, it is not easy to produce the fine Ti2O3 particles in a large amount because most of the Ti2O3 particles are easy to cluster and float out.
The inclusions after Mg treatment show a stronger tendency to disperse evenly [11]. It has certainly been known that the complex deoxidation of Ti followed by the strong deoxidant (Mg and Zr) is a promising method to precisely control the composition, size, and number of deoxidation particles [12]. Mg deoxidation is an effective method to control inclusions, contributing to the refine, dispersion, and modification of Ti-based inclusions [13,14]. In the Ti-containing steel with the addition of an appropriate amount of Mg, the well-developed IAF microstructures were formed in HAZs by MgO-Ti2O3+MnS inclusions acting as the effective nucleants of IAF [15]. As a result, the excellent HAZ toughness was obtained by inclusion control with Mg deoxidation [16]. It was also reported that the Ti2O3 content in inclusions was decreased with the increase of Mg content in steel [13,14]. However, the influence of Mg content on the behavior of Ti-based inclusions in HAZ microstructural control should be studied further.
Inhibition of the coarsening of austenite grains is also an effective method to improve HAZ toughness [17]. Kato et al. [18] found that Ca addition inhibited the growth of TiN particles and increased the number of fine TiN particles, resulting in a fine-grained microstructure of HAZ and excellent HAZ toughness. Compared with the nitrides of Al, Nb, or V, titanium nitride (TiN) particles have higher thermal stability, which is one of the most effective particles in pinning the austenite grain boundaries [19,20]. Mg deoxidation was also used to improve the HAZ toughness by the refinement of austenite grains [21,22]. Zhu et al. [23] found that Mg deoxidation was effective to promote the formation of TiN particles with the sizes of 20–200 nm. In addition, with the increase of Mg content in steel, the sizes of austenite grains in HAZ after high-heat-input welding of 400 kJ/cm decreased [24]. It is currently unclear about the influence of Mg content on characteristics of TiN particles in HAZs of steel plates after high-heat-input welding. Furthermore, the relationship between TiN particles and HAZ toughness is needed to be illustrated.
Nowadays, in situ observation with high-temperature laser scanning confocal microscopy (HT-LSCM) has been utilized extensively to investigate the behavior of austenite grain growth during the thermal cycle of HAZ [25,26]. Wan et al. [27] found that the growth process of austenite grains in Ti-micro-alloyed steel was a continuous process during heating, isothermal holding, and cooling in the simulated HAZ thermal cycling. Zhu et al. [28] reported that the austenite grain size of steel with Mg deoxidation could hold the fine-grained structure after 1400 °C heating for 300 s. This inhibition of austenite grain growth was mainly attributed to the formation of pinning particles after the addition of Mg, resulting in the improvement of HAZ toughness [23]. To clarify the austenite grain growth behavior during the HAZ thermal cycle is helpful to deeply understand the mechanism of improving the HAZ toughness of steel plate with Mg deoxidation.
In the present work, two kinds of experimental steels have been developed based on the principle of oxide metallurgy technology. The roles of micron size inclusions and nanoscale particles on improving the HAZ toughness of steel plate after high-heat-input welding were investigated. Moreover, the growth behavior of austenite grains during the thermal cycles of HAZ was studied by use of HT-LSCM. Furthermore, the effect of Mg content in steel on the control mechanism of formation IAF and the inhibition of austenite grain growth was comprehensively illustrated.
2. Experimental Methods
2.1. Experimental Steel Preparation
Table 1 shows the chemical compositions of three experimental steel samples with conventional Al deoxidation, low-content Mg deoxidation, and high-content Mg deoxidation termed as A, LM, and HM, respectively. A 50 kg vacuum induction furnace with the sintered magnesia lining was used to melt those steel samples. The proper amount of deoxidants, Mn, Si, Al, Ti, and Mg were added to obtain the target steel compositions. Then, the melt was cast into an ingot with the size of 120 × 180 × 240 mm3. Each ingot was then hot rolled into a steel plate of the thickness of 50 mm.

Table 1.
Chemical compositions of experimental steels (wt%).
2.2. Welding Thermal Simulation Experiments
To evaluate the HAZ toughness of the experimental steel plates, the simulating welding experiments were carried out by use of a Gleeble 3800 thermal simulation tester (Dynamic system INC., Poestenkill, NY, USA). The steel sample for simulating welding experiments were with the size of 11 × 11 × 71 mm3. The peak temperature of the thermal cycle was 1400 °C and lasted for 3 s, followed by cooling from 800 to 500 °C in 385 s, which was designed to simulate the HAZ of an electrogas arc welding with heat input of 400 kJ/cm for a 50 mm thick plate. The Charpy absorbed energy of HAZs were measured at −20 °C.
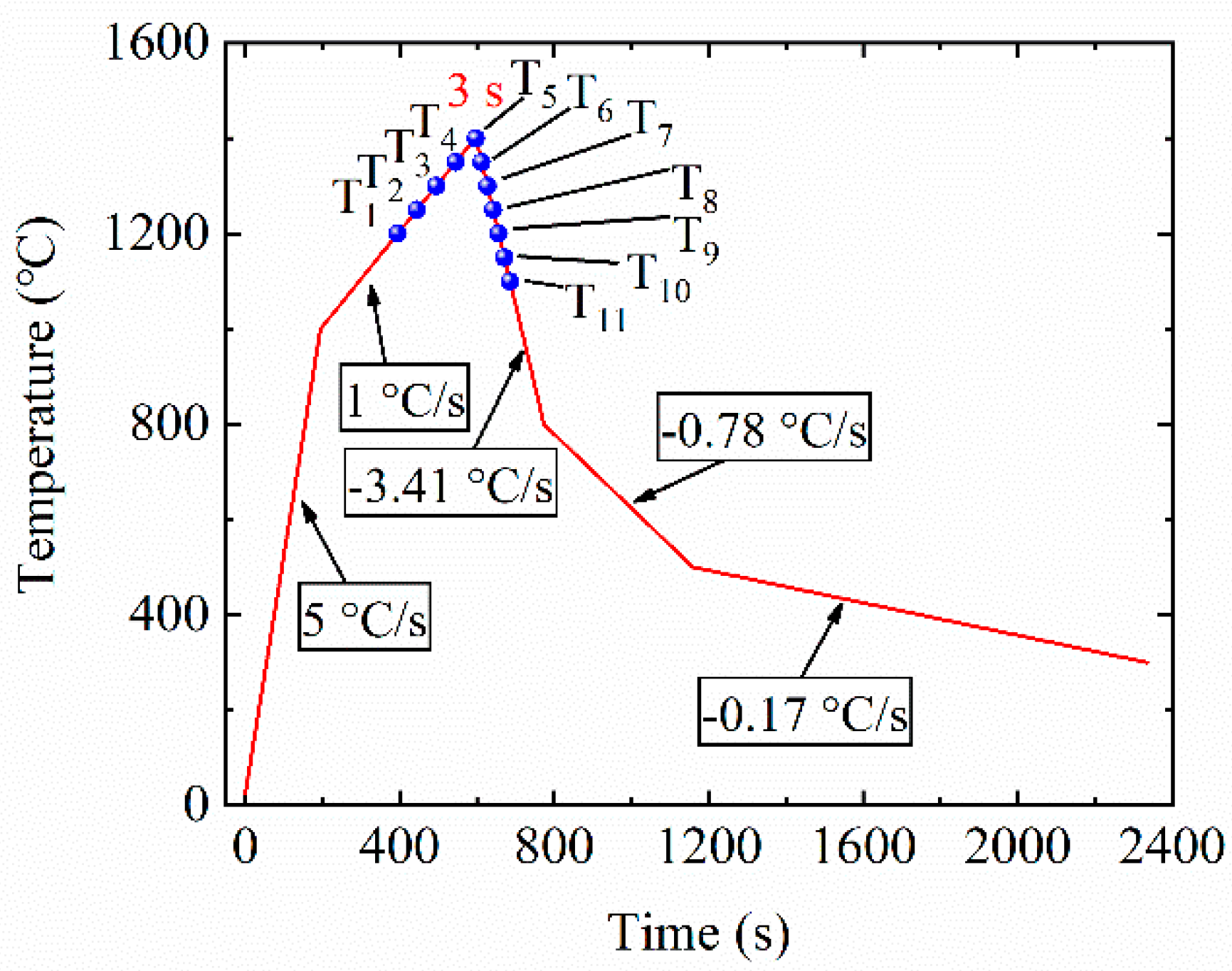
The growth behavior of austenite grains during the thermal cycles in HAZ of 50 mm thick steel plate after simulated welding with a heat input of 400 kJ/cm was studied through in situ observation with HT-LSCM (VL2000DX, Lasertech Corporation, Yokohama, Japan). A more detailed description was given elsewhere [24]. Figure 1 shows the thermal cycle of in situ observation experiment. As shown in Figure 1, the size of austenite grains was measured at 11 selected temperature points that were termed as T1~T11, respectively. All the temperature intervals were 50 °C. Five points (T1~T5) among them were from 1200 to 1400 °C during the heating stage and six points (T6~T11) during the cooling stage from 1350 to 1100 °C. Then, the sizes of austenite grains were measured with the image-pro plus software by the method of averaging the long axis and short axis in the grain from the screenshots observed during in situ observation.
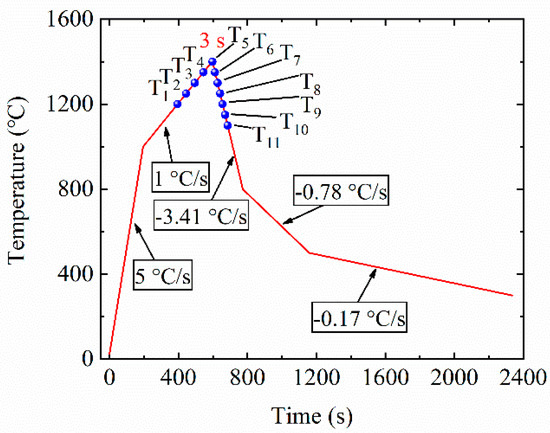
Figure 1.
Thermal cycle of in situ observation experiment simulated heat-affected zone (HAZ) during high-heat-input welding by HT-LSCM.
2.3. Microstructure and Inclusion/Precipitate Analysis
The surfaces in parallel with the fracture cross-sections of HAZ after the Charpy impact test were polished and then etched by using a 4% Nital solution. The characteristics of inclusions and the microstructure in HAZ of the steel samples were investigated by scanning electron microscopy (SEM, EVO MA25, Carl Zeiss, Oberkochen, Germany) equipped with energy dispersive spectrometer (EDS, Oxford Instruments, Oxon, UK) and optical microscopy (OM, DM 2500M, Leica Microsystems, Wetzlar, Germany).
Thin carbon film was deposited on HAZ samples and then extracted. The characteristics of nanoscale particles collected by carbon replica were observed by transmission electron microscopy (TEM, JEM-2100, JEOL Ltd., Tokyo, Japan) operated at 200 kV.
3. Experimental Results
3.1. Microstructure and Toughness of Base Metal and HAZ
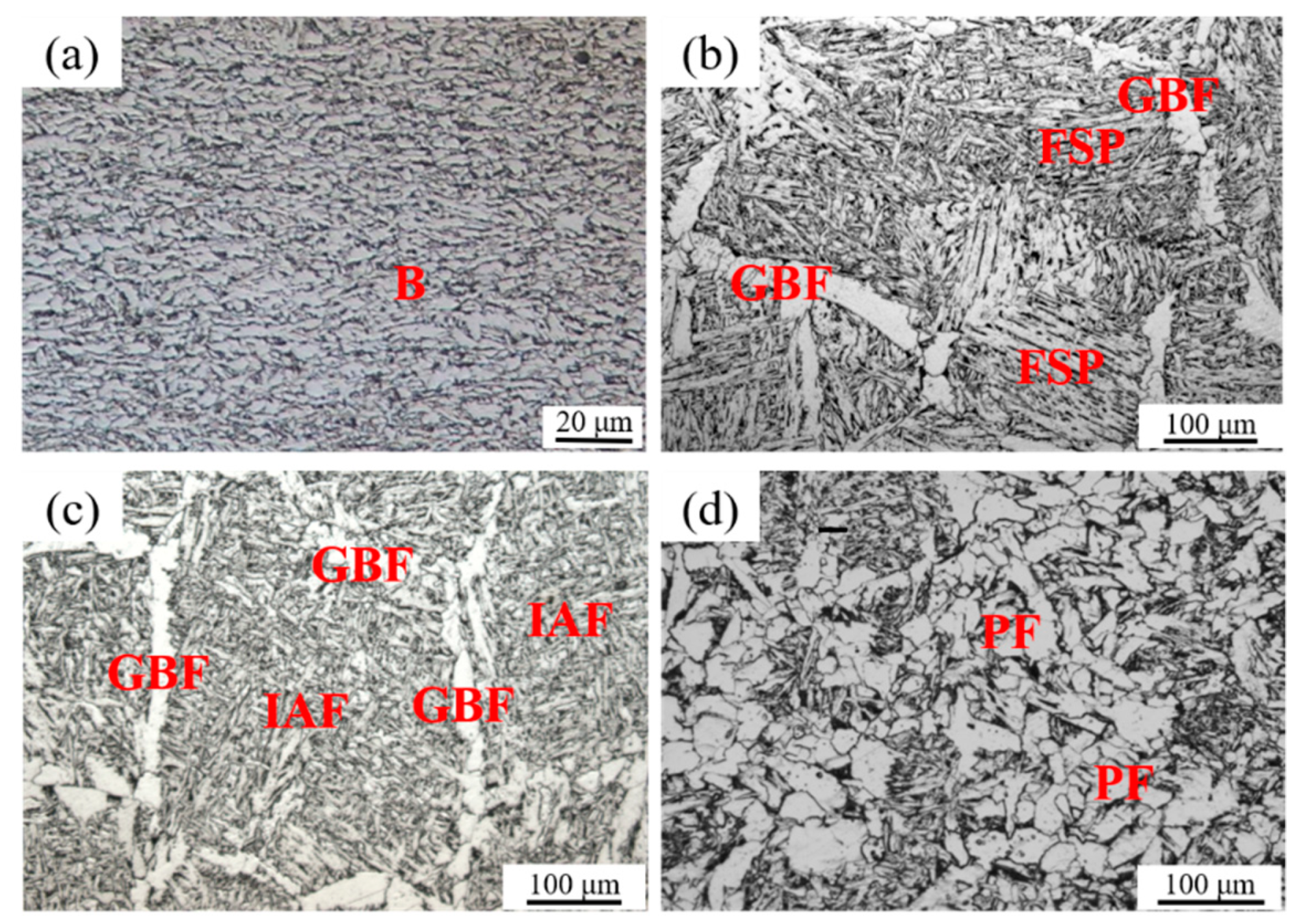
Experimental steel samples had similar chemical compositions and were manufactured by the same TMCP process. The optical microstructures of base metals of all three experimental steel samples were fine bainite (B), as shown in Figure 2a. The optical HAZ microstructures of A, LM, and HM after the simulated high-heat-input welding are illustrated in Figure 2b–d, respectively. The microstructure has changed completely after the thermal cycle of HAZ. It is seen from Figure 2b that the HAZ microstructure of A was mainly composed of the coarse grain boundary ferrites (GBFs) and the parallel ferrite side plates (FSPs). Differently, the HAZ microstructure of LM mainly consisted of the fine interlocked IAFs and the narrow GBFs, as shown in Figure 2c. In HM, the HAZ microstructures mainly contained the polygonal ferrites (PFs), as well as a few IAFs, as shown in Figure 2d.
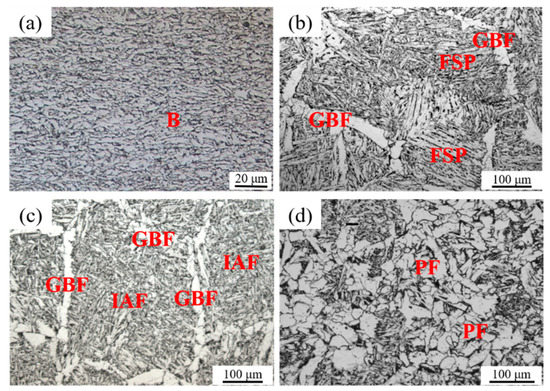
Figure 2.
Microstructures of base metal (a) and HAZs after simulated high-heat-input welding of 400 kJ/cm for (b) Al deoxidation (A), (c) low-content Mg deoxidation (LM), and (d) high-content Mg deoxidation (HM).
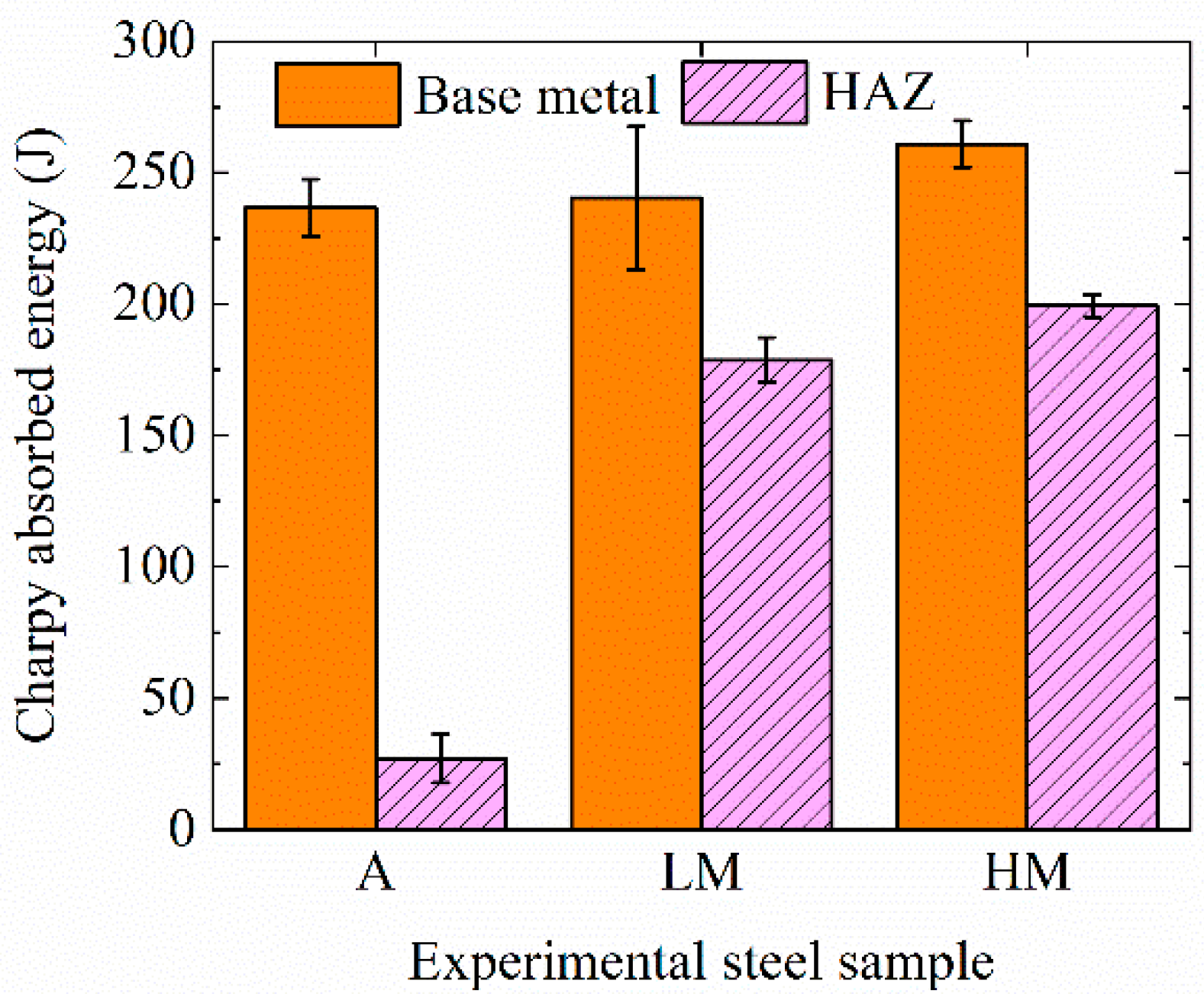
Figure 3 shows the impact toughness of base metals at −40 °C and HAZs at −20 °C of experimental steel samples. The average Charpy absorbed energies of base metals of A, LM, and HM were 227, 240, and 261 J, respectively. Base metals of all three steel samples had excellent impact toughness, resulting from fine bainite microstructures, as shown in Figure 2a. The average Charpy absorbed energies of HAZs of A, LM, and HM are 27, 179, and 199 J, respectively. The values were 88.59%, 25.66%, and 23.63% lower than those of base metals, respectively. Consequently, the HAZ toughness of A was greatly deteriorated and the steel samples with Mg deoxidation, LM and HM, still had excellent HAZ toughness after high-heat-input welding.
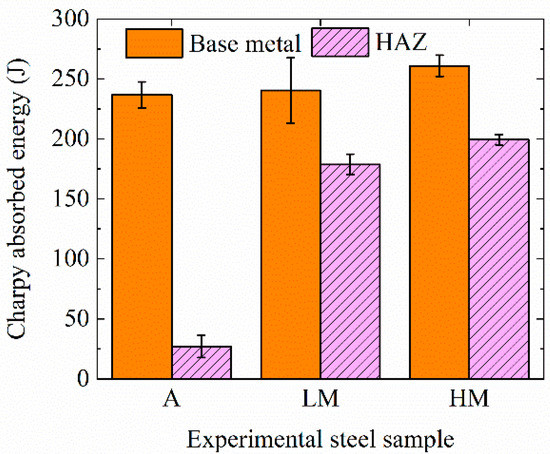
Figure 3.
Impact toughness of base metals at −40 °C and HAZs at −20 °C of experimental steel samples.
3.2. Characteristics of Inclusions/Precipitates in HAZ Microstructures
3.2.1. Characteristics of Micron Size Inclusions
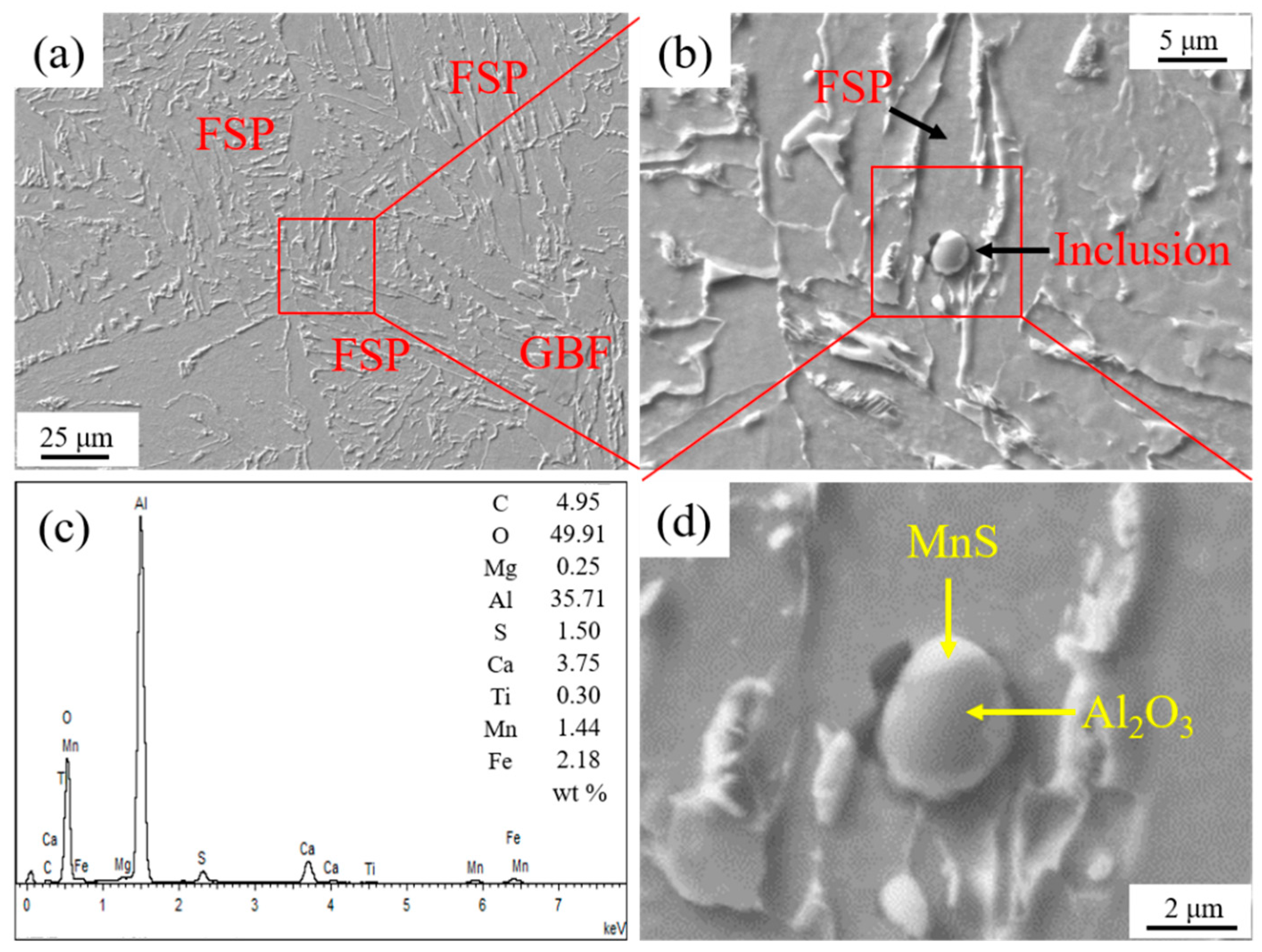
Figure 4 shows the inclusion related to the HAZ microstructure in experimental steel A. It is seen in Figure 4a,b that an ellipsoidal inclusion with the size about 3.30 μm was located in an FSP. According to the EDS analysis result, as shown in Figure 4c, its main component was Al2O3 and MnS. This complex inclusion was composed of the central Al2O3 part and the MnS phase covering outside, as shown in Figure 4d.
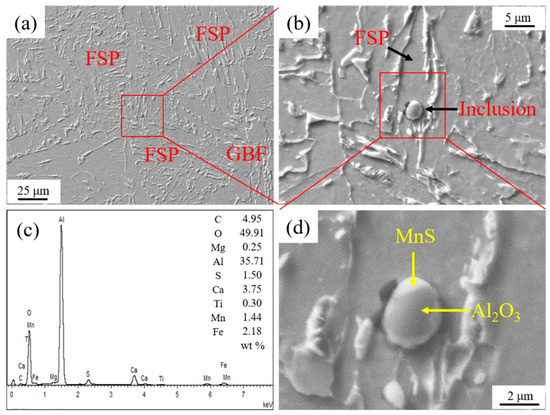
Figure 4.
Inclusion related to HAZ microstructure in experimental steel A. (a) HAZ microstructure, (b) inclusion, (c) energy dispersive spectrometer (EDS) analysis result of inclusion, and (d) component of inclusion.
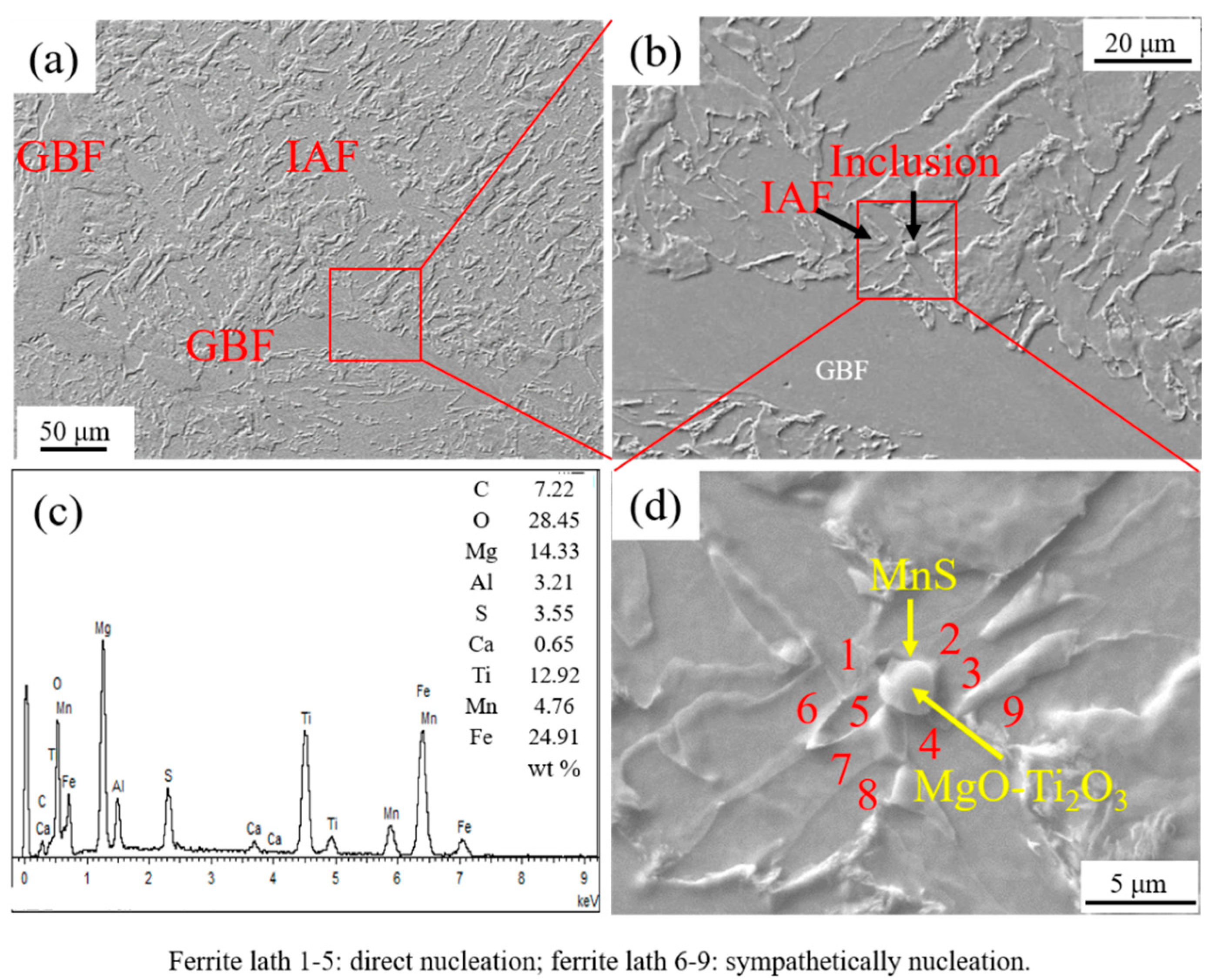
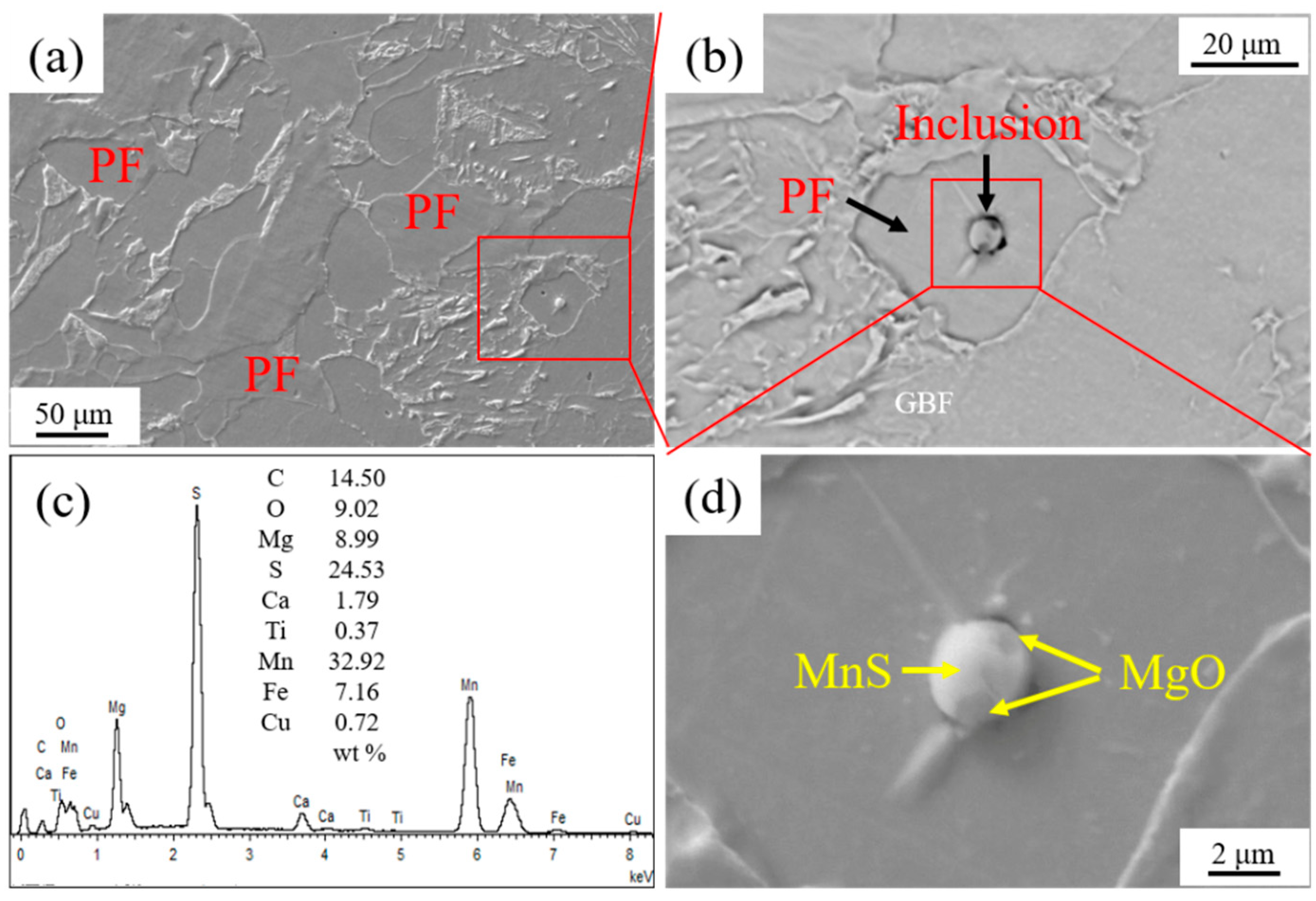
Figure 5 shows the IAFs nucleated by intragranular inclusion nearby GBF in HAZ of the experimental steel LM. It is seen that a near-spherical MgO-Ti2O3 + MnS complex inclusion with the size around 2.70 μm acted as the directly heterogeneous nucleation site of five acicular ferrite plates. Moreover, as shown in Figure 5d, three acicular ferrite plates were nucleated sympathetically at the broad face of the pre-formed acicular ferrite laths [20,21]. Figure 6 shows the inclusion related with the microstructure in HAZ of the experimental steel HM. It is found that the spherical MgO + MnS complex inclusion with a size of about 2.80 μm was located in a PF. This kind of complex inclusion contained two MgO particles with sizes less than 0.50 μm entrapped in a continuous MnS phase.
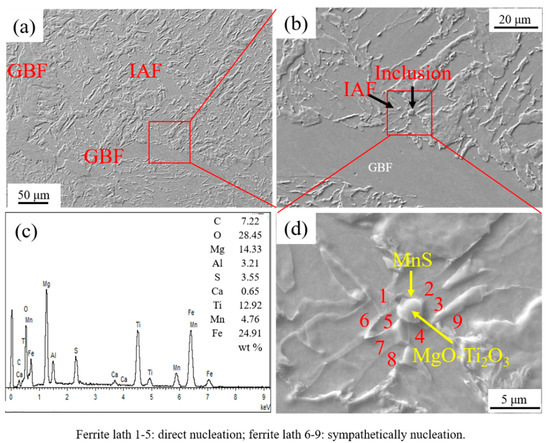
Figure 5.
Intragranular acicular ferrites (IAFs) nucleated by intragranular inclusion nearby grain boundary ferrite (GBF) in HAZ of experimental steel LM. (a) HAZ microstructure, (b) inclusion, (c) energy dispersive spectrometer (EDS) analysis result of inclusion, and (d) component of inclusion.
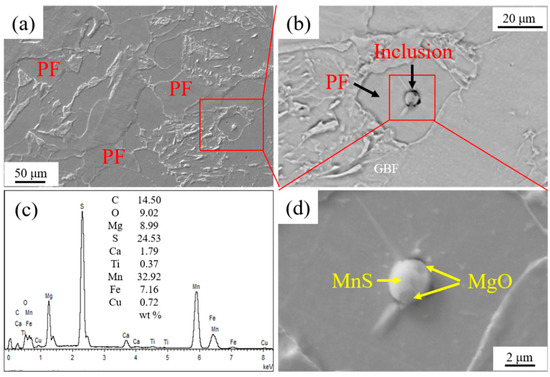
Figure 6.
Inclusion related to microstructure in HAZ of experimental steel HM. (a) HAZ microstructure, (b) inclusion, (c) energy dispersive spectrometer (EDS) analysis result of inclusion, and (d) component of inclusion.
3.2.2. Characteristics of Nanoscale Particles
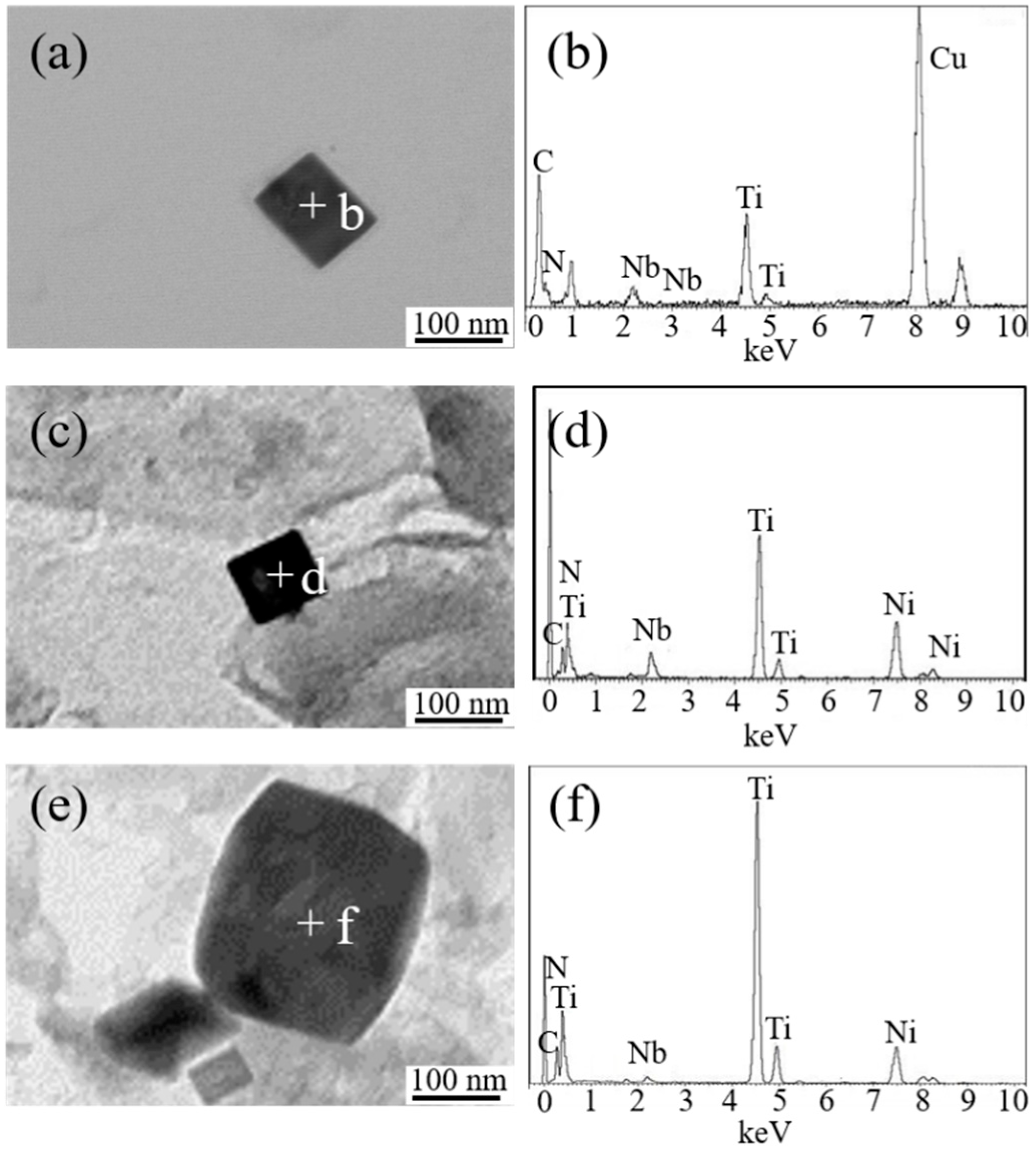
Figure 7 shows the typical TEM images and the corresponding EDS analysis of nanoscale particles in simulated HAZs of experimental steel samples. It is observed that nanoscale precipitates in A, LM, and HM were all rectangular TiN particles containing a small amount of Nb (C, N).
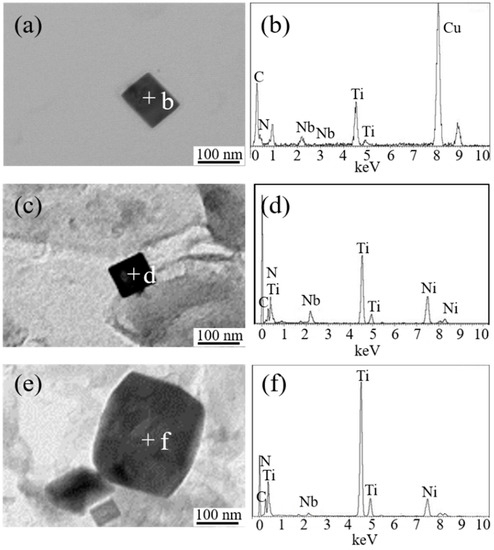
Figure 7.
TEM image and corresponding energy dispersive spectrometer (EDS) analysis of the nanoscale particles in simulated HAZs. (a,b) A, (c,d) LM, and (e,f) HM.
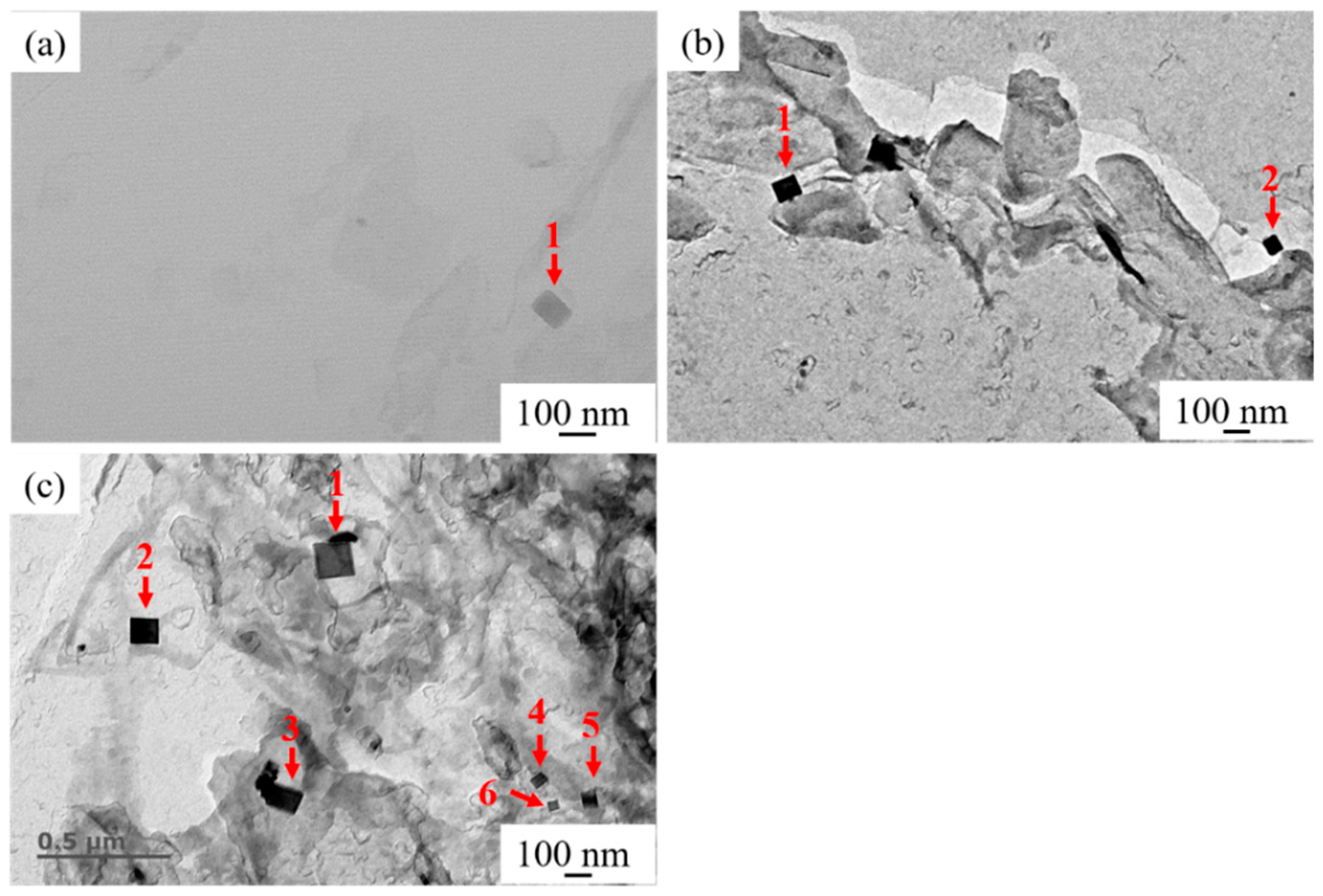
Figure 8 shows the typical distribution of nanoscale TiN particles in HAZs of experimental steel samples collected by carbon replica. Those fields of view were observed by TEM at 50,000 times. It can be seen from Figure 8a that only one regular TiN particle with a size of 56 nm could be observed in the field of view. As shown in Figure 8b, two regular TiN particles could be observed in the typical field of view in the HAZ of LM, which were marked as 1 and 2 and with sizes of 88 and 62 nm, respectively. It is seen in Figure 8c that six regular TiN particles marked as 1–6 with the sizes of 135, 103, 98, 45, 63, and 37 nm could be observed in the typical field of view in HAZ of HM. Compared with A and LM as shown in Figure 8a,b, respectively, it can be seen that the number of nanoscale TiN particles in the HAZ of HM was significantly more than those of A and LM. It can be inferred that when the Mg content in Mg deoxidized steel was at a low level (0.0027%), compared with steel with conventional Al deoxidation, Mg deoxidation did not play a significant role in promoting the formation of nanoscale TiN particles. However, when Mg content was at a high level (0.0099%), it played a significant role in promoting the formation of nanoscale TiN particles, even after high-heat-input welding. These fine inclusions can prevent the movement of the austenite grain boundary by the pinning effect and then restrain the austenite grain growth during the welding process [24]. In the present study, only a qualitative explanation is given to clarify the characteristics of nanoscale precipitates in HAZs of all three steel samples. The quantitative statistical results will be available in our further study.
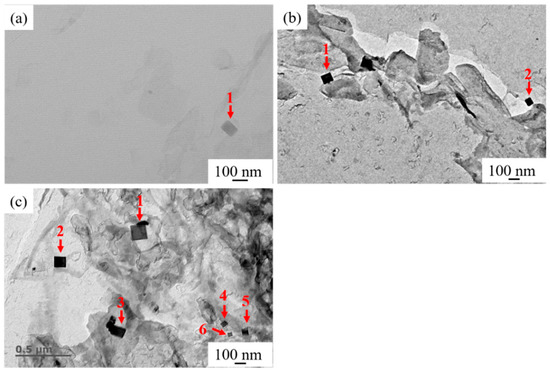
Figure 8.
Distribution of the nanoscale titanium nitride (TiN) particles in HAZs of (a) A, (b) LM, and (c) HM.
3.2.3. Thermodynamic Calculation of Evolution of Inclusions/Precipitates
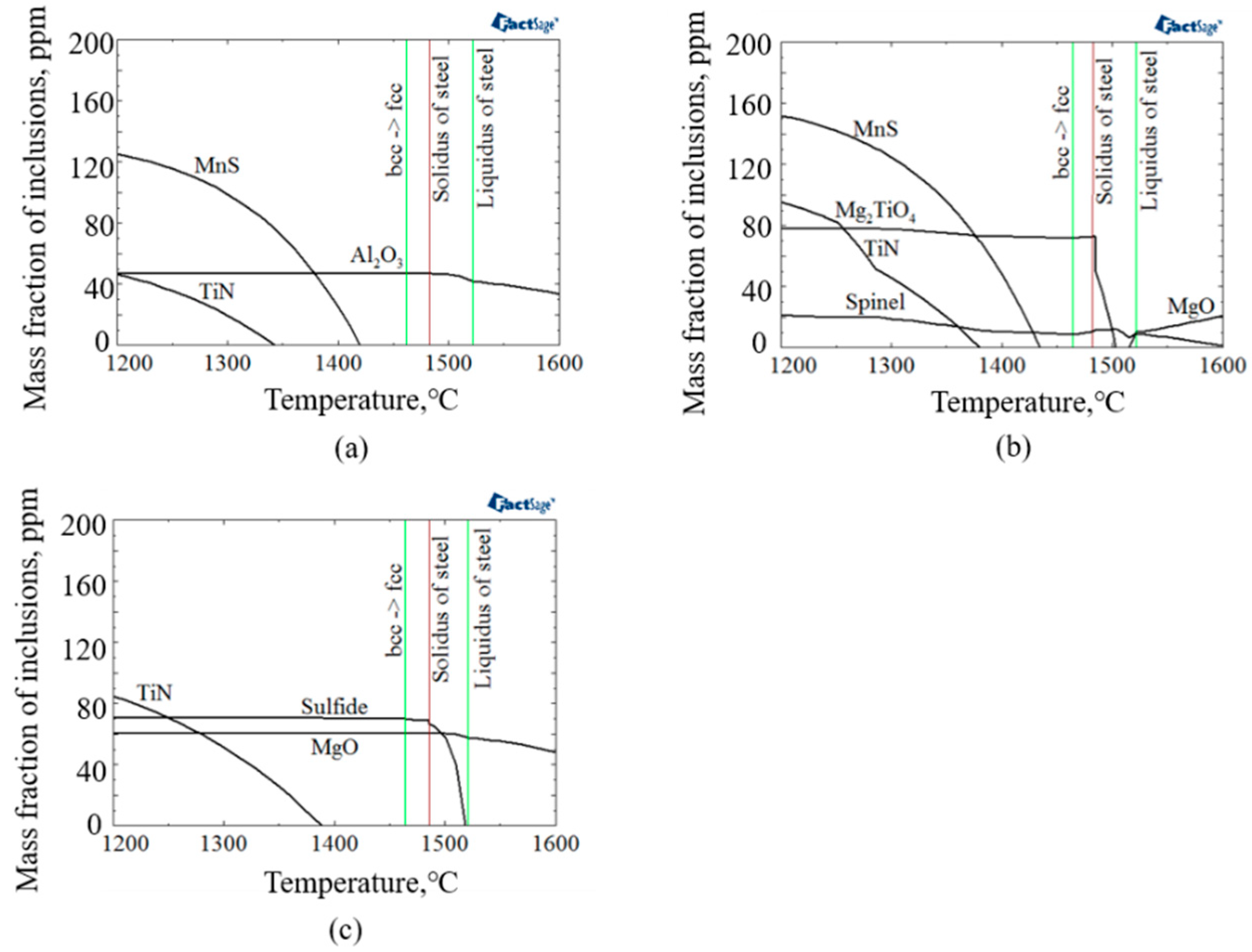
The evolution of inclusions/precipitates in the experimental steel samples compositional systems were thermodynamically calculated by the use of FactSage 7.3 software with FToxid, FTmisc, and FSstel databases. Figure 9 shows the thermodynamic calculation results for inclusion/precipitates evolution during solidification of experimental steel samples. As shown in Figure 9, the stable oxide phase in molten steel of A, LM, and HM were Al2O3, Mg-Al-Ti-O, and MgO, respectively. In addition, the initial precipitation temperature of TiN in A, LM, and HM were 1340, 1379, and 1387 °C, respectively, which were lower than the solid phase line temperature. It is indicated that the single-phase TiN could not be generated in the molten steel. The thermodynamic condition for the formation of nanoscale TiN particles was possible by the precipitation of TiN in the solid phase. Moreover, with the increase of Mg content, the initial precipitation temperature of TiN increased gradually, which could promote the precipitation of TiN particles.
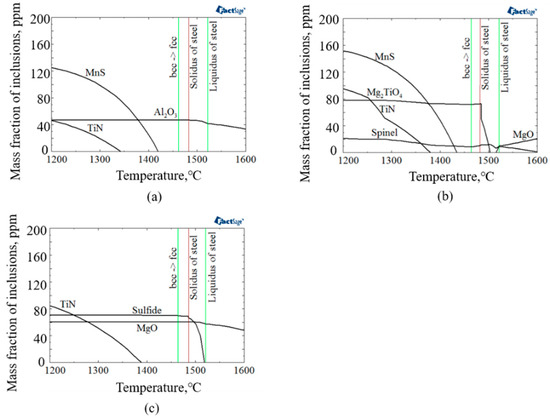
Figure 9.
Thermodynamic calculation results for inclusion evolution during solidification of (a) A, (b) LM, and (c) HM.
3.3. Growth Behavior of Austenite Grains
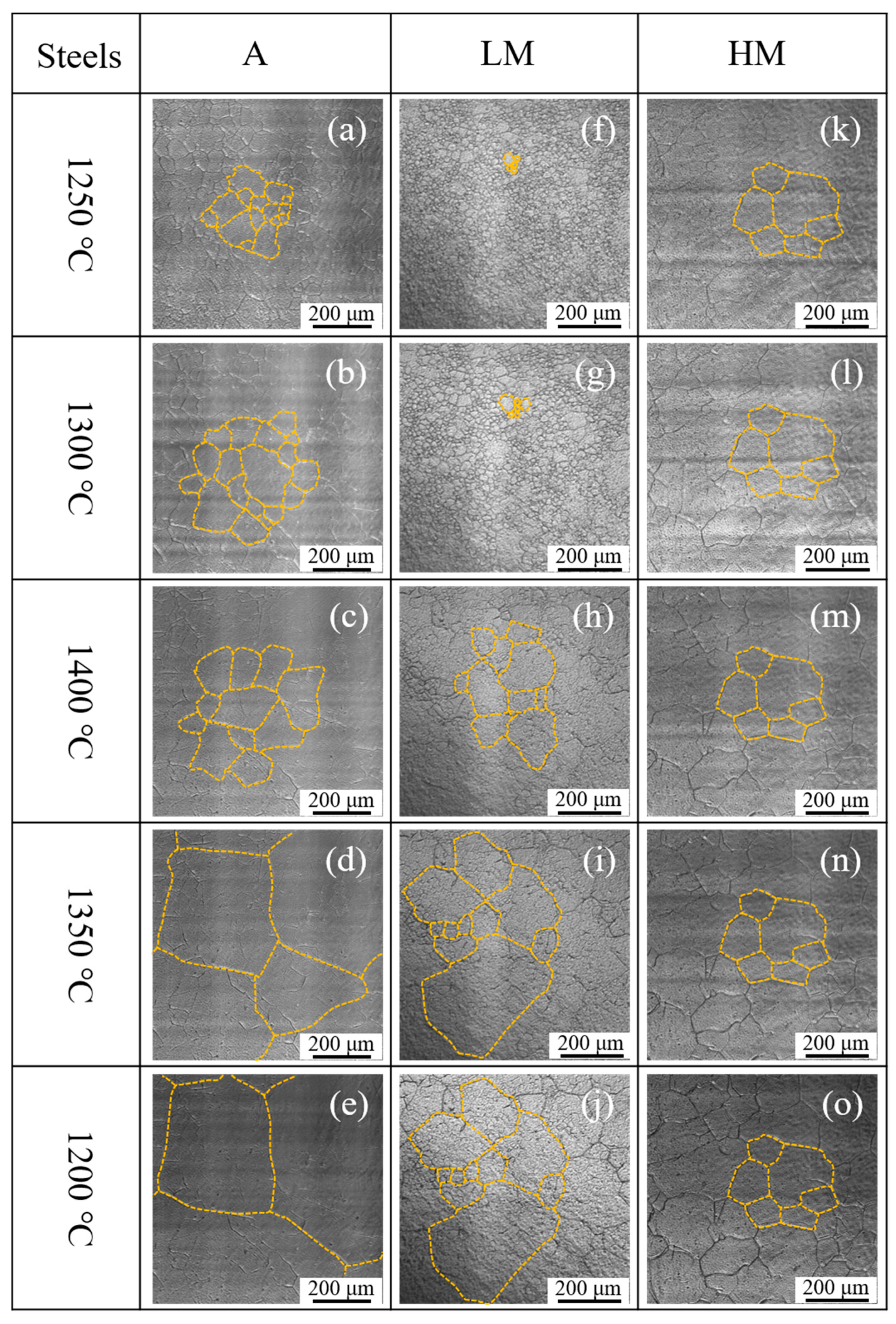
Figure 10 shows the in situ observation of austenite grain growth of experimental steel samples in the high-temperature stage during the thermal cycle of HAZ. When the temperature increased up to 1250 °C, the austenite grains were observed clearly in all three steel samples, as shown in Figure 10a,f,k. It is seen in Figure 10a–e that the austenite grain in A was growing continuously during the heating and cooling stage. In LM, as shown in Figure 10f–j, when the temperature increased up to 1400 °C, the austenite grains were grown sharply and even cooled down to 1350 °C. The growth of austenite grains in HM showed different behavior, as shown in Figure 10k–o. It is observed that the austenite grains hardly grew up with increasing the temperature from 1250 to 1400 °C and decreasing the temperature from 1400 to 1200 °C.
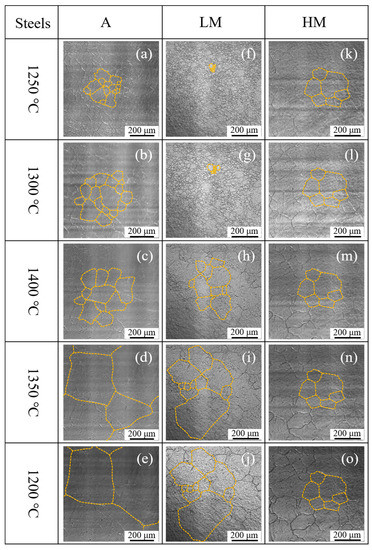
Figure 10.
In situ observation of austenite grain growth during the high-temperature stage: (a–e) A, (f–j) LM, and (k–o) HM.
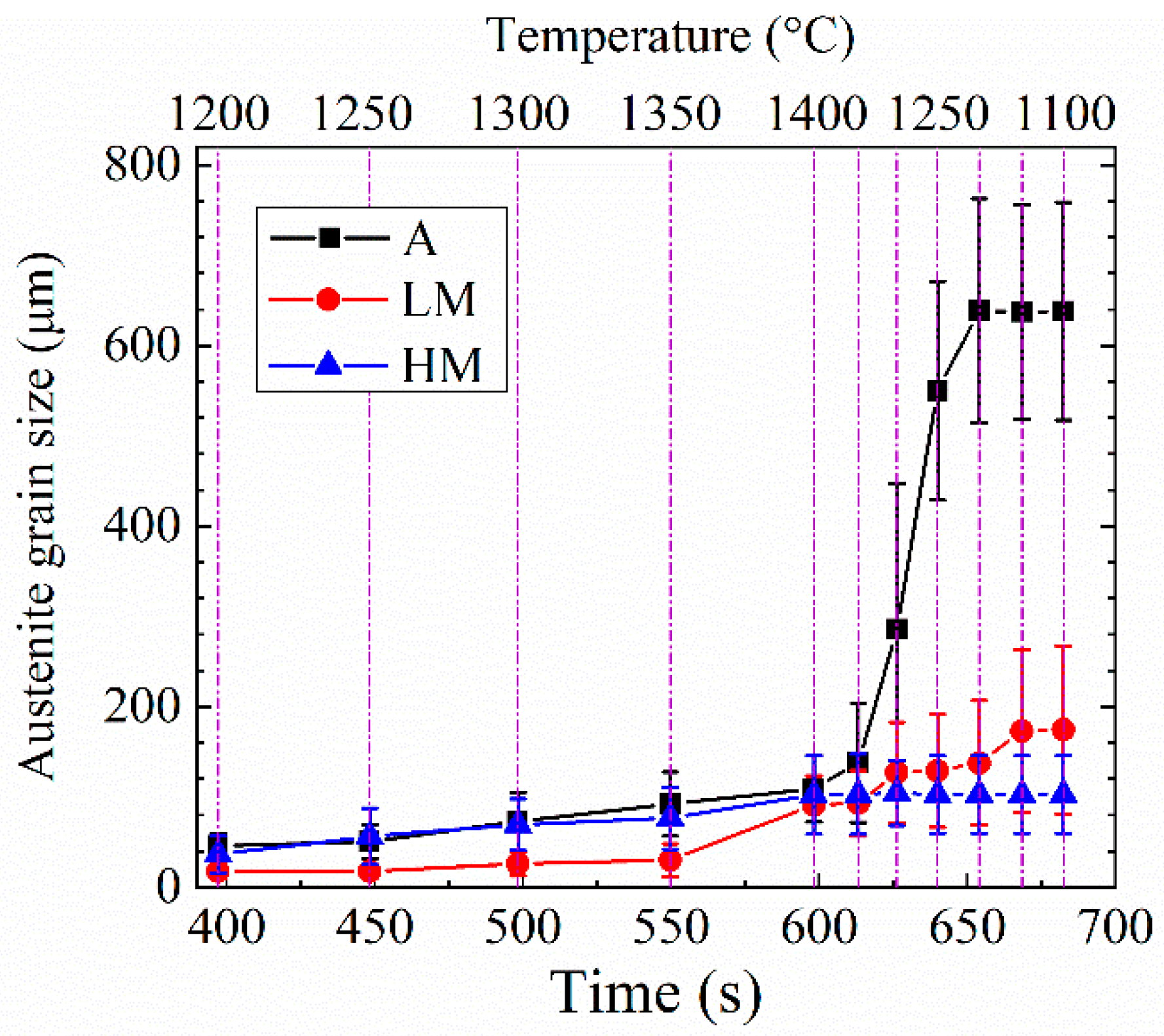
Figure 11 shows the change in the average austenite grain size with the increase of high-temperature residence time of experimental steel samples at the high-temperature stage of the HAZ thermal cycle. The growth rate of austenite grains at the high-temperature stage of the HAZ thermal cycle is shown in Table 2. With the temperature increase from 1200 to 1400 °C, the average austenite grain sizes of A, LM, and HM increased from 45.90 to 109.40 μm, 17.01 to 88.76 μm, and 35.48 to 99.75 μm with the average growth rates of 0.32, 0.36, and 0.32 μm/s, respectively. As shown in Table 1, the Ti and N contents in LM were lower and higher than those in A and HM, respectively. According to the results reported by Baker [29], the dense dispersed TiN particles with sizes less than 20 nm was easy to be formed in the Ti microalloyed steel containing low Ti and high N. These nanoscale TiN particles were effective to retard the growth of austenite grain below 1350 °C. Therefore, the austenite grain size for LM at T1~T4 (1200~1350 °C) was much smaller than those for A and HM, as shown in Figure 11. During the cooling process from 1400 to 1250 °C, the average austenite grain sizes of A, LM, and HM were further increased from 109.40 to 549.98 μm, 88.76 to 125.80 μm, and 99.75 to 100.32 μm with the average growth rates of 10.55, 0.89, and 0.01 μm/s, respectively. With the temperature further decreasing from 1250 to 1100 °C, the average austenite grain sizes of A and LM increased slowly with the growth rates of 2.06 and 1.02 μm/s, respectively. It is noted that the austenite grains sizes of HM stopped increasing at the whole cooling stage from 1400 to 1100 °C. It is inferred that the growth of austenite grains in HM was finished when the temperature increased up to the peak value of the thermal cycle.
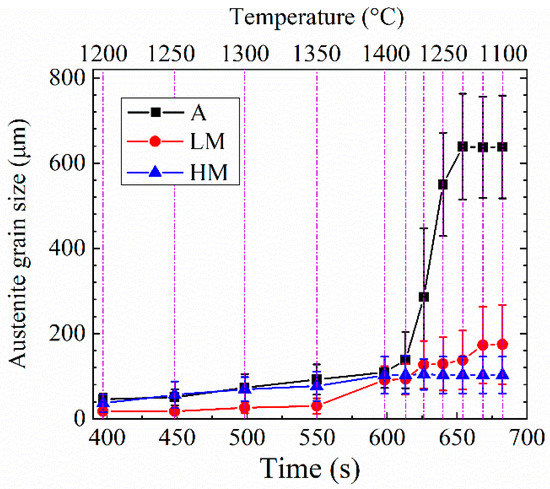
Figure 11.
Change in the average austenite grain size with the increase of high-temperature residence time.

Table 2.
The growth rate of austenite grains at the high-temperature stage of the HAZ thermal cycle (μm/s).
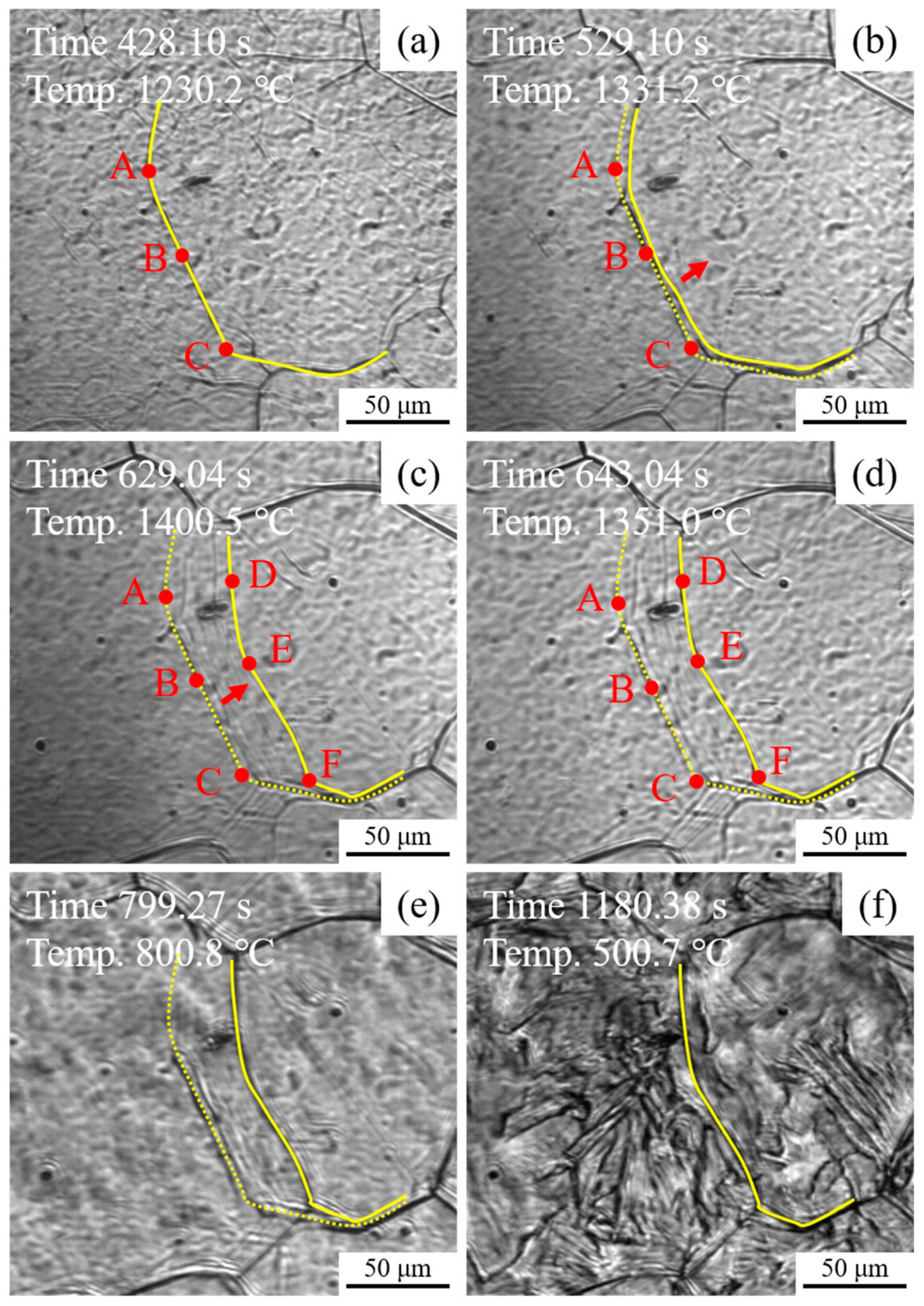
Figure 12 shows the in situ observation of the migration of austenite grain boundary during the heating and cooling process of experimental steel HM. As shown in Figure 12a, the austenite grain boundary was observed clearly in 1230.2 °C, which was marked by a yellow solid line. To illustrate the migration of austenite grain boundary, three points, termed as A, B, and C, on the austenite grain boundary were selected as the reference points. It is seen in Figure 12b that with the temperature increase to 1331.2 °C, the austenite grain boundary started to migrate along the direction indicated by the red arrow. As shown in Figure 12c, with the further increase up to the peak temperature 1400.5 °C, three reference points A, B and C were moved to D, E, and F with average growth rates of 0.33, 0.27, and 0.33 μm/s. During the cooling process from 1351.0 to 800.8 °C, the migration phenomenon of austenite grain boundary has not been observed, as shown in Figure 12d,e. After the γ → α phase transformation, the traces of the migration of austenite grain boundary were covered and only the prior austenite grain boundary remained, as shown in Figure 12f. It is noted in Figure 12 that micron size inclusions were not observed on the migrating trajectories of austenite grain boundaries. Thus, it might be reasonable to infer that the effective particles for pinning the migration of austenite grain boundaries in HM are those particles with sizes in nanoscale.
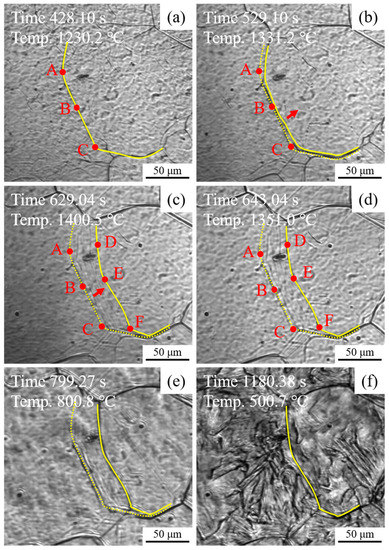
Figure 12.
In situ observation of migration of austenite grain boundary during the heating and cooling process of experimental steel HM: (a) austenite grain boundary, (b) migration start, (c) migration finish, and (d–f) cooling process.
4. Discussion
4.1. Effect of Mg Content on the Behavior of Ti-Containing Inclusions
The main complex inclusion, Al2O3+MnS, in conventional steel with Al deoxidation is a kind of micron size inclusion without the ability to induce the formation of IAF [15]. There are two kinds of existing forms of Ti in inclusions/precipitate, namely oxide form of Ti2O3 and nitride form of TiN. The micron size Ti2O3 plays a key role in the formation of IAF [6,9]. Additionally, the nanoscale TiN is an effective pinning particle for retarding the growth of austenite grain [4,18]. In the present study, Mg oxidation has been applied to control the existing forms of Ti within inclusions/precipitates in steel. Mg has a stronger affinity with oxygen than Al and Ti [30,31]. Thus, Al2O3 and Ti2O3 inclusions are easy to be reduced by Mg in steel. In both the developed steels, LM and HM, Mg deoxidation was used for inclusion control, instead of Al deoxidation. It was intended to avoid the formation of Al2O3 inclusion, which was helpless to the formation of IAF [15].
When Mg was added into molten steel, the dissolved Mg in the melt reacted with the dissolved oxygen to form MgO particles, which could be expressed by Equation (1):
[Mg] + [O] = MgO(s)
In LM, the Mg content in steel was at the low level (0.0027%), and the primary Ti2O3 inclusions were partly reduced by [Mg] to form MgO-Ti2O3, which could be expressed as Equation (2):
3[Mg] + 4Ti2O3(s) = 3MgO-Ti2O3(s)+ 2 [Ti]
During the solidification and cooling process, [Ti] was combined with nitrogen in steel to form nanoscale TiN particles, which could be expressed as Equation (3):
[Ti] + [N] = TiN(s)
Thus, the Ti within inclusions/precipitates in LM existed in both forms of micron size MgO-Ti2O3 and nanoscale TiN, as shown in Figure 5 and Figure 7c,d, respectively. The experimental results are consistent with the thermodynamic calculation result shown in Figure 9b. It also confirms the results reported by Chai et al. [14] and Song et al. [4]. However, in HM, the Mg content in steel was at a high level (0.0099%), and then the primary Ti2O3 inclusions were completely reduced by Mg, which could be expressed as Equation (4):
3[Mg] + Ti2O3(s) = 3MgO(s) + 2[Ti]
As a result, the Ti within inclusions/precipitates in HM existed only in the form of nanoscale TiN and the oxide phase of inclusion was only MgO, as shown in Figure 6 and Figure 7e,f, respectively. It is indicated that the formation of nanoscale TiN particles was prompted in steel with Mg deoxidation under the condition without an increase of Ti and N content. Consequently, it benefits to avoid the formation of coarse TiN particles with the size over 0.5 μm, which may act as cleavage nucleation sites [17].
4.2. Role of Mg Deoxidation on Improving HAZ Toughness
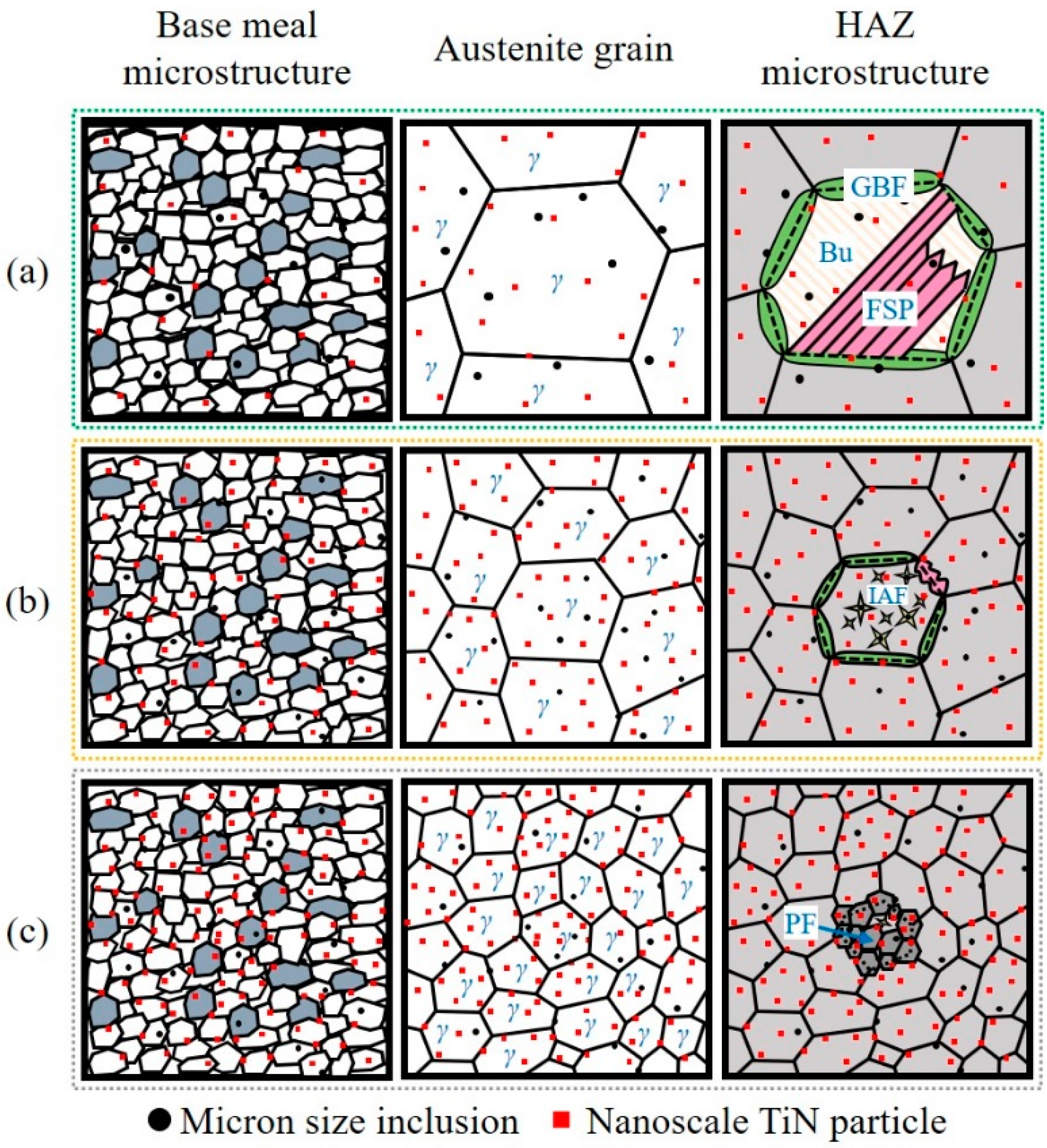
Figure 13 shows the schematic diagram of the mechanism for improving the HAZ toughness by inclusion control with Mg deoxidation. As shown in Figure 13a, in the conventional steel with Al deoxidation, A, micron size inclusions mainly were Al2O3+MnS without the ability to induce the formation of IAF. Meanwhile, the nanoscale TiN particles acting as pinning the growth of austenite grains were inadequate, as shown in Figure 8a. At the high-temperature stage of the HAZ thermal cycle, austenite grains were coarsened resulting from the weak pinning effect. Subsequently, GBF and FSP were nucleated on austenite grain boundaries during the γ → α phase transformation process, then grown along the austenite grain boundaries towards intragranular, respectively [24]. Thus, the brittle microstructure in HAZ of A mainly composed of GBF and FSP were formed, resulting in the deterioration of HAZ toughness [15]. It has been concluded that there are two keys to improve the HAZ toughness: (1) Retard the growth of austenite grains by nanoscale pinning particles, (2) induce the nucleation of IAF microstructure by micron size inclusions, instead of brittle microstructure.
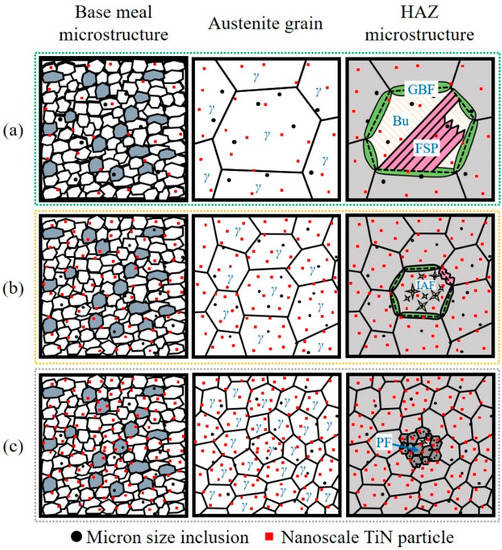
Figure 13.
Schematic diagram of the mechanism for improving the HAZ toughness by inclusion control with Mg deoxidation: (a) A, (b) LM, and (c) HM.
As shown in Figure 8, the number density of nanoscale TiN particles in LM increased comparing with that in A. As a result, the pinning effect for retarding the growth of austenite grains in LM was stronger than that in A. Therefore, the size of austenite grains of A was larger than that of LM as shown in Figure 13a,b, respectively. Moreover, those micron size inclusions, MgO-Ti2O3+MnS, acted as the effective nucleus of IAF and then promoted the formation of well-developed IAFs within the austenite grains. Meanwhile, the formation of GBF and FSP was inhibited. As a result, the HAZ toughness of steel sample LM was improved compared with steel sample A.
In HM, the formation of nanoscale TiN particles was obviously promoted by Mg deoxidation as shown in Figure 8c, resulting in a strong pinning effect for inhibiting the growth of austenite grains. As a consequence, the austenite grain growth rate at the high-temperature stage (1400~1200 °C) of HM was much smaller than those of A and LM, as shown in Figure 11. The austenite grains with the small sizes provided adequate triple junctions as the preferential nucleating sites for γ → α phase transformation [24,32]. Furthermore, the relatively slow cooling rate during the phase transformation process was beneficial to the formation of PFs [33]. Therefore, the fine PF microstructure instead of brittle microstructures were formed in the HAZ of HM, as shown in Figure 2d and Figure 13c. This kind of PF microstructure greatly promoted the HAZ toughness of steel plate after high-heat-input welding [34].
5. Conclusions
In the present study, the mechanism of improving the heat-affected zone (HAZ) toughness of steel plate with Mg deoxidation after high-heat-input welding with 400 kJ/cm were investigated based on experimental study and thermodynamic calculation. The obtained results were summarized as follows:
- The microstructure in the base metal of steels with Mg deoxidation containing low and high-level Mg contents (LM and HM) were both fine bainite, as well as the steel with the conventional Al deoxidation (A), resulting in the excellent toughness of base metals. After the simulated welding with the heat-input of 400 kJ/cm, compared with base metals, the toughness of HAZ of A, LM, and HM were decreased by 88.59%, 25.66%, and 23.63%, respectively. The intragranular acicular ferrite (IAF) and polygonal ferrite (PF) contributed to the improvements of HAZ toughness of LM and HM, respectively.
- With the increase of Mg content in steel with Mg deoxidation, the oxide in micron size inclusion was firstly changed to MgO-Ti2O3, then to MgO with the further increase in Mg content in steel. The formation of nanoscale TiN particles was promoted more obviously with the higher Mg content in the steel.
- The micron size MgO-Ti2O3+MnS inclusion could effectively induce the formation of IAF. The growth rates of austenite grains at the high-temperature stage (1400~1250 °C) during the HAZ thermal cycle of A, LM, and HM were 10.55, 0.89, 0.01 μm/s, respectively. It was indicated that nanoscale TiN particles formed in steel with Mg deoxidation were effective to inhibit the growth of austenite grain.
- The excellent HAZ toughness of steel plates after welding with a heat input of 400 kJ/cm could be obtained by control of the Mg content in steel to selectively promote the formation of IAF or retard the growth of austenite grain.
Author Contributions
L.X. performed the experiments, analyzed the experimental results, and wrote this manuscript. J.Y. contributed the guidance, conceived, and designed this research. J.P. and H.O. provided useful discussions and suggestions. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. U1960202).
Acknowledgments
The authors are grateful to X.F. Jiang and R.Z. Wang for the assistance in steelmaking.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, J.; Zhu, K.; Wang, G.D. Progress in the Technological Development of Oxide Metallurgy for Manufacturing Steel Plates with Excellent Haz Toughness. Baosteel Technol. Res. 2008, 2, 43–50. [Google Scholar]
- Xu, L.Y.; Yang, J.; Wang, R.Z. Influence of Al Content on the Inclusion-Microstructure Relationship in the Heat-Affected Zone of a Steel Plate with Mg Deoxidation After High-Heat-Input Welding. Metals 2018, 8, 1027. [Google Scholar] [CrossRef]
- Lou, H.N.; Wang, C.; Wang, B.X.; Wang, Z.D.; Li, Y.Q.; Chen, Z.G. Inclusion Evolution Behavior of Ti-Mg Oxide Metallurgy Steel and its Effect on a High Heat Input Welding HAZ. Metals 2018, 8, 534. [Google Scholar] [CrossRef]
- Song, M.M.; Hu, C.L.; Song, B.; Zhu, H.Y.; Xue, Z.L.; Xu, R.S. Effect of Ti-Mg Treatment on the Impact Toughness of Heat Affected Zone in 0.15%C-1.31%Mn Steel. Steel Res. Int. 2018, 89, 1700355. [Google Scholar] [CrossRef]
- Sarma, D.S.; Karasev, A.V.; Jönsson, P.G. On the Role of Non-Metallic Inclusions in the Nucleation of Acicular Ferrite in Steels. ISIJ Int. 2009, 49, 1063–1074. [Google Scholar] [CrossRef]
- Lee, J.L.; Pan, Y.T. Effect of Sulfur Content on the Microstructure and Toughness of Simulated Heat-Affected Zone in Ti-Killed Steels. Metall. Trans. A 1993, 24, 1399–1408. [Google Scholar] [CrossRef]
- Mabuchi, H.; Uemori, R.; Fujioka, M. The Role of Mn Depletion in Intra-Granular Ferrite Transformation in the Heat Affected Zone of Welded Joints with Large Heat Input in Structural Steels. ISIJ Int. 1996, 36, 1406–1412. [Google Scholar] [CrossRef]
- Shim, J.H.; Byun, J.S.; Cho, Y.W.; Oh, Y.J.; Shim, J.D.; Lee, D.N. Mn Absorption Characteristics of Ti2O3 Inclusions in Low Carbon Steels. Scr. Mater. 2001, 44, 49–54. [Google Scholar] [CrossRef]
- Kang, Y.J.; Jang, J.H.; Park, J.H.; Lee, C.H. Influence of Ti On Non-Metallic Inclusion Formation and Acicular Ferrite Nucleation in High-Strength Low-Alloy Steel Weld Metals. Met. Mater. Int. 2014, 20, 119–127. [Google Scholar] [CrossRef]
- Hou, Y.H.; Zheng, W.; Wu, Z.H.; Li, G.Q.; Moelans, N.; Guo, M.X.; Khan, B.S. Study of Mn Absorption by Complex Oxide Inclusions in Al-Ti-Mg Killed Steels. Acta Mater. 2016, 118, 8–16. [Google Scholar] [CrossRef]
- Kimura, S.; Nakajima, K.; Mizoguchi, S. Behavior of Alumina-Magnesia Complex Inclusions and Magnesia Inclusions on the Surface of Molten Low-Carbon Steels. Metall. Mater. Trans. B 2001, 32, 79–85. [Google Scholar] [CrossRef]
- Karasev, A.V.; Suito, H. Characteristics of Fine Oxide Particles Produced by Ti/M (M=Mg and Zr) Complex Deoxidation in Fe-10 Mass%Ni Alloy. ISIJ Int. 2008, 48, 1507–1516. [Google Scholar] [CrossRef]
- Kim, H.S.; Chang, C.H.; Lee, H.G. Evolution of Inclusions and Resultant Microstructural Change with Mg Addition in Mn/Si/Ti Deoxidized Steels. Scr. Mater. 2005, 53, 1253–1258. [Google Scholar] [CrossRef]
- Chai, F.; Yang, C.F.; Hang, S.; Zhang, Y.Q.; Xu, Z. Effect of Magnesium on Inclusion Formation in Ti-Killed Steels and Microstructural Evolution in Welding Induced Coarse-Grained Heat Affected Zone. J. Iron Steel Res. Int. 2009, 16, 69–74. [Google Scholar] [CrossRef]
- Xu, L.Y.; Yang, J.; Wang, R.Z.; Wang, W.L.; Wang, Y.N. Effect of Mg Addition on Formation of Intragranular Acicular Ferrite in Heat-Affected Zone of Steel Plate After High-Heat-Input Welding. J. Iron Steel Res. Int. 2018, 25, 433–441. [Google Scholar] [CrossRef]
- Yang, J.; Xu, L.Y.; Zhu, K.; Wang, R.Z.; Zhou, L.J.; Wang, W.L. Improvement of HAZ Toughness of Steel Plate for High Heat Input Welding by Inclusion Control with Mg Deoxidation. Steel Res. Int. 2015, 86, 619–625. [Google Scholar] [CrossRef]
- Zhang, L.P.; Davis, C.L.; Strangwood, M. Effect of TiN Particles and Microstructure on Fracture Toughness in Simulated Heat-Affected Zones of a Structural Steel. Metall. Mater. Trans. A 1999, 30, 2089–2096. [Google Scholar] [CrossRef]
- Kato, T.; Sato, S.; Ohta, H.; Shiwaku, T. Effects of Ca Addition on Formation Behavior of TiN Particles and HAZ Toughness in Large Heat Input Welding. Kobe Steel Eng. Rep. 2011, 61, 32–35. [Google Scholar]
- Sennour, M.; Esnouf, C. Contribution of Advanced Microscopy Techniques to Nano-Precipitates Characterization: Case of AlN Precipitation in Low-Carbon Steel. Acta Mater. 2003, 51, 943–957. [Google Scholar] [CrossRef]
- Karmakar, A.; Kundu, S.; Roy, S.; Neogy, S.; Srivastava, D.; Chakrabarti, D. Effect of Microalloying Elements on Austenite Grain Growth in Nb-Ti and Nb-V Steels. Mater. Sci. Technol. 2014, 30, 653–664. [Google Scholar] [CrossRef]
- Li, X.B.; Zhang, T.S.; Min, Y.; Liu, C.J.; Jiang, M.F. Effect of Magnesium Addition in Low-Carbon Steel Part 1: Behaviour of Austenite Grain Growth. Ironmak. Steelmak. 2017, 46, 292–300. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, X.L.; Li, G.Q.; Wang, Y.; Zheng, W.; Hou, Y.H. Grain Refinement in Coarse-Grained Heat-Affected Zone of Al-Ti-Mg Complex Deoxidised Steel. Sci. Technol. Weld. Join. 2018, 24, 43–51. [Google Scholar] [CrossRef]
- Kai, Z.; Jian, Y.; Wang, R.Z.; Yang, Z.G. Effect of Mg Addition on Inhibiting Austenite Grain Growth in Heat Affected Zones of Ti-Earing Low Carbon Steels. J. Iron Steel Res. Int. 2011, 18, 60–64. [Google Scholar]
- Xu, L.Y.; Yang, J.; Wang, R.Z.; Wang, Y.N.; Wang, W.L. Effect of Mg Content on the Microstructure and Toughness of Heat-Affected Zone of Steel Plate After High Heat Input Welding. Metall. Mater. Trans. A 2016, 47, 3354–3364. [Google Scholar] [CrossRef]
- Yan, N.; Yu, S.; Chen, Y. In Situ Observation of Austenite Grain Growth and Transformation Temperature in Coarse Grain Heat Affected Zone of Ce-Alloyed Weld Metal. J. Rare Earth. 2017, 35, 203–210. [Google Scholar] [CrossRef]
- Cao, Y.; Wan, X.; Hou, Y.; Niu, C.; Liu, Y.; Li, G. In Situ Observation of Grain Refinement in the Simulated Heat-Affected Zone of Al-Ti-0.05% Ce-Deoxidized Steel. Steel Res. Int. 2019, 90, 1900084. [Google Scholar] [CrossRef]
- Wan, X.L.; Wu, K.M.; Huang, G.; Wei, R.; Cheng, L. In Situ Observation of Austenite Grain Growth Behavior in the Simulated Coarse-Grained Heat-Affected Zone of Ti-Microalloyed Steels. Int. J. Miner. Metall. Mater. 2014, 21, 878–885. [Google Scholar] [CrossRef]
- Zhu, K.; Yang, Z.G. Effect of Magnesium on the Austenite Grain Growth of the Heat-Affected Zone in Low-Carbon High-Strength Steels. Metall. Mater. Trans. A 2011, 42, 2207–2213. [Google Scholar] [CrossRef]
- Baker, T.N. Titanium Microalloyed Steels. Ironmak. Steelmak. 2019, 46, 1–55. [Google Scholar] [CrossRef]
- Cao, L.; Wang, G.C.; Yuan, X.H.; Jin, P.L.; Sridhar, S. Thermodynamics and Agglomeration Behavior on Spinel Inclusion in Al-Deoxidized Steel Coupling with Mg Treatment. Metals 2019, 9, 900. [Google Scholar] [CrossRef]
- Zhang, T.S.; Liu, C.J.; Jiang, M.F. Effect of Mg on Behavior and Particle Size of Inclusions in Al-Ti Deoxidized Molten Steels. Metall. Mater. Trans. B 2016, 47, 2253–2262. [Google Scholar] [CrossRef]
- Zou, X.D.; Sun, J.C.; Matsuura, H.; Wang, C. Documenting Ferrite Nucleation Behavior Differences in the Heat-Affected Zones of Eh36 Shipbuilding Steels with Mg and Zr Additions. Metall. Mater. Trans. A 2019, 50, 4506–4512. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.B.; Ma, H. Enhancement of Heat-Affected Zone Toughness of a Low Carbon Steel by TiN Particle. Metall. Mater. Trans. B 2016, 47, 2148–2156. [Google Scholar] [CrossRef]
- Zou, Z.Y.; Li, Y.X. Effect of Inclusions on Toughness and Microstructures in Simulated Coarse-Grain Heat-Affected Zones of Al/Ti Deoxidised and Nb/V Microalloyed Steels. Ironmak. Steelmak. 2019, 46, 574–583. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).