Kinetic Models for the in Situ Reaction between Cu-Ti Melt and Graphite
Abstract
:1. Introduction
2. Model Descriptions
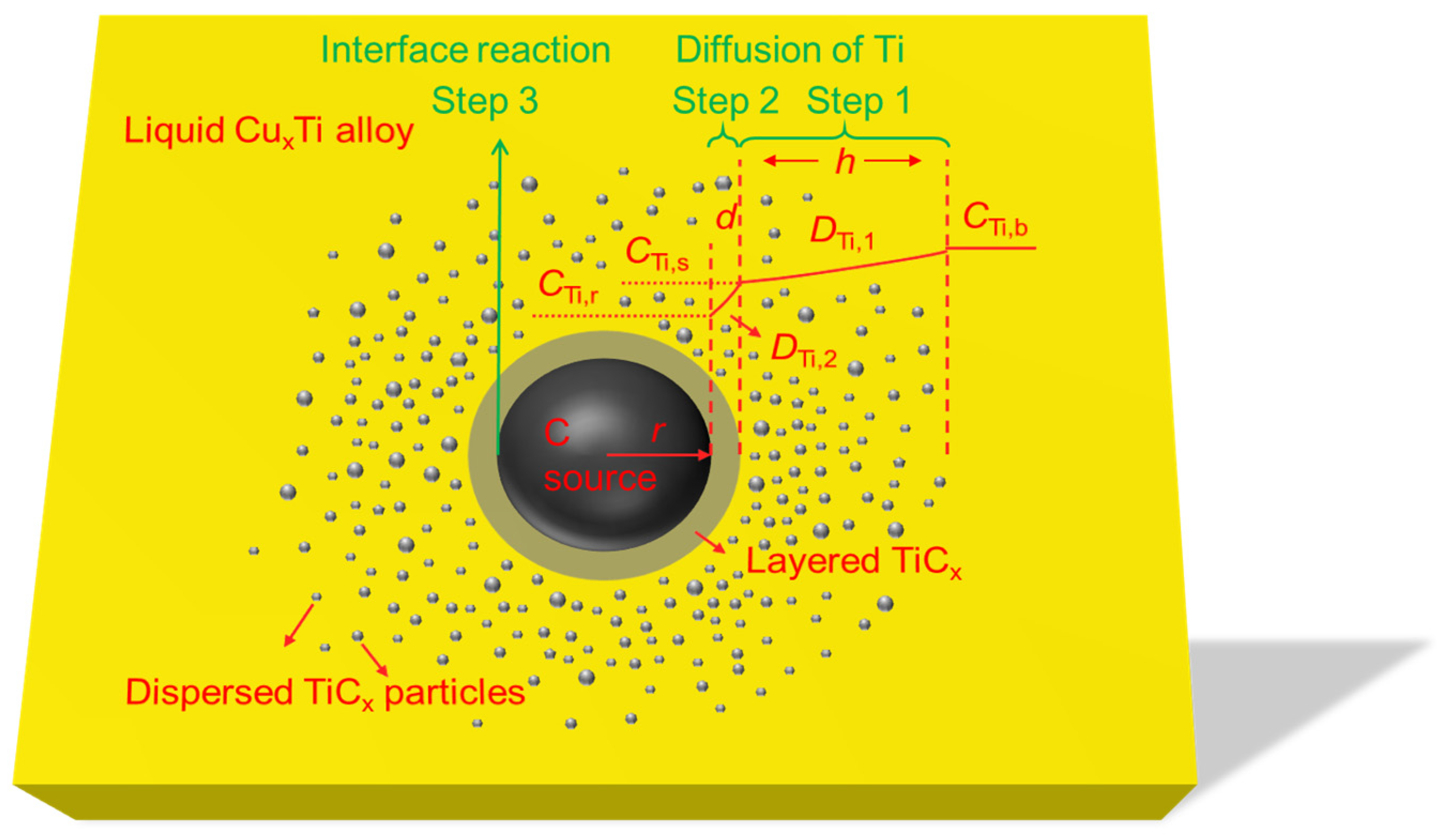
2.1. Basic Assumptions
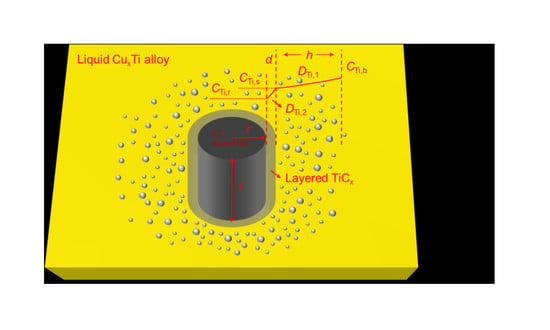
2.2. Spherical C Source
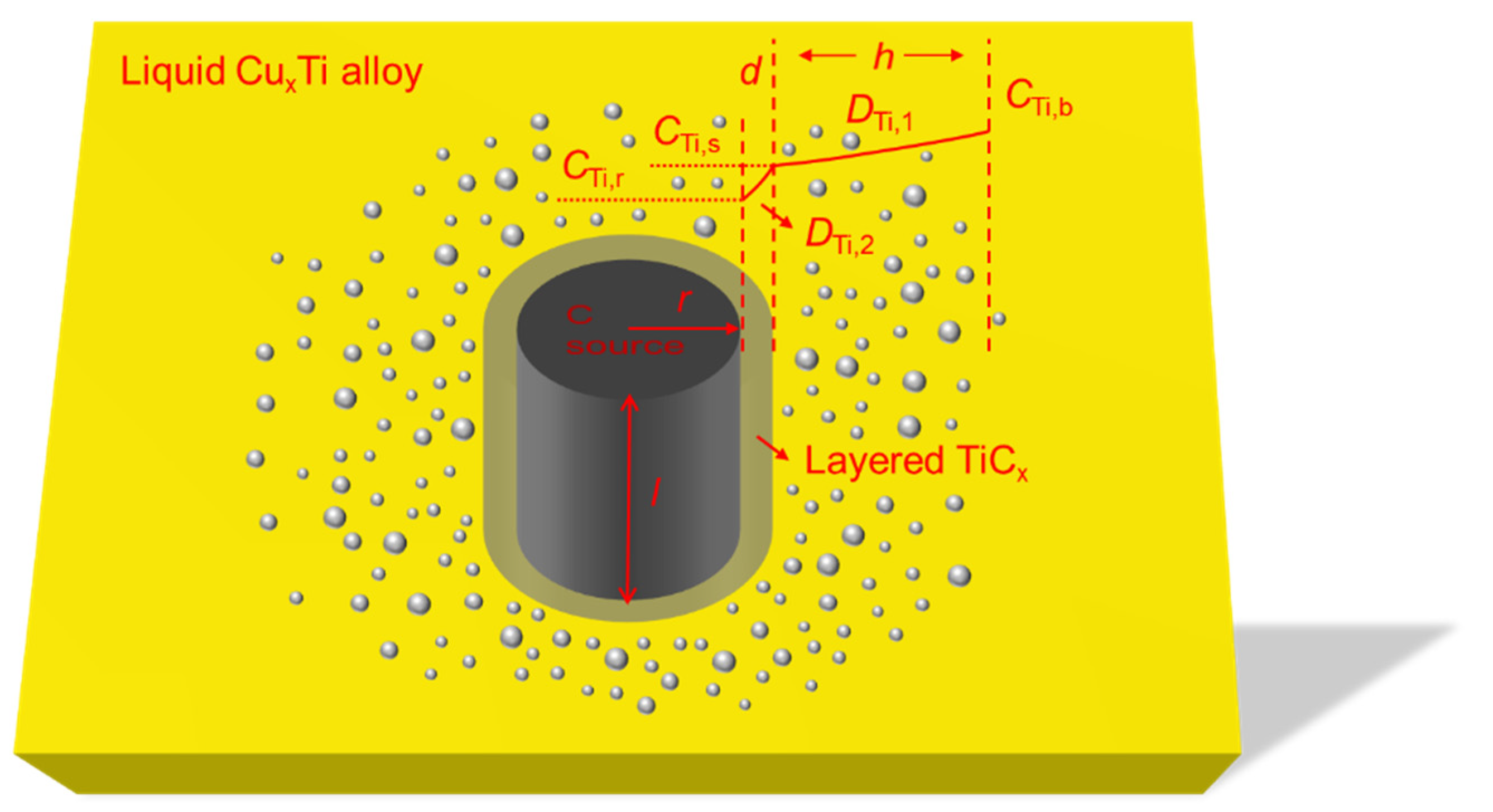
2.3. Cylindrical C Source
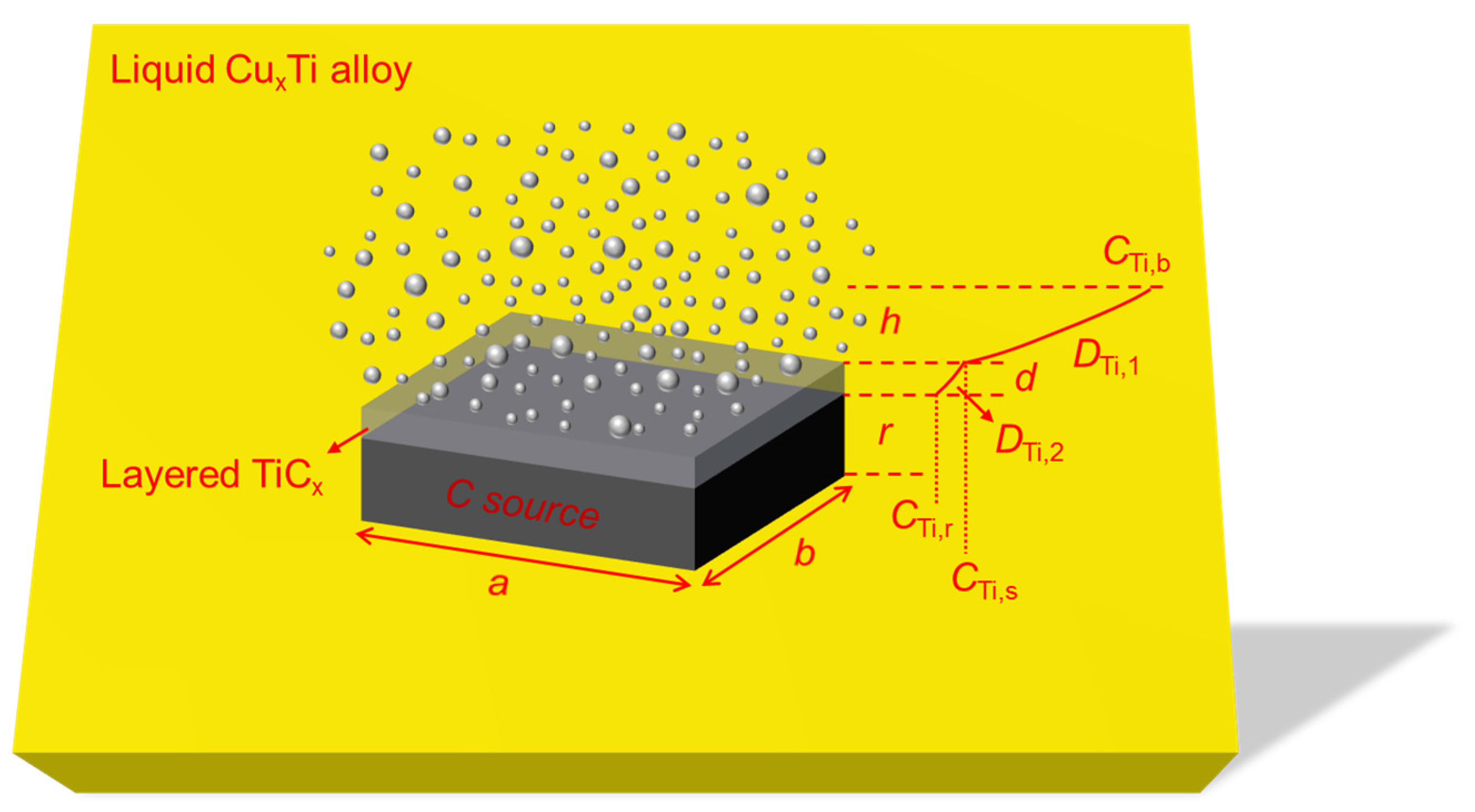
2.4. Flat C Source
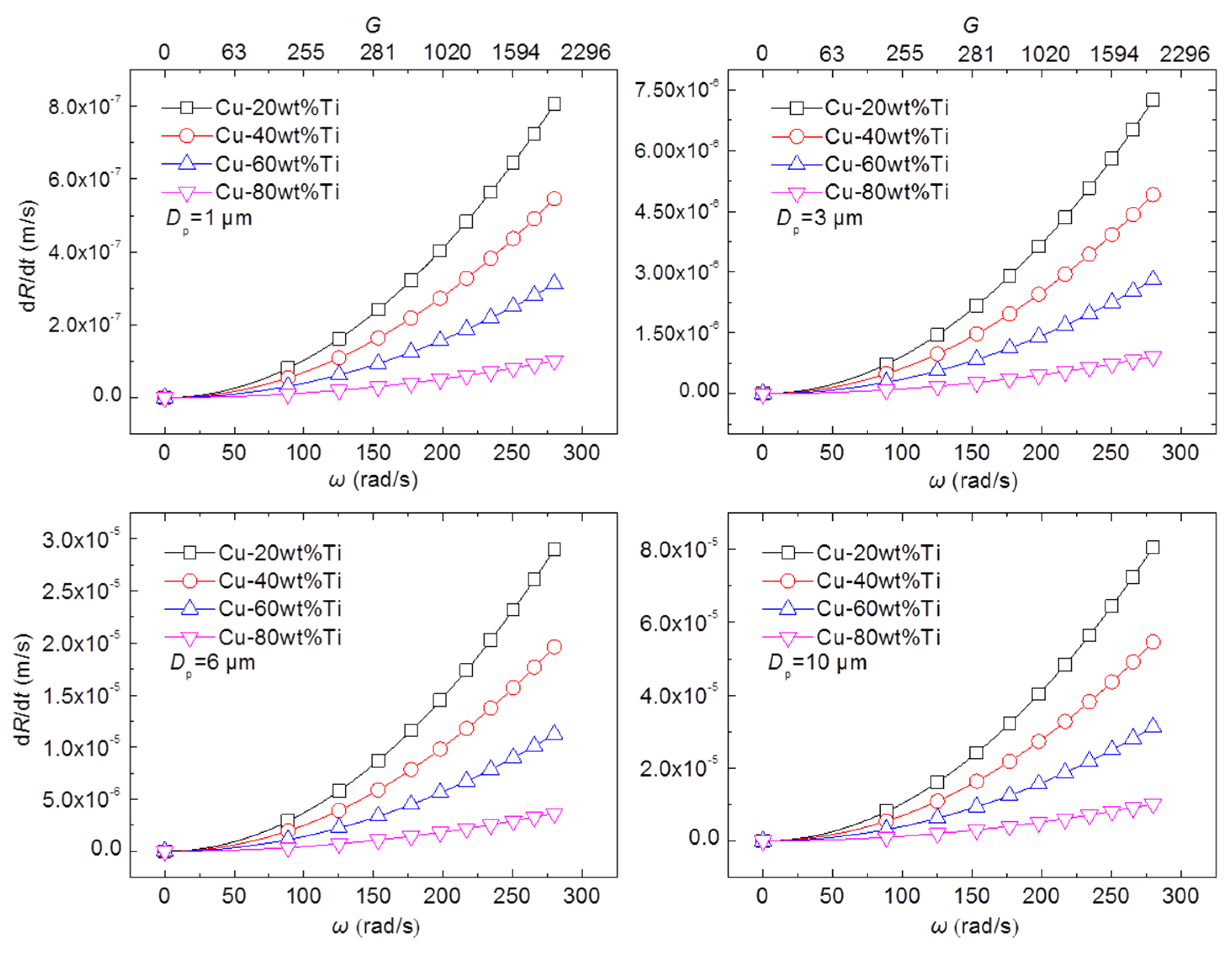
2.5. The Reaction Behavior in Super-Gravity Field
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ṅTi,1 | Diffusion flux of Ti atoms from the distant Cu-Ti melt to the layered TiCx surface (mol/s); |
| r | Radius of the C source particles (m); |
| DTi,1 | Diffusion coefficient of Ti atoms in the Cu-Ti melt (m2/s); |
| CTi | Concentration of Ti atoms (mol/m3); |
| CTi,b | Concentration of Ti atoms in the Cu-Ti melt (mol/m3); |
| CTi,s | Concentration of Ti atoms on the layered TiCx surface (mol/m3); |
| d | Thickness of the layered TiCx (m); |
| h | Concentration transition layer thickness of Ti atom in the Cu-Ti melt (m); |
| ṅTi,2 | Diffusion flux of Ti atoms through the layered TiCx product layer (mol/s); |
| DTi,2 | Diffusion coefficient of Ti atoms in the layered TiCx product layer (m2/s); |
| CTi,r | Ti atom concentration on the surface of the solid C source particle (mol/m3); |
| ṅTi,r | Reaction rate of TiCx generated at the surface of C source (mol/s); |
| kr | Chemical reaction rate constant; |
| rTi | Ti atom consumption rate of the overall process (mol/s); |
| rc | Consumption rate of solid C source during the reaction (mol/s); |
| Molar density of the solid C source (mol/m3); | |
| x | Stoichiometry of the product TiCx; |
| r0 | Initial radius of the solid C source particles (m); |
| t | Reaction time (s); |
| a, b | Length and width of the flat solid C source (m); |
| Rate of reaction defined by the consuming molar rate of Ti per unit time per unit surface area of C source (mol/(s·m2)); | |
| S | Surface area of the C source particle (m2); |
| Ap | Original surface area of the C source; |
| Fp | Shape factor of the C source; |
| Vp | Original volume of the C source; |
| R | Distance between the particles and the rotating axis of the centrifugal machine (m); |
| ρTiCx | Density of TiCx particles (kg/m3); |
| ρCu-Ti | Density of molten Cu-Ti alloy (kg/m3); |
| △ρ | |ρTiCx − ρCu-Ti | |
| Dp | Diameter of TiCx particles (m); |
| G | Super-gravity coefficient; |
| g | Gravitational acceleration (m/s2); |
| η | Viscosity of liquid (Pa·s); |
| ηCu | Viscosity of molten Cu (Pa·s); |
| ηTi | Viscosity of molten Ti (Pa·s); |
| ηCu-Ti | Viscosity of Cu-Ti melt (Pa·s); |
| ω | Rotational angular velocity (rad/s); |
| fCu | Mass fraction of Cu in the Cu-Ti alloy; |
| fTi | Mass fraction of Ti in the Cu-Ti alloy; |
| η0 | Viscosity of the molten metal without particles (Pa·s); |
| ε | Particle volume fraction of TiCx; |
| εmax | Maximum packing fraction of TiCx; |
| VTiCx | Volume of TiCx produced per unit time; |
| Vocc | Volume of TiCx particles precipitated in the melt per unit time. |
References
- Gotoh, Y.; Fujimura, K.; Koike, M.; Ohkoshi, Y.; Nagura, M.; Akamatsu, K.; Deki, S. Synthesis of titanium carbide from a composite of TiO2 nanoparticles/methyl cellulose by carbothermal reduction. Mater. Res. Bull. 2001, 36, 2263–2275. [Google Scholar] [CrossRef]
- AlMangour, B.; Grzesiak, D.; Yang, J.M. In situ formation of TiC-particle-reinforced stainless steel matrix nanocomposites during ball milling: Feedstock powder preparation for selective laser melting at various energy densities. Powder Technol. 2018, 326, 467–478. [Google Scholar] [CrossRef]
- Sen, W.; Xu, B.Q.; Yang, B.; Sun, H.Y.; Song, J.X.; Wan, H.L.; Dai, Y.N. Preparation of TiC powders by carbothermal reduction method in vacuum. Trans. Nonferrous Met. Soc. China 2011, 21, 185–190. [Google Scholar] [CrossRef]
- Lee, J.; Thadhani, N.N. Reaction synthesis mechanism in dynamically densified Ti+C powder compacts. Scripta Mater. 1997, 37, 1979–1985. [Google Scholar] [CrossRef]
- Boutefnouchet, H.; Curfs, C.; Triki, A.; Boutefnouchet, A.; Vrel, D. Self-propagating high-temperature synthesis mechanisms within the Ti–C–Ni system: A time resolved X-ray diffraction study. Powder Technol. 2012, 217, 443–450. [Google Scholar] [CrossRef]
- Contreras, L.; Turrillas, X.; Mas-Guindal, M.J.; Vaughan, G.B.M.; Kvick, Å.; Rodríguez, M.A. Synchrotron diffraction studies of TiC/FeTi cermets obtained by SHS. J. Solid State Chem. 2005, 178, 1595–1600. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, Q.; Zhang, Z.; Li, X.; Ren, L. Preparation and characterization of TiC particulate locally reinforced steel matrix composites from Cu–Ti–C system with various C particles. J. Asian Ceram. Soc. 2014, 2, 281–288. [Google Scholar] [CrossRef]
- Wei, S.F.; Feng, K.Q.; Chen, H.S.; Xiong, J.; Fan, H.Y.; Zhang, G.M.; Wang, H. Combustion synthesis of TiC/Fe–Cu composites under an electric field. J. Alloys Compd. 2012, 541, 186–191. [Google Scholar] [CrossRef]
- Zhang, M.X.; Hu, Q.D.; Huang, B.; Li, J.Z.; Li, J.G. Study of formation behavior of TiC in the Fe–Ti–C system during combustion synthesis. Int. J. Refract. Met. Hard Mater. 2011, 29, 356–360. [Google Scholar] [CrossRef]
- Thi Hoang Oanh, N.; Hoang Viet, N.; Kim, J.; Moreira Jorge Junior, A. Characterization of In-Situ Cu–TiH2–C and Cu–Ti–C Nanocomposites Produced by Mechanical Milling and Spark Plasma Sintering. Metals 2017, 7, 117. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Li, Y.; Wakoh, K.; Koizumi, Y.; Chiba, A. Cu–Ti–C alloy with high strength and high electrical conductivity prepared by two-step ball-milling processes. Mater. Des. 2014, 61, 70–74. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Yamanaka, K.; Wakon, K.; Harata, K.; Chiba, A. Influence of two-step ball-milling condition on electrical and mechanical properties of TiC-dispersion-strengthened Cu alloys. Mater. Des. 2014, 64, 441–449. [Google Scholar] [CrossRef]
- Zhu, H.; Dong, K.; Wang, H.; Huang, J.; Li, J.; Xie, Z. Reaction mechanisms of the TiC/Fe composite fabricated by exothermic dispersion from Fe–Ti–C element system. Powder Technol. 2013, 246, 456–461. [Google Scholar] [CrossRef]
- Ding, H.; Chu, W.; Wang, Q.; Miao, W.; Wang, H.; Liu, Q.; Glandut, N.; Li, C. The in-situ synthesis of TiC in Cu melts based on Ti–C–Si system and its mechanism. Mater. Des. 2019, 182, 108007. [Google Scholar] [CrossRef]
- Rambo, C.R.; Travitzky, N.; Zimmermann, K.; Greil, P. Synthesis of TiC/Ti–Cu composites by pressureless reactive infiltration of TiCu alloy into carbon preforms fabricated by 3D-printing. Mater. Lett. 2005, 59, 1028–1031. [Google Scholar] [CrossRef]
- Wen, X.C.; Guo, L.; Bao, Q.P.; Gao, J.T.; Guo, Z.C. Efficient separation of lead and antimony metals from the Pb-Sb alloy by super-gravity technology. J. Alloys Compd. 2019, 806, 1012–1021. [Google Scholar] [CrossRef]
- Zhang, R.; He, X.; Liu, Q.; Qu, X. Improvement in Mechanical and Thermal Properties of Graphite Flake/Cu Composites by Introducing TiC Coating on Graphite Flake Surface. Metals 2019, 9, 519. [Google Scholar] [CrossRef] [Green Version]
- Tuan, N.Q.; Khoa, H.X.; Vieta, N.H.; Lee, Y.H.; Lee, B.H.; Kim, J.S. Fabrication of Fe-TiC Composite by High-Energy Milling and Spark-Plasma Sintering. J. Korean Powder Metall. Inst. 2013, 20, 338–344. [Google Scholar] [CrossRef] [Green Version]
- Rao, P.P.; Kumar, J.P.; Rahul, R. Production of Copper Metal Matrix Composite through Powder Metallurgy Route. Int. J. Eng. Technol. Sci. Res. 2017, 4, 855–864. [Google Scholar] [CrossRef]
- Akhtar, F.; Askari, S.J.; Shah, K.A.; Du, X.; Guo, S. Microstructure, mechanical properties, electrical conductivity and wear behavior of high volume TiC reinforced Cu-matrix composites. Mater. Charact. 2009, 60, 327–336. [Google Scholar] [CrossRef]
- Rathod, S.; Modi, O.P.; Prasad, B.K.; Chrysanthou, A.; Vallauri, D.; Deshmukh, V.P.; Shah, A.K. Cast in situ Cu–TiC composites: Synthesis by SHS route and characterization. Mater. Sci. Eng. A 2009, 502, 91–98. [Google Scholar] [CrossRef]
- Tjong, S.C.; Ma, Z.Y. Microstructural and mechanical characteristics of in situ metal matrix composites. Mater. Sci. Eng. R: Rep. 2000, 29, 49–113. [Google Scholar] [CrossRef]
- Westwood, A.R.C. Materials for advanced studies and devices. Metall. Trans. A 1988, 19, 749–758. [Google Scholar] [CrossRef]
- Mao, W.; Yamaki, T.; Miyoshi, N.; Shinozaki, N.; Ogawa, T. Wettability of Cu-Ti alloys on graphite in different placement states of copper and titanium at 1373 K (1100 °C). MMTA 2015, 46, 2262–2272. [Google Scholar] [CrossRef]
- Casati, R.; Vedani, M. Metal Matrix Composites Reinforced by Nano-Particles—A Review. Metals 2014, 4, 65–83. [Google Scholar] [CrossRef]
- Dudina, D.V.; Vidyuk, T.M.; Korchagin, M.A.; Gavrilov, A.I.; Bulina, N.V.; Esikov, M.A.; Datekyu, M.; Kato, H. Interaction of a Ti–Cu Alloy with Carbon: Synthesis of Composites and Model Experiments. Materials 2019, 12, 1482. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Yang, Y.R.; Wen, X.C.; Gao, H.; Wang, Z.; Guo, Z. Synthesis of Cu-based TiCx composites via in-situ reaction between CuxTi melt and dissolvable solid carbon. Powder Technol. 2020, 362, 375–385. [Google Scholar] [CrossRef]
- Li, C.; Gao, J.T.; Wang, Z.; Ren, H.R.; Guo, Z.C. Separation of Fe-bearing and P-bearing phase from the steelmaking slag by super fravity. ISIJ Int. 2017, 57, 767–769. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Gao, J.T.; Wang, F.Q.; Guo, Z.C. Separation of anosovite from modified Titanium-bearing slag melt in a reducing atmosphere by supergravity. Metall. Mater. Trans. B 2017, 48, 749–753. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Z.; Guo, L.; Meng, L.; Guo, Z.C. Supergravity process for enriching and separating Ag from Sn–Ag–Zn melts. Chem. Eng. Process. Process Intensif. 2019, 143, 107591. [Google Scholar] [CrossRef]
- Meng, L.; Zhong, Y.W.; Guo, L.; Wang, Z.; Chen, K.Y.; Guo, Z.C. Recovery of Cu and Zn from waste printed circuit boards using super-gravity separation. Waste Manag. 2018, 78, 559–565. [Google Scholar] [CrossRef]
- Guo, L.; Wen, X.C.; Bao, Q.P.; Guo, Z.C. Removal of Tramp Elements within 7075 Alloy by Super-Gravity Aided Rheorefining Method. Metals 2018, 8, 701. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.T.; Zhong, Y.W.; Guo, L.; Guo, Z.C. Separation of Iron Phase and P-Bearing Slag Phase from Gaseous-Reduced, High-Phosphorous Oolitic Iron Ore at 1473 K (1200 °C) by Super Gravity. Metall. Mater. Trans. B 2016, 47, 1080–1092. [Google Scholar] [CrossRef]
- Guo, L.; Gao, J.T.; Li, C.; Guo, Z.C. Removal of Fine SiO2/Composite Inclusions from 304 Stainless Steel Using Super-gravity. ISIJ Int. 2020. [Google Scholar] [CrossRef] [Green Version]
- Kivi, M.; Holappa, L.; Louhenkilpi, S.; Nakamoto, M.; Tanaka, T. Studies on Interfacial Phenomena in Titanium Carbide/Liquid Steel Systems for Development of Functionally Graded Material. Metall. Mater. Trans. B 2016, 47, 2114–2122. [Google Scholar] [CrossRef]
- Hayashi, T.; Matsuura, K.; Ohno, M. TiC coating on titanium by carbonization reaction using spark plasma sintering. Mater. Trans. 2013, 54, 2098–2101. [Google Scholar] [CrossRef] [Green Version]
- Szekely, J.; Evans, J.W.; Sohn, H.Y. Gas-Solid Reactions; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Watanabe, Y.; Kawamoto, A.; Matsuda, K. Particle size distributions in functionally graded materials fabricated by the centrifugal solid-particle method. Compos. Sci. Technol. 2002, 62, 881–888. [Google Scholar] [CrossRef]
- Watanabe, Y.; Yamanaka, N.; Fukui, Y. Control of composition gradient in a metal-ceramic functionally graded material manufactured by the centrifugal method. Compos. Part A 1998, 29, 595–601. [Google Scholar] [CrossRef]
- Ishikawa, T.; Paradis, P.; Okada, J.T.; Watanabe, Y. Viscosity measurements of molten refractory metals using an electrostatic levitator. Meas. Sci. Technol. 2012, 23, 25305. [Google Scholar] [CrossRef]
- Kaptay, G. A unified equation for the viscosity of pure liquid metals. Mater. Res. Adv. Tech. 2005, 96, 24–31. [Google Scholar] [CrossRef]
- Battezzati, L.; Greer, A.L. The viscosity of liquid metals and alloys. Acta Metall. 1989, 37, 1791–1802. [Google Scholar] [CrossRef]
- Brinkman, H.C. The Viscosity of Concentrated Suspensions and Solutions. J. Chem. Phys. 1952, 20, 571. [Google Scholar] [CrossRef]

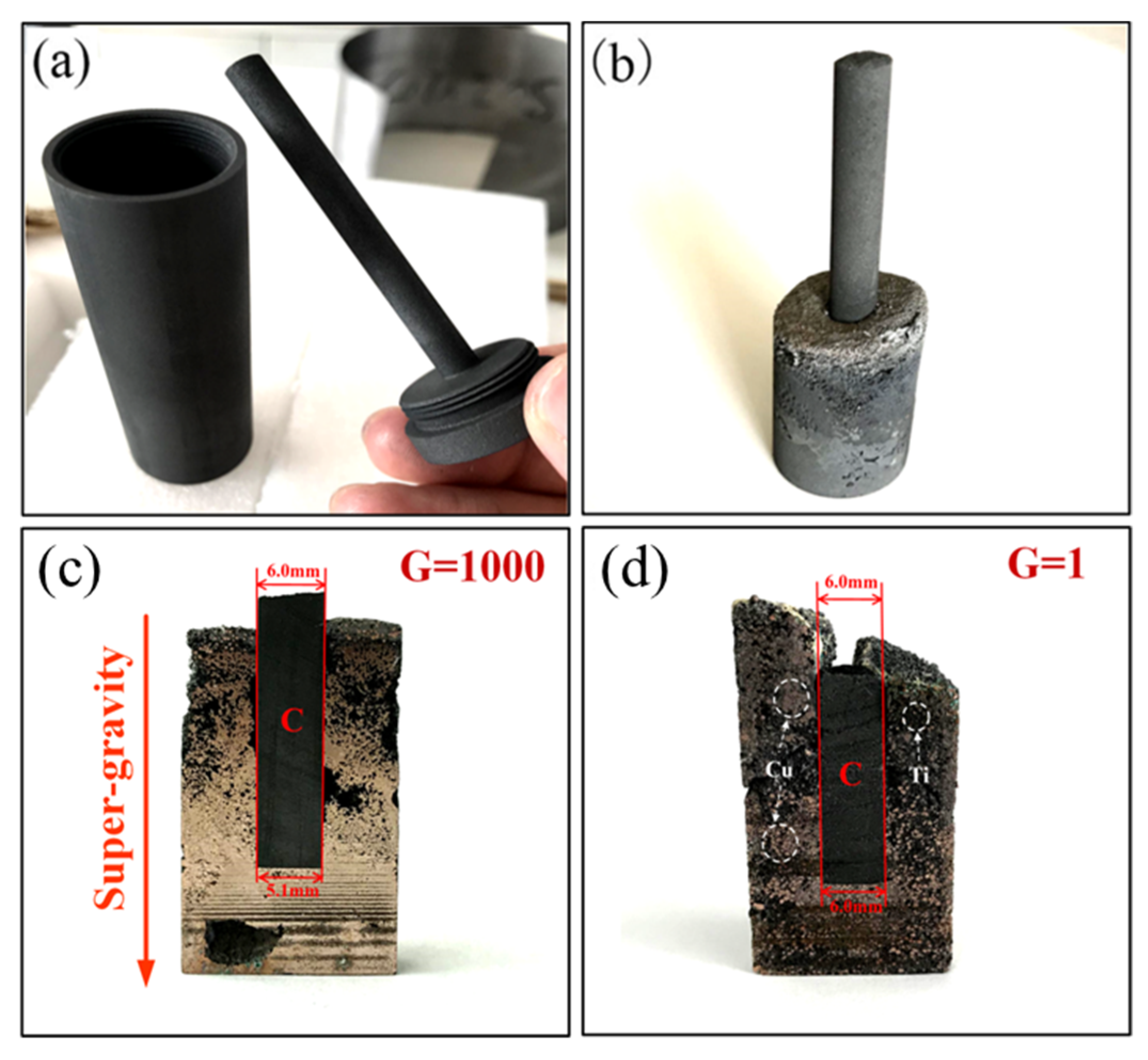
| Cu-20 wt.% Ti | Cu-40 wt.% Ti | Cu-60 wt.% Ti | Cu-80 wt.% Ti | |
|---|---|---|---|---|
| ρCu-Ti (kg/m3) | 8068 | 7176 | 6284 | 5392 |
| △ρ (kg/m3) | 3138 | 2246 | 1354 | 462 |
| ηCu-Ti (× 10−3 Pa·s) | 4.24 | 4.48 | 4.72 | 4.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Wen, X.; Guo, Z. Kinetic Models for the in Situ Reaction between Cu-Ti Melt and Graphite. Metals 2020, 10, 267. https://doi.org/10.3390/met10020267
Guo L, Wen X, Guo Z. Kinetic Models for the in Situ Reaction between Cu-Ti Melt and Graphite. Metals. 2020; 10(2):267. https://doi.org/10.3390/met10020267
Chicago/Turabian StyleGuo, Lei, Xiaochun Wen, and Zhancheng Guo. 2020. "Kinetic Models for the in Situ Reaction between Cu-Ti Melt and Graphite" Metals 10, no. 2: 267. https://doi.org/10.3390/met10020267