Hydride Rim Formation in E110 Zirconium Alloy during Gas-Phase Hydrogenation
Abstract
:1. Introduction
2. Materials and Research Methods
3. Results and Discussion
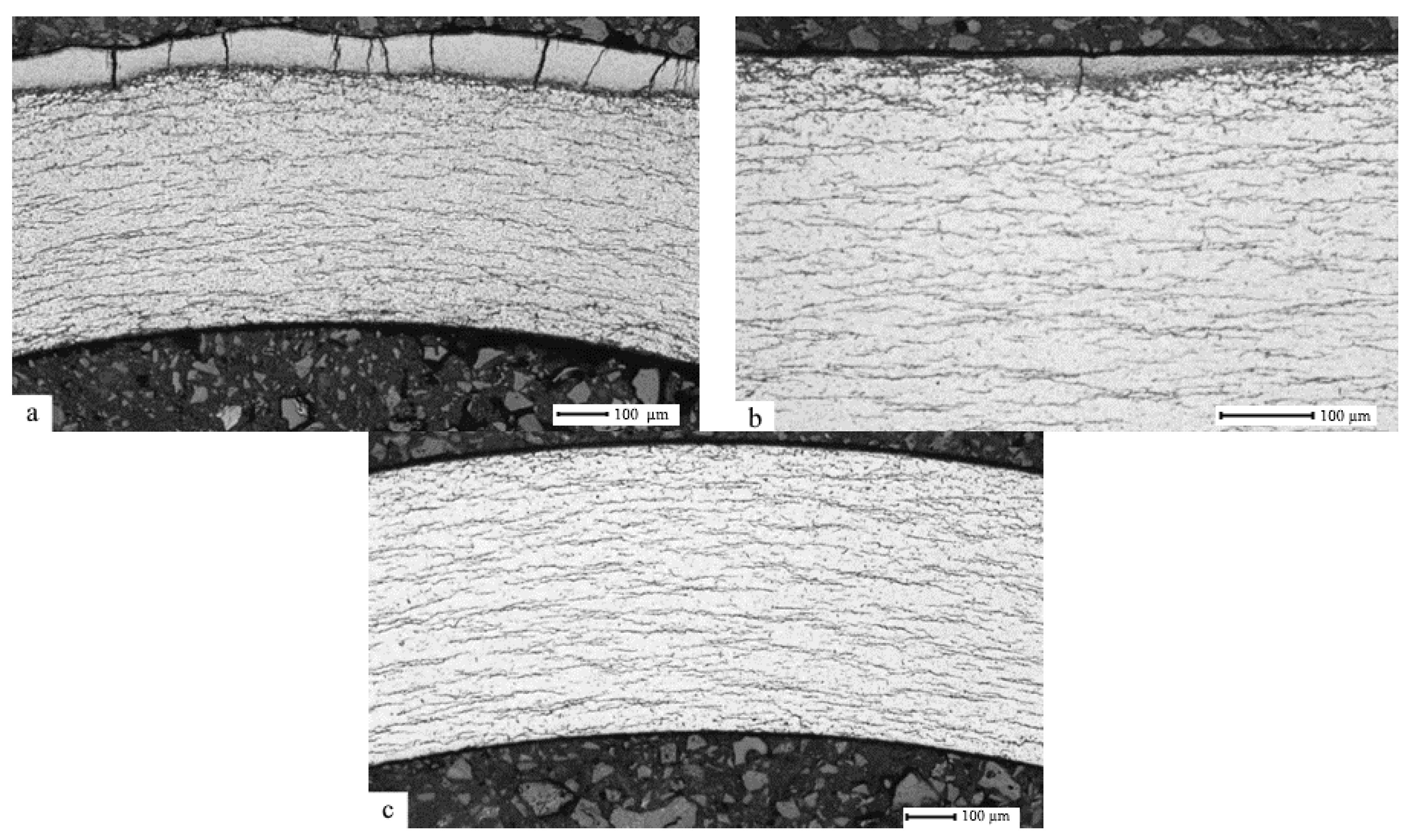
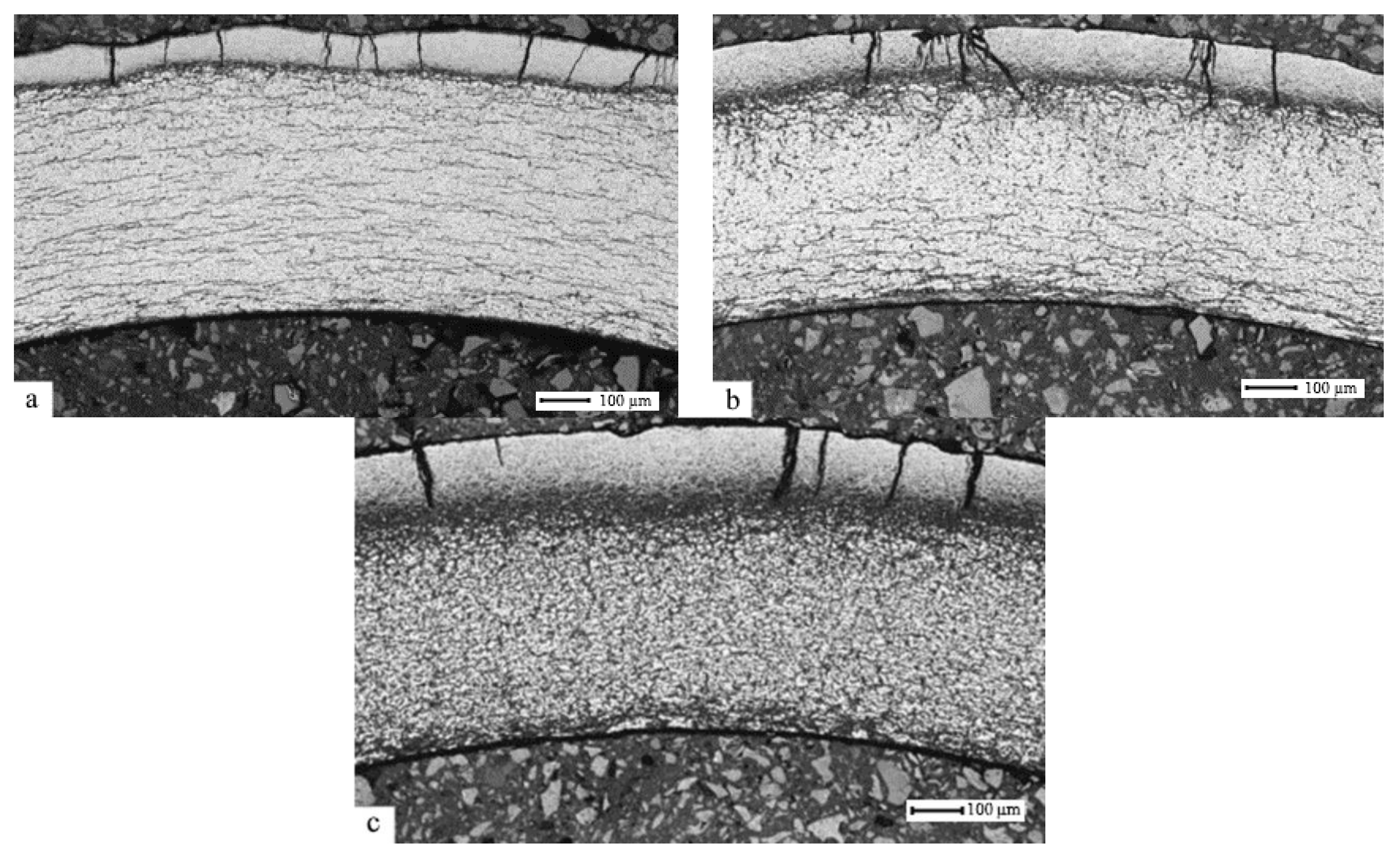
3.1. The Effect of the Gas-Phase Hydrogenation Temperature on the Value of the Threshold Temperature of Hydride Rim Formation
3.2. The Effect of Hydrogen Concentration on the Hydride Rim Thickness
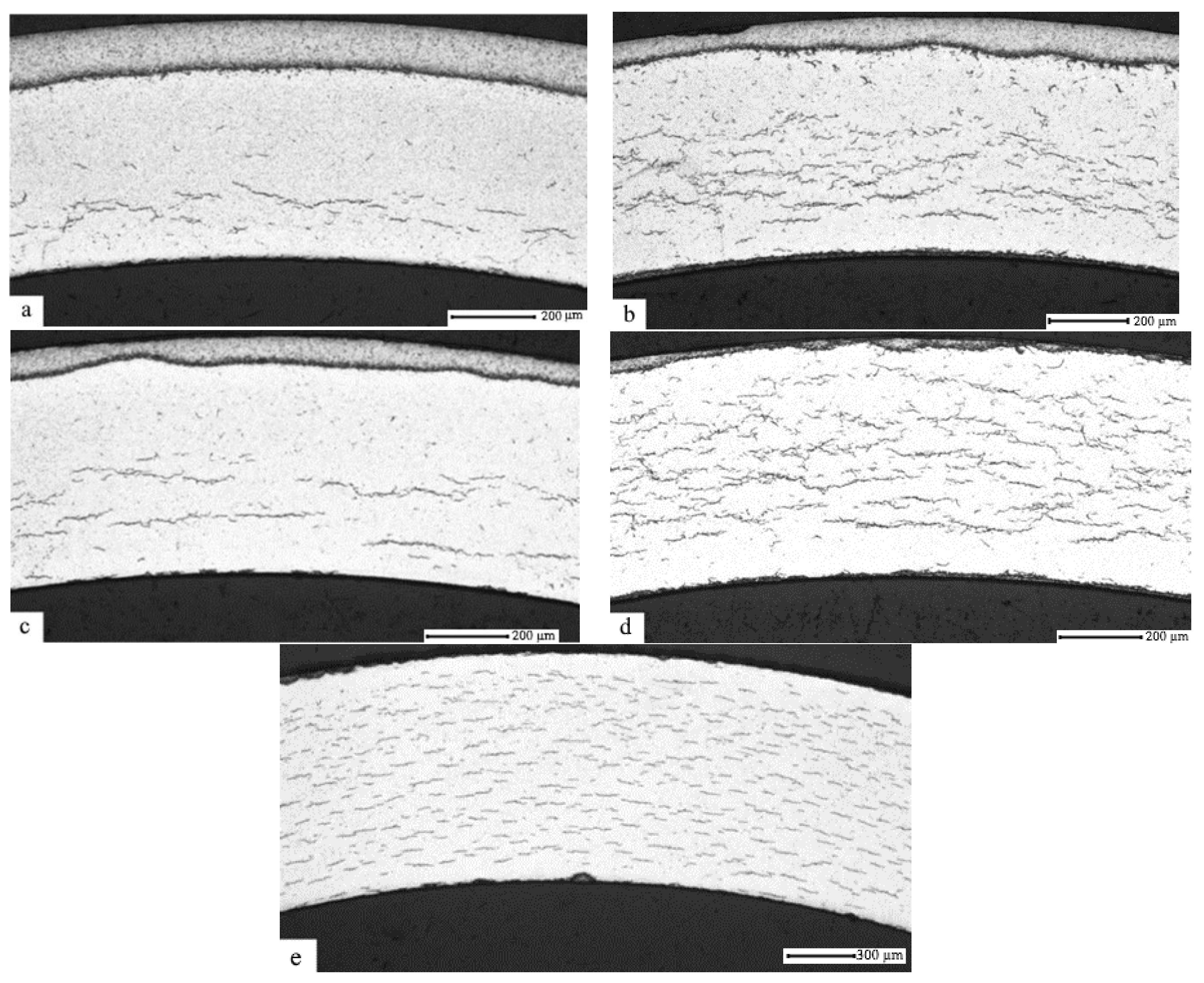
3.3. The Effect of the Gas-Phase Hydrogenation Temperature on the Value of the Threshold Temperature of Hydride Rim Formation in E110 Zirconium Alloy Subjected to Ion-Beam Cleaning and Nickel Coating
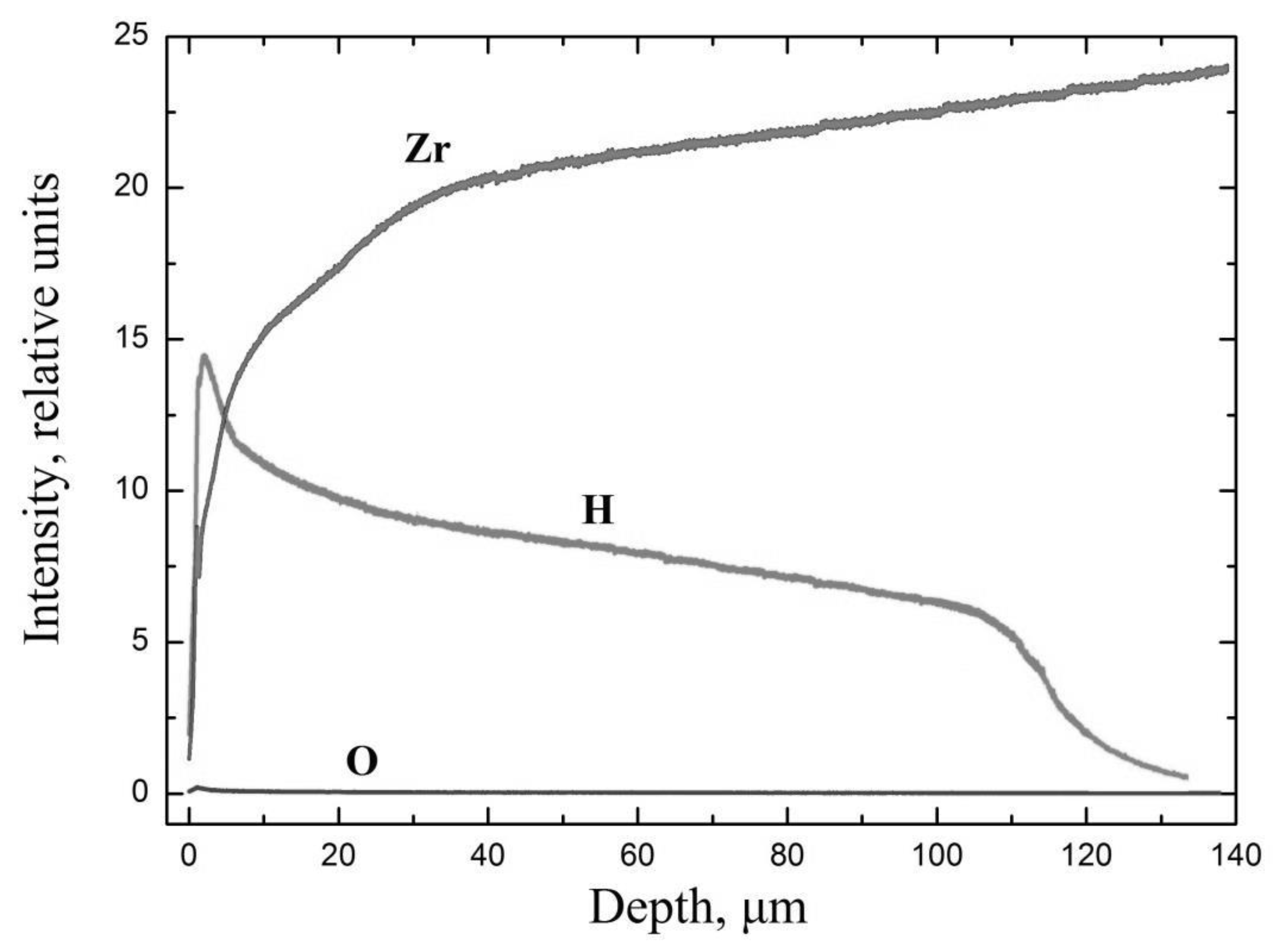
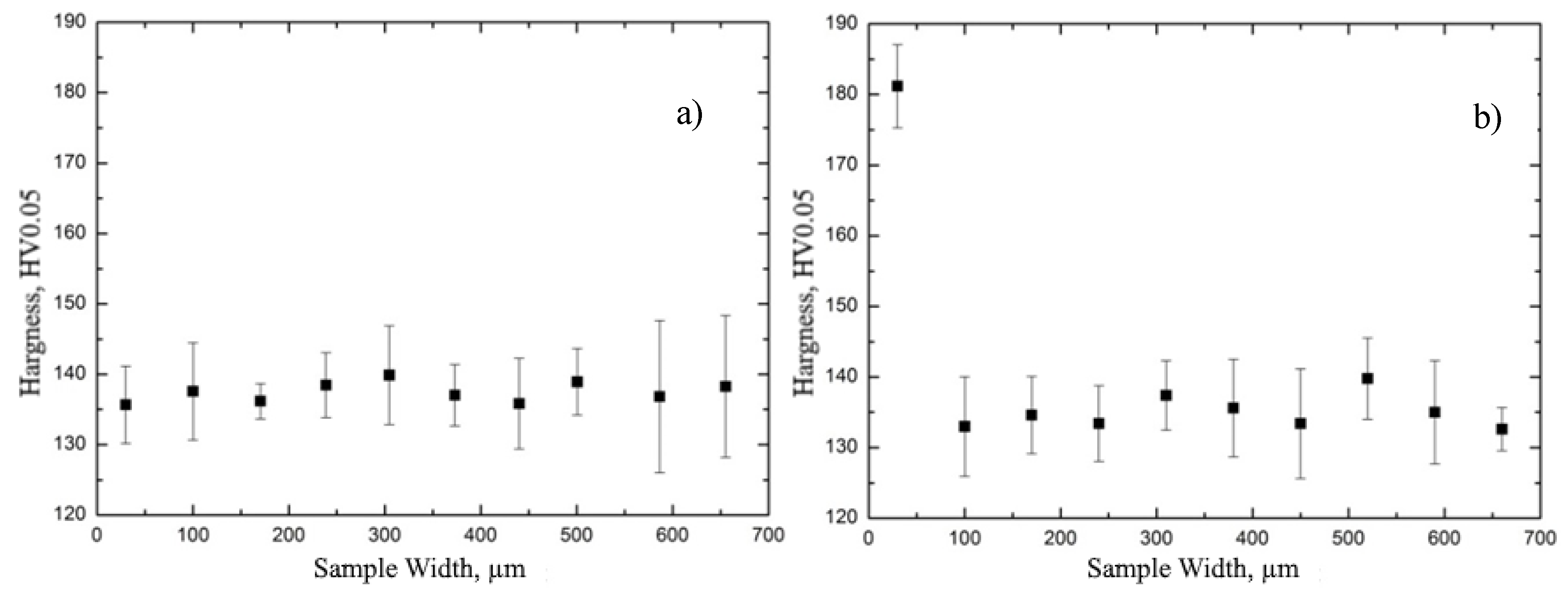
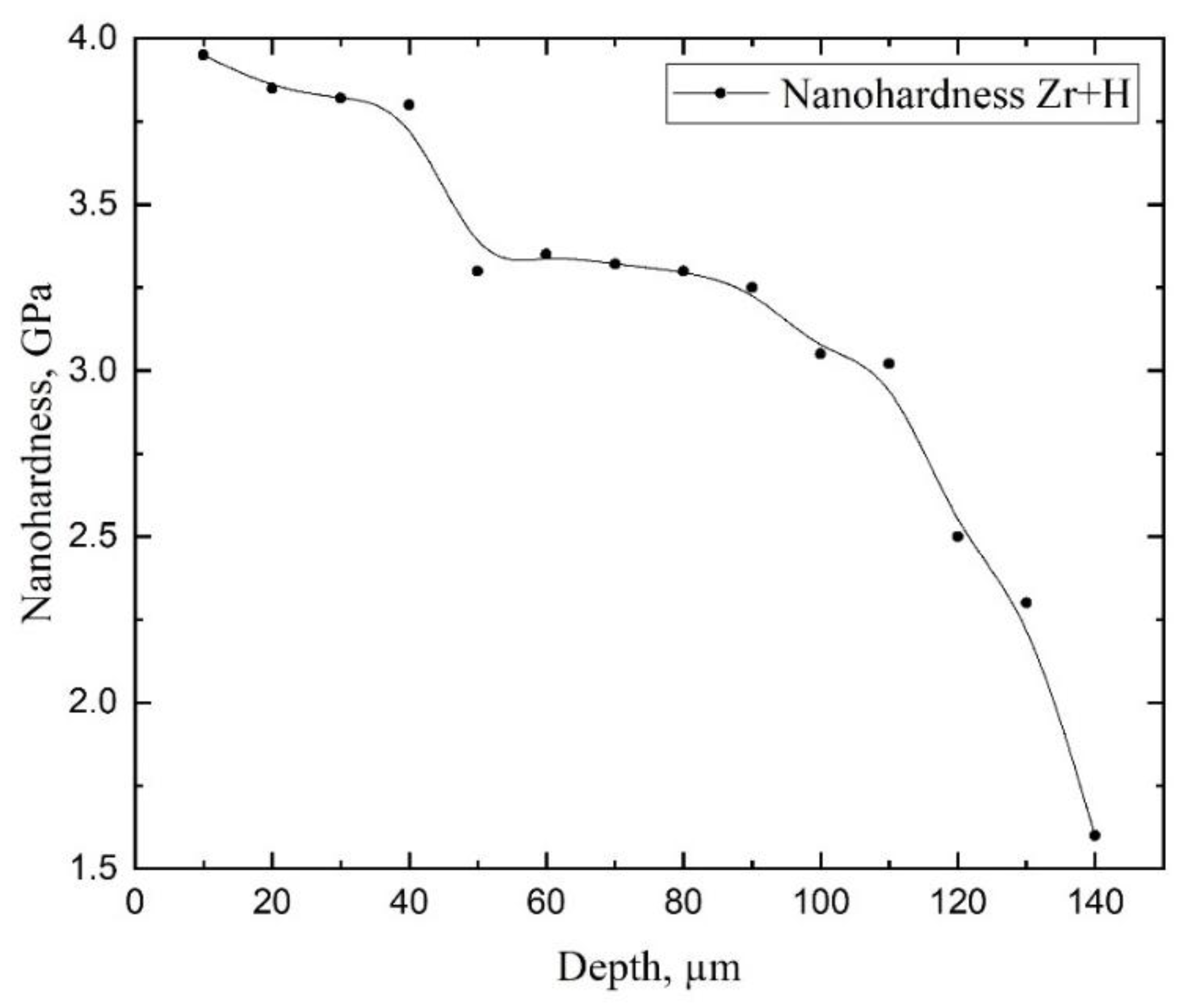
3.4. Studies of the Properties of the Hydride Rim Formed during Gas-Phase Hydrogenation in E110 Zirconium Alloy
4. Summary
- It has been established that, during gas-phase hydrogenation of the E110 zirconium alloy, the cladding tubes in the delivery condition under the constant pressure of 2 atm., within the temperature range (400–550) °C, up to hydrogen concentrations (0.1–1) wt.%, and subsequent slow cooling is where the formation of hydrides in the bulk of the material takes place.
- It has been demonstrated that in the E110 zirconium alloy cladding tubes, the formation of hydride rims of different thicknesses is achieved due to hydrogenation at the temperatures lower than the threshold temperature (400 ± 20) °C up to different hydrogen concentrations. The observed specifics of hydride distribution in the volume of zirconium cladding coincide with the data available as a result of the analysis of hydride distribution in the cladding after the operation.
- It has been experimentally proven that the hydride rim formed in the E110 zirconium alloy cladding tubes is characterized by the nonuniform distribution of hardness and hydrogen concentration through the thickness.
- It has been experimentally proven that E110 zirconium alloy surface modification, using the method of argon ion-beam cleaning (under the voltage 2000 V, the power 1000 W, the current 0.5 A, and the pressure 6 ×·10−2 Pa during 5 min) and subsequent nickel coating using the method of magnetron sputtering (under the voltage 500 V, the power 2000 W, the current 3 A, and the pressure 1 ×·10−1 Pa) with the thickness of ~1 µm on the E110 zirconium alloy cladding tubes, leads to the increase of the threshold temperature value of hydride rim formation in 100 °C, which is related to the significant increase of the hydrogen absorption rate.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Azevedo, C.R.F. Selection of Fuel Cladding Material for Nuclear Fission Reactors. Eng. Fail. Anal. 2011, 18, 1943–1962. [Google Scholar] [CrossRef]
- Hallstadius, L.; Johnson, S.; Lahoda, E. Cladding fo high performance fuel. Prog. Nucl. Energy 2012, 57, 71–76. [Google Scholar] [CrossRef]
- Primakov, N.G.; Rudenko, V.A.; Kazarnikov, V.V.; Bespalov, A.G. Nonuniform Swelling and Hydrogen Redistribution in Zirconium Hydride under Neutron Irradiation. Int. J. Hydrog. Energy 1999, 24, 805–811. [Google Scholar] [CrossRef]
- Ribeiro, R.M.; Woyames, C.B.; de Almeida, L.H.; dos Santos, D.S. Effect of Microstructure and Addition of Alloying Elements on Hydriding Kinetics of Zr–Nb-based Alloys. Int. J. Hydrog. Energy 2015, 40, 17118–17127. [Google Scholar] [CrossRef]
- Kazarnikov, V.V.; Primakov, N.G.; Rudenko, V.A. Effect of neutron irradiation on the microstructure of zirconium hydride. Int. J. Hydrog. Energy 1997, 22, 169–173. [Google Scholar] [CrossRef]
- Zhao, C.; Song, X.; Yang, Y.; Zhang, B. Hydrogen Absorption Cracking of Zirconium Alloy in the Application of Nuclear Industry. Int. J. Hydrog. Energy 2013, 38, 10903–10911. [Google Scholar] [CrossRef]
- Ivanova, S.V. Hydrogen Effected Defects Evolution in Zirconium Items of Light-water Reactors. Int. J. Hydrog. Energy 2006, 31, 295–300. [Google Scholar] [CrossRef]
- Tang, R.; Yang, X. Dissolution and Precipitation Behaviors of Hydrides in N18, Zry-4 and M5 alloys. Int. J. Hydrog. Energy 2009, 34, 7269–7274. [Google Scholar] [CrossRef]
- Zielinski, A.; Sobieszczyk, S. Hydrogen-enhanced Degradation and Oxide Effects in Zirconium Alloys for Nuclear Applications. Int. J. Hydrog. Energy 2011, 36, 8619–8629. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Gong, W.; Zhang, H. Effect of Yttrium Addition on Microstructure and Orientation of Hydride Precipitation in Zr-1Nb Alloy. Int. J. Hydrog. Energy 2014, 39, 21116–21126. [Google Scholar] [CrossRef]
- Kearns, J.J. Terminal Solubility and Partitioning of Hydrogen in the Alpha–phase of Zirconium. Zircaloy-2 and Zircaloy-4. J. Nucl. Mater. 1967, 22, 292–303. [Google Scholar] [CrossRef]
- Nagase, F. Hydride Behavior in Zircaloy Cladding Tube during High-temperature Transients. J. Nucl. Mater. 2011, 415, 117–122. [Google Scholar] [CrossRef]
- Daum, R.S. The Influence of a Hydrided layer on the Fracture of Zircaloy-4 cladding Tubes. In Hydrogen Effects on Material Behavior and Corrosion Deformation Interactions; Moody, N.R., Thompson, A.W., Was, G.S., Jones, R.H., Eds.; The Minerals and Materials Society, Argonne National Lab.: Lemont, IL, USA, 2003; pp. 249–259. [Google Scholar]
- Motta, A.T.; Chen, L.Q. Hydride Formation in Zirconium Alloys. J. Miner. Met. Mater. Soc. 2012, 64, 1403–1408. [Google Scholar] [CrossRef]
- Nagase, F.; Fuketa, T. Investigation of Hydride Rim Effect on Failure of Zircaloy-4 Cladding with Tube Burst Test. J. Nucl. Sci. Technol. 2005, 42, 58–65. [Google Scholar] [CrossRef]
- Terrani, K.A.; Balooch, M.; Wongsawaeng, D.; Jaiyen, S.; Olander, D.R. The Kinetics of Hydrogen Desorption from and Adsorption on Zirconium Hydride. J. Nucl. Mater. 2010, 397, 61–68. [Google Scholar] [CrossRef]
- Suman, S.; Khan, M.K.; Pathak, M.; Singh, R.N. 3D Simulation of Hydride-assisted Crack Propagation in Zircaloy-4 Using XFEM. Int. J. Hydrog. Energy 2017, 42, 18668–18673. [Google Scholar] [CrossRef]
- Hanson, B.; Shimskey, R.; Lavender, C.; MacFarlan, P.; Eslinger, P. Hydride Rim Formation in Unirradiated Zircaloy. Available online: http://www.energy.gov/sites/prod/files/2013/08/f2/HydrideRimFormationZircaloy.pdf (accessed on 30 December 2019).
- Shimskey, R.; Hanson, B.; MacFarlan, P. Optimization of Hydride Rim Formation in Unirradiated Zr-4 Cladding. Available online: http://www.pnnl.gov/main/publications/external/technical_reports/PNNL-22835.pdf (accessed on 30 December 2019).
- Pushilina, N.S.; Lider, A.M.; Kudiiarov, V.N.; Chernov, I.P.; Ivanova, S.V. Hydrogen effect on zirconium alloy surface treated by pulsed electron beam. J. Nucl. Mater. 2015, 456, 311–315. [Google Scholar] [CrossRef] [Green Version]
- Motta, A.T.; Capulongo, L.; Chen, L.-Q.; Cinbiz, M.; Daymond, M.; Koss, D.A.; Lacroix, E.; Pastore, G.; Simin, P.-C.; Tonks, M.R.; et al. Hydrogen in Zirconium Alloys: A review. J. Nucl. Mater. 2019, 518, 440–460. [Google Scholar] [CrossRef] [Green Version]
- Pushilina, N.S.; Kudiiarov, V.N.; Laptev, R.S.; Lider, A.M.; Teresov, A.D.R. Microstructure Changes in Zr–1Nb Alloy after Pulsed Electron Beam Surface Modification and Hydrogenation. Surf. Coat. Technol. 2015, 284, 63–68. [Google Scholar] [CrossRef]
- Kudiiarov, V.N.; Kashkarov, E.B.; Syrtanov, M.S.; Lider, A.M. Hydrogen Sorption by Ni-coated Titanium alloy VT1-0. Int. J. Hydrog. Energy 2017, 42, 10604–10610. [Google Scholar] [CrossRef]
- Kido, T.; Sugano, M. Development of a Method to Charge Hydrogen into Zirconium Alloys. Nippon Genshiryoku Gakkai Wabun Ronbunshi 2002, 1, 469–471. [Google Scholar] [CrossRef] [Green Version]
- Zuzek, E.; Abriata, J.P.; San-Martin, A.; Manchester, F.D. The H-Zr (hydrogen-zirconium) System. Bull. Alloy Phase Diagr. 1990, 11, 385–395. [Google Scholar] [CrossRef]
- Sindelar, R.L.; Louthan, M.R.; Hanson, B.D. White Paper Summary of 2nd ASTM International Workshop on Hydrides in Zirconium Alloy Cladding. Available online: http://sti.srs.gov/fulltext/FCRD-UFD-2015-000533_SRNL-STI-2015-00256.pdf (accessed on 12 February 2020).
| Hydrogenation Temperature | Observed Phases | Phase Content, Volume.% | Lattice Parameters |
|---|---|---|---|
| 350 °C | Zirconium hydride | 55 | a = 4.7776 |
| Zirconium | 45 | a = 3.2299; c = 5.1380 | |
| 380 °C | Zirconium hydride | 19 | a = 4.7656 |
| Zirconium | 81 | a = 3.2294; c = 5.1398 | |
| 420 °C | Zirconium hydride | 5 | a = 4.7632 |
| Zirconium | 95 | a = 3.2243; c = 5.1401 |
| Hydrogen Concentration | Observed Phases | Phase Content, Volume.% | Lattice Parameters |
|---|---|---|---|
| 0.1 wt.% | Zirconium hydride | 55 | a = 4.7776 |
| Zirconium | 45 | a = 3.2299; c = 5.1380 | |
| 0.25 wt.% | Zirconium hydride | 27 | a = 4.7668 |
| Zirconium | 73 | a = 3.2294; c = 5.1398 | |
| 0.4 wt.% | Zirconium hydride | 83 | a = 4.7768 |
| Zirconium | 17 | a = 3.2300; c = 5.1450 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudiiarov, V.; Sakvin, I.; Syrtanov, M.; Slesarenko, I.; Lider, A. Hydride Rim Formation in E110 Zirconium Alloy during Gas-Phase Hydrogenation. Metals 2020, 10, 247. https://doi.org/10.3390/met10020247
Kudiiarov V, Sakvin I, Syrtanov M, Slesarenko I, Lider A. Hydride Rim Formation in E110 Zirconium Alloy during Gas-Phase Hydrogenation. Metals. 2020; 10(2):247. https://doi.org/10.3390/met10020247
Chicago/Turabian StyleKudiiarov, Viktor, Ivan Sakvin, Maxim Syrtanov, Inga Slesarenko, and Andrey Lider. 2020. "Hydride Rim Formation in E110 Zirconium Alloy during Gas-Phase Hydrogenation" Metals 10, no. 2: 247. https://doi.org/10.3390/met10020247
APA StyleKudiiarov, V., Sakvin, I., Syrtanov, M., Slesarenko, I., & Lider, A. (2020). Hydride Rim Formation in E110 Zirconium Alloy during Gas-Phase Hydrogenation. Metals, 10(2), 247. https://doi.org/10.3390/met10020247







