Influence of Martensite Deformation on Cu Precipitation Strengthening
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
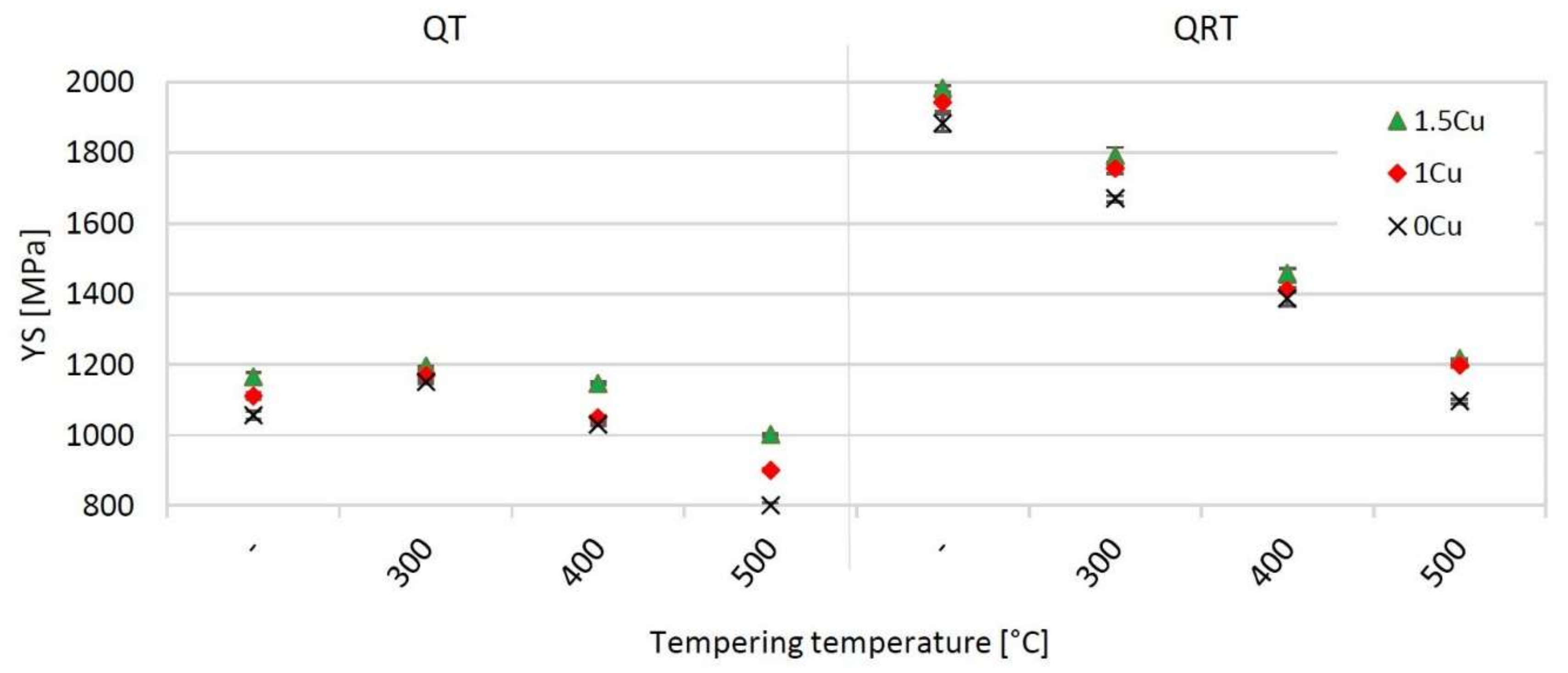
4.1. QT Samples–Yield Stress
4.2. QRT Samples—Yield Stress
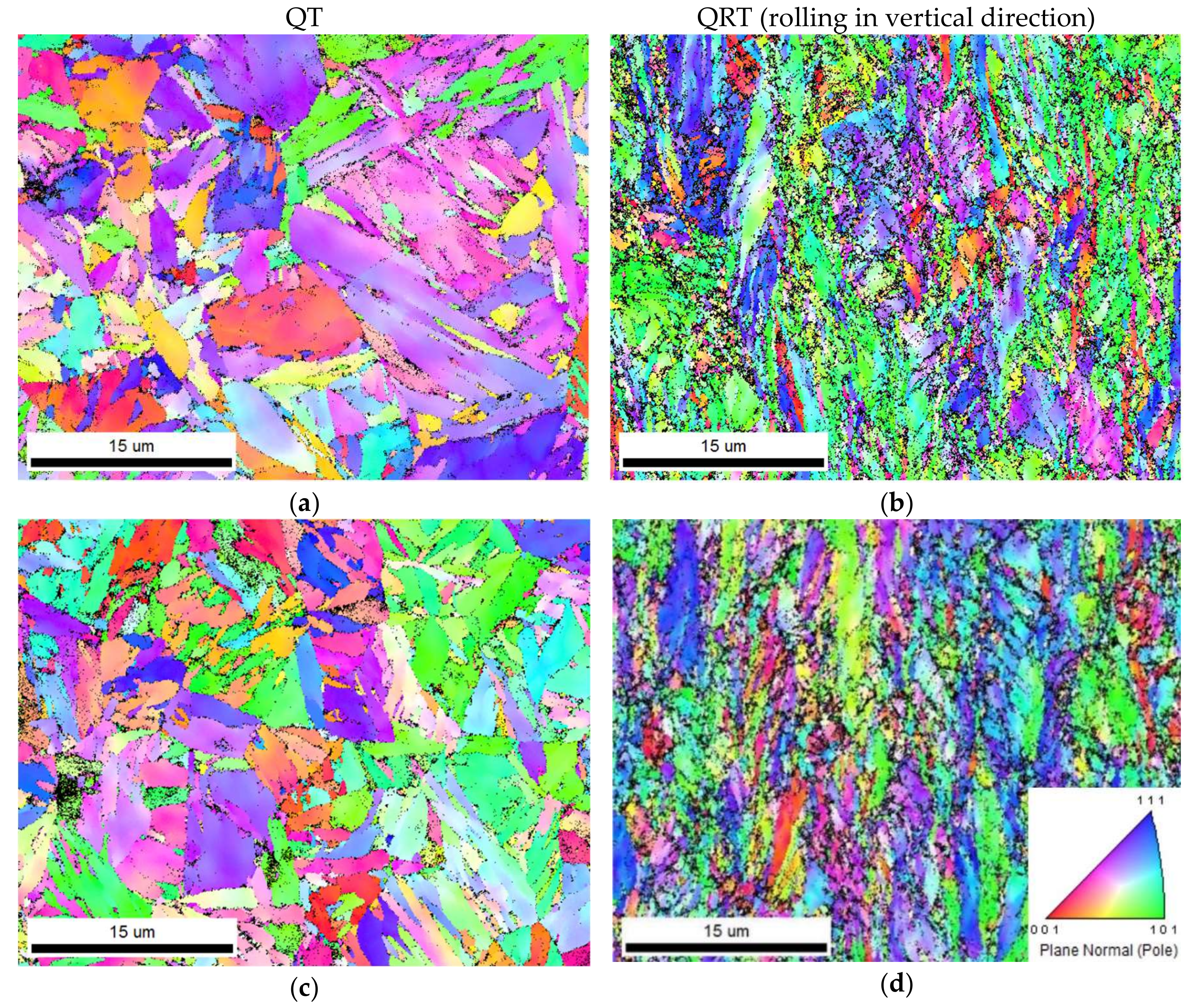
4.3. Subgrain Structure
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Regime | Material | Tempering Time [min] | YS [MPa] | UTS [MPa] | Ag [%] | A5 [%] | Z [%] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QT | 0Cu | 0 | 1057 | ± | 11 | 1447 | ± | 11 | 3.6 | ± | 0.1 | 13.9 | ± | 0.2 | 43.7 | ± | 0.1 |
| QT | 0Cu | 15 | 1062 | ± | 4 | 1101 | ± | 2 | 2.6 | ± | 0.3 | 12.6 | ± | 0.2 | 56.2 | ± | 0.8 |
| QT | 0Cu | 30 | 1030 | ± | 2 | 1051 | ± | 6 | 1.6 | ± | 0.7 | 11.9 | ± | 1.0 | 56.3 | ± | 1.2 |
| QT | 0Cu | 60 | 982 | ± | 12 | 1010 | ± | 19 | 2.8 | ± | 0.6 | 12.0 | ± | 0.1 | 57.0 | ± | 2.7 |
| QT | 0Cu | 120 | 1100 | ± | 2 | 1156 | ± | 8 | 2.2 | ± | 0.5 | 11.1 | ± | 2.0 | 52.2 | ± | 2.3 |
| QT | 1Cu | 0 | 1105 | ± | 3 | 1495 | ± | 11 | 3.1 | ± | 0.3 | 11.2 | ± | 0.5 | 46.8 | ± | 1.0 |
| QT | 1Cu | 15 | 1097 | ± | 1 | 1137 | ± | 7 | 2.6 | ± | 0.3 | 10.2 | ± | 0.7 | 54.3 | ± | 1.8 |
| QT | 1Cu | 30 | 1071 | ± | 8 | 1091 | ± | 1 | 2.1 | ± | 0.6 | 10.4 | ± | 0.8 | 55.4 | ± | 1.8 |
| QT | 1Cu | 60 | 1043 | ± | 3 | 1059 | ± | 11 | 2.2 | ± | 0.9 | 11.4 | ± | 0.4 | 51.5 | ± | 1.1 |
| QT | 1Cu | 120 | 1038 | ± | 5 | 1050 | ± | 6 | 2.5 | ± | 0.1 | 12.6 | ± | 0.3 | 51.5 | ± | 1.7 |
| QT | 1.5Cu | 0 | 1166 | ± | 4 | 1520 | ± | 5 | 1.9 | ± | 0.2 | 12.9 | ± | 1.5 | 48.6 | ± | 0.8 |
| QT | 1.5Cu | 15 | 1156 | ± | 7 | 1203 | ± | 5 | 3.1 | ± | 0.1 | 11.2 | ± | 0.8 | 54.6 | ± | 0.0 |
| QT | 1.5Cu | 30 | 1153 | ± | 2 | 1183 | ± | 1 | 3.4 | ± | 0.1 | 12.0 | ± | 0.7 | 51.4 | ± | 0.5 |
| QT | 1.5Cu | 60 | 1147 | ± | 3 | 1157 | ± | 2 | 1.7 | ± | 0.1 | 11.7 | ± | 0.6 | 54.2 | ± | 0.4 |
| QT | 1.5Cu | 120 | 1128 | ± | 2 | 1142 | ± | 3 | 3.9 | ± | 0.6 | 15.4 | ± | 0.7 | 51.5 | ± | 1.0 |
| QRT | 0Cu | 0 | 1884 | ± | 25 | 1911 | ± | 24 | 0.4 | ± | 0.1 | 4.5 | ± | 1.0 | 29.8 | ± | 14.9 |
| QRT | 0Cu | 15 | 1411 | ± | 25 | 1419 | ± | 22 | 0.3 | ± | 0.1 | 4.5 | ± | 0.4 | 18.9 | ± | 8.7 |
| QRT | 0Cu | 30 | 1393 | ± | 12 | 1402 | ± | 11 | 0.3 | ± | 0.1 | 4.8 | ± | 1.2 | 16.9 | ± | 8.1 |
| QRT | 0Cu | 60 | 1388 | ± | 23 | 1396 | ± | 26 | 0.3 | ± | 0.1 | 3.8 | ± | 0.5 | 9.0 | ± | 0.8 |
| QRT | 0Cu | 120 | 1328 | ± | 12 | 1334 | ± | 12 | 0.4 | ± | 0.1 | 6.4 | ± | 0.5 | 16.0 | ± | 9.5 |
| QRT | 1Cu | 0 | 1943 | ± | 27 | 1976 | ± | 22 | 0.4 | ± | 0.1 | 4.4 | ± | 0.7 | 30.1 | ± | 4.0 |
| QRT | 1Cu | 15 | 1451 | ± | 39 | 1456 | ± | 36 | 0.4 | ± | 0.1 | 6.4 | ± | 1.0 | 12.2 | ± | 9.0 |
| QRT | 1Cu | 30 | 1456 | ± | 29 | 1458 | ± | 25 | 0.3 | ± | 0.1 | 6.1 | ± | 0.7 | 24.1 | ± | 4.2 |
| QRT | 1Cu | 60 | 1410 | ± | 7 | 1412 | ± | 11 | 0.3 | ± | 0.1 | 5.1 | ± | 0.4 | 17.3 | ± | 3.8 |
| QRT | 1Cu | 120 | 1376 | ± | 23 | 1380 | ± | 22 | 0.3 | ± | 0.1 | 6.6 | ± | 0.5 | 13.2 | ± | 1.7 |
| QRT | 1.5Cu | 0 | 1983 | ± | 7 | 2006 | ± | 10 | 0.4 | ± | 0.1 | 4.3 | ± | 0.4 | 22.8 | ± | 3.7 |
| QRT | 1.5Cu | 15 | 1496 | ± | 19 | 1500 | ± | 18 | 0.3 | ± | 0.1 | 5.6 | ± | 0.2 | 15.4 | ± | 0.7 |
| QRT | 1.5Cu | 30 | 1505 | ± | 5 | 1506 | ± | 5 | 0.3 | ± | 0.1 | 4.8 | ± | 0.1 | 12.0 | ± | 0.9 |
| QRT | 1.5Cu | 60 | 1459 | ± | 14 | 1462 | ± | 14 | 0.3 | ± | 0.1 | 4.7 | ± | 0.9 | 16.3 | ± | 2.9 |
| QRT | 1.5Cu | 120 | 1413 | ± | 7 | 1415 | ± | 7 | 0.2 | ± | 0.1 | 6.0 | ± | 0.4 | 8.8 | ± | 4.2 |
| Regime | Material | Tempering Temperature [°C] | YS [MPa] | UTS [MPa] | Ag [%] | A5 [%] | Z [%] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QT | 0Cu | - | 1057 | ± | 11 | 1447 | ± | 11 | 3.6 | ± | 0.1 | 13.9 | ± | 0.2 | 43.7 | ± | 0.1 |
| QT | 0Cu | 300 | 1151 | ± | 4 | 1252 | ± | 3 | 2.5 | ± | 0.1 | 12.6 | ± | 0.2 | 54.7 | ± | 1.8 |
| QT | 0Cu | 400 | 1030 | ± | 2 | 1051 | ± | 6 | 1.6 | ± | 0.7 | 11.9 | ± | 1.0 | 56.3 | ± | 1.2 |
| QT | 0Cu | 500 | 802 | ± | 7 | 832 | ± | 3 | 4.1 | ± | 0.2 | 16.7 | ± | 0.2 | 61.1 | ± | 0.8 |
| QT | 1Cu | - | 1110 | ± | 9 | 1495 | ± | 11 | 3.1 | ± | 0.4 | 11.2 | ± | 0.9 | 46.8 | ± | 2.3 |
| QT | 1Cu | 300 | 1170 | ± | 9 | 1284 | ± | 11 | 2.7 | ± | 0.2 | 10.5 | ± | 1.0 | 51.2 | ± | 1.2 |
| QT | 1Cu | 400 | 1049 | ± | 8 | 1059 | ± | 11 | 2.2 | ± | 0.6 | 11.4 | ± | 0.8 | 51.5 | ± | 1.8 |
| QT | 1Cu | 500 | 901 | ± | 4 | 937 | ± | 3 | 7.1 | ± | 0.9 | 16.8 | ± | 0.7 | 56.4 | ± | 1.5 |
| QT | 1.5Cu | - | 1166 | ± | 12 | 1520 | ± | 14 | 1.9 | ± | 0.1 | 12.9 | ± | 0.6 | 48.6 | ± | 1.5 |
| QT | 1.5Cu | 300 | 1196 | ± | 9 | 1292 | ± | 7 | 1.5 | ± | 0.1 | 12.0 | ± | 0.6 | 52.4 | ± | 3.2 |
| QT | 1.5Cu | 400 | 1147 | ± | 3 | 1157 | ± | 2 | 1.7 | ± | 0.1 | 11.7 | ± | 0.6 | 54.2 | ± | 0.4 |
| QT | 1.5Cu | 500 | 1003 | ± | 4 | 1024 | ± | 2 | 5.7 | ± | 0.1 | 16.7 | ± | 0.4 | 54.1 | ± | 0.5 |
| QRT | 0Cu | - | 1884 | ± | 25 | 1911 | ± | 24 | 0.4 | ± | 0.1 | 4.5 | ± | 1.0 | 29.8 | ± | 14.9 |
| QRT | 0Cu | 300 | 1670 | ± | 9 | 1671 | ± | 8 | 0.2 | ± | 0.1 | 6.0 | ± | 0.8 | 12.6 | ± | 7.0 |
| QRT | 0Cu | 400 | 1388 | ± | 23 | 1396 | ± | 26 | 0.3 | ± | 0.1 | 3.8 | ± | 0.5 | 9.0 | ± | 0.8 |
| QRT | 0Cu | 500 | 1095 | ± | 7 | 1101 | ± | 4 | 0.2 | ± | 0.1 | 7.8 | ± | 1.0 | 20.0 | ± | 6.1 |
| QRT | 1Cu | - | 1943 | ± | 27 | 1976 | ± | 22 | 0.4 | ± | 0.1 | 4.4 | ± | 0.7 | 30.1 | ± | 4.0 |
| QRT | 1Cu | 300 | 1757 | ± | 15 | 1761 | ± | 15 | 0.2 | ± | 0.1 | 5.0 | ± | 0.9 | 8.7 | ± | 1.4 |
| QRT | 1Cu | 400 | 1410 | ± | 7 | 1412 | ± | 11 | 0.3 | ± | 0.1 | 5.1 | ± | 0.4 | 17.3 | ± | 3.8 |
| QRT | 1Cu | 500 | 1197 | ± | 4 | 1201 | ± | 5 | 0.2 | ± | 0.1 | 8.6 | ± | 1.1 | 21.2 | ± | 2.7 |
| QRT | 1.5Cu | - | 1983 | ± | 7 | 2006 | ± | 10 | 0.4 | ± | 0.1 | 4.3 | ± | 0.4 | 22.8 | ± | 3.7 |
| QRT | 1.5Cu | 300 | 1792 | ± | 23 | 1796 | ± | 25 | 0.1 | ± | 0.1 | 4.1 | ± | 0.3 | 10.4 | ± | 3.4 |
| QRT | 1.5Cu | 400 | 1459 | ± | 14 | 1462 | ± | 14 | 0.3 | ± | 0.1 | 4.7 | ± | 0.9 | 16.3 | ± | 2.9 |
| QRT | 1.5Cu | 500 | 1216 | ± | 1 | 1221 | ± | 3 | 0.1 | ± | 0.1 | 8.8 | ± | 1.0 | 24.0 | ± | 6.2 |
References
- Isheim, D.; Kolli, R.P.; Morris, E.F.; Seidman, D.N. An atom-probe tomographic study of the temporal evolution of the nanostructure of Fe–Cu based high-strength low-carbon steels. Scr. Mater. 2006, 55, 35–40. [Google Scholar] [CrossRef]
- Takaki, S.; Fujioka, M.; Aihara, S.; Nagataki, Y.; Yamashita, T.; Sano, N.; Adachi, Y.; Nomura, M.; Yaguchi, H. Effect of Copper on Tensile Properties and Grain-Refinement of Steel and its Relation to Precipitation Behavior. Mater. Trans. 2004, 45, 2239–2294. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.K.; Haldar, A.; Chattopadhyay, P.P. Effect of ageing on the mechanical properties of directly quenched copper bearing microalloyed steels. Mater. Chem. Phys. 2010, 119, 436–441. [Google Scholar] [CrossRef]
- Rana, R.; Bleck, W.; Singh, S.B.; Mohanty, O.N. Development of high strength interstitial free steel by copper precipitation hardening. Mater. Lett. 2007, 61, 2919–2922. [Google Scholar] [CrossRef]
- Von Goldbeck, O.K. Iron—Copper Fe—Cu. In IRON—Binary Phase Diagrams; Springer: Berlin, Germany, 1982. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, W.; Liu, Z.; Wang, G. Direct observations on the crystal structure evolution of nano Cu-precipitates in an extremely low carbon steel. Mater. Lett. 2017, 187, 49–52. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, S.J.; Liu, Q. The effect of size of Cu precipitation on the mechanical properties of microalloyed steel. Mater. Sci. Eng. A 2012, 556, 140–146. [Google Scholar] [CrossRef]
- Holzer, I.; Kozeschnik, E. Computer simulation of the yield strength evolution in Cu-precipitation strengthened ferritic steel. Mater. Sci. Eng. A 2010, 527, 546–3551. [Google Scholar] [CrossRef]
- Takahashi, J.; Kawakami, K.; Kobayashi, Y. Consideration of particle-strengthening mechanism of copper-precipitation-strengthened steels by atom probe tomography analysis. Mater. Sci. Eng. A 2012, 535, 144–152. [Google Scholar] [CrossRef]
- Guo, H.; Cheng, J.; Yang, S.; He, X. Influence of combined Cu and Nb addition on the quenched microstructure and precipitation during tempering in ultra-low carbon steels. J. Alloys Compd. 2013, 557, S619–S625. [Google Scholar] [CrossRef]
- Dlouhy, J.; Podany, P.; Dzugan, J. Strengthening from Cu Addition in 0.2C-(1–2)Mn Steels during Tempering. Materials 2019, 12, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.K.; Bhowmik, N.; Haldar, A.; Chattopadhyay, P.P. Effects of Cu addition on the synergistic effects of Ti–B in thermomechanically processed low carbon steels. Mater. Sci. Eng. A 2010, 527, 1082–1088. [Google Scholar] [CrossRef]
- Kapoor, M.; Isheim, D.; Ghosh, G.; Vaynman, S.; Fine, M.E.; Chung, Y.W. Aging characteristics and mechanical properties of 1600 MPa body-centered cubic Cu and B2-NiAl precipitation-strengthened ferritic steel. Acta Mater. 2014, 73, 56–74. [Google Scholar] [CrossRef]
- Podany, P.; Martinek, P. Thermomechanical processing of micro-alloyed steel. Mater. Tehnol. 2014, 48, 855–859. [Google Scholar]
- Podany, P.; Martinek, P.; Balcar, M. Mechanical properties of steel with various microalloying addition after particular thermomechanical processing. In Proceedings of the METAL 2011—20th International Conference on Metallurgy and Materials, Brno, Czech Republic, 18–20 May 2011; pp. 646–649. [Google Scholar]
- Ghosh, S.K.; Haldar, A.; Chattopadhyay, P.P. On the Cu precipitation behavior in thermomechanically processed low carbon microalloyed steels. Mater. Sci. Eng. A 2009, 519, 88–93. [Google Scholar] [CrossRef]
- International Standard EN ISO 6892-1. Metallic Materials—Tensile Testing—Part1: Method of Test at Room Temperature; International Organization for Standardization: Geneva, Switzerland, 2016. [Google Scholar]
- Lefebvre, S.; Devincre, B.; Hoc, T. Yield stress strengthening in ultrafine-grained metals: A two-dimensional simulation of dislocation dynamics. J. Mech. Phys. Solids 2007, 55, 788–802. [Google Scholar] [CrossRef]
- Wang, Y.M.; Ma, E. Strain hardening, strain rate sensitivity, and ductility of nanostructured metals. Mater. Sci. Eng. A 2004, 375, 46–52. [Google Scholar] [CrossRef]
- Pascuet, M.I.; Monnet, G.; Bonny, G.; Martinez, E.; Lim, J.J.H.; Burke, M.G.; Malerba, L. Solute precipitation on a screw dislocation and its effects on dislocation mobility in bcc Fe. J. Nucl. Mater. 2019, 519, 265–273. [Google Scholar] [CrossRef]
- Xu, S.S.; Zhao, Y.; Tong, X.; Guo, H.; Chen, L.; Sun, L.W.; Sun, G.A. Independence of work hardening and precipitation strengthening in ananocluster strengthened steel. J. Alloys Compd. 2017, 712, 573–578. [Google Scholar] [CrossRef] [Green Version]











| Material | C | Cu | Mn | Si | Ti | B | N |
|---|---|---|---|---|---|---|---|
| 0Cu | 0.22 | 0.12 | 0.98 | 0.07 | 0.022 | 0.0014 | 0.0056 |
| 1Cu | 0.21 | 1.08 | 0.98 | 0.08 | 0.025 | 0.0013 | 0.0063 |
| 1.5Cu | 0.21 | 1.49 | 0.99 | 0.10 | 0.022 | 0.0013 | 0.0054 |
| Regime | 15 min | 30 min | 60 min | 120 min |
|---|---|---|---|---|
| 300 °C | X | |||
| 400 °C | X | X | X | X |
| 500 °C | X |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dlouhy, J.; Podany, P.; Džugan, J. Influence of Martensite Deformation on Cu Precipitation Strengthening. Metals 2020, 10, 282. https://doi.org/10.3390/met10020282
Dlouhy J, Podany P, Džugan J. Influence of Martensite Deformation on Cu Precipitation Strengthening. Metals. 2020; 10(2):282. https://doi.org/10.3390/met10020282
Chicago/Turabian StyleDlouhy, Jaromir, Pavel Podany, and Ján Džugan. 2020. "Influence of Martensite Deformation on Cu Precipitation Strengthening" Metals 10, no. 2: 282. https://doi.org/10.3390/met10020282
APA StyleDlouhy, J., Podany, P., & Džugan, J. (2020). Influence of Martensite Deformation on Cu Precipitation Strengthening. Metals, 10(2), 282. https://doi.org/10.3390/met10020282






