Selective Copper Recovery by Acid Leaching from Printed Circuit Board Waste Sludge
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
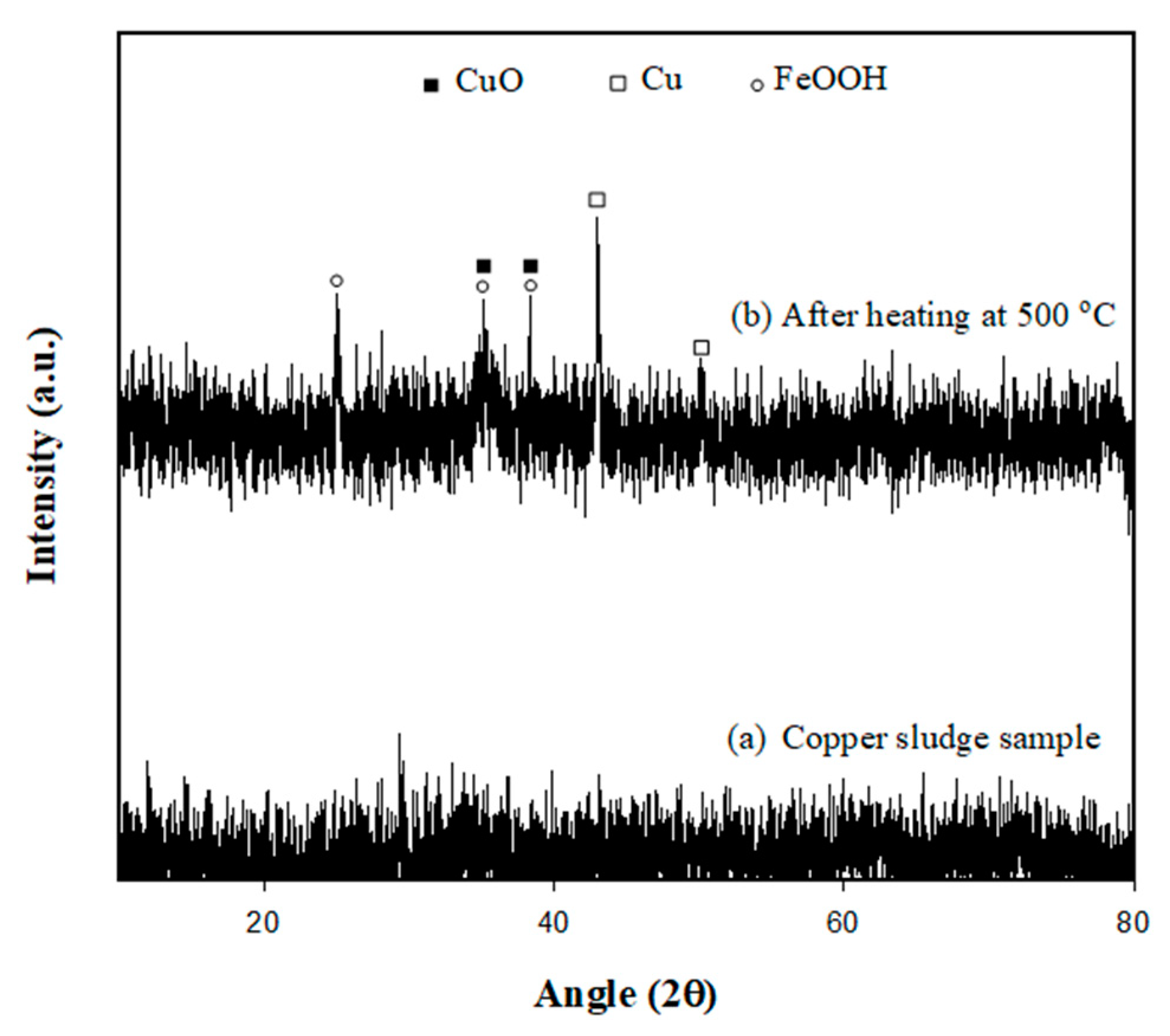
3.1. Characteristics of Copper Sludge
3.2. Potential of Selective Copper Leaching from Sludge using Different Acids
3.3. Sulfuric Leaching of Copper Sludge
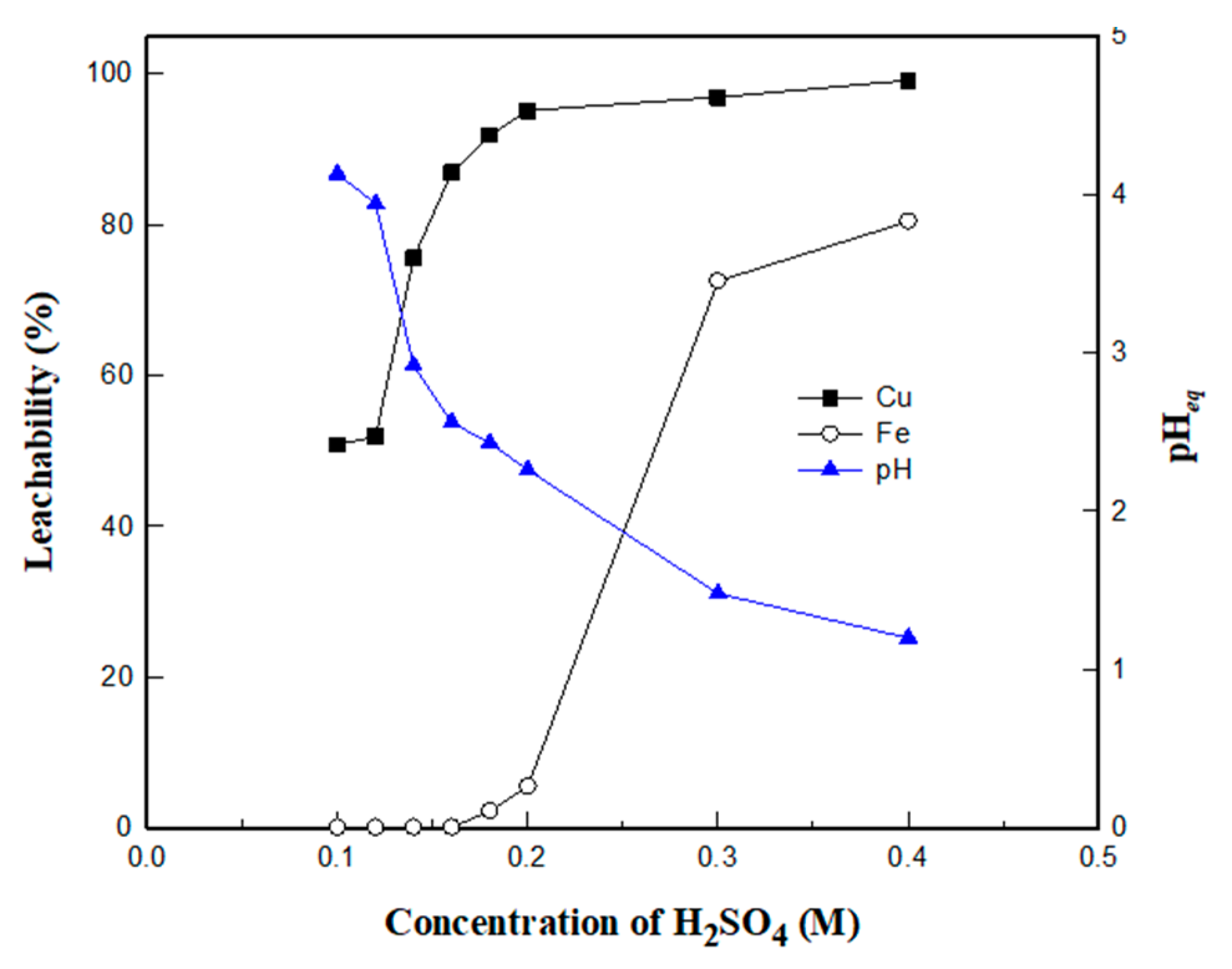
3.3.1. Effect of H2SO4 Concentration
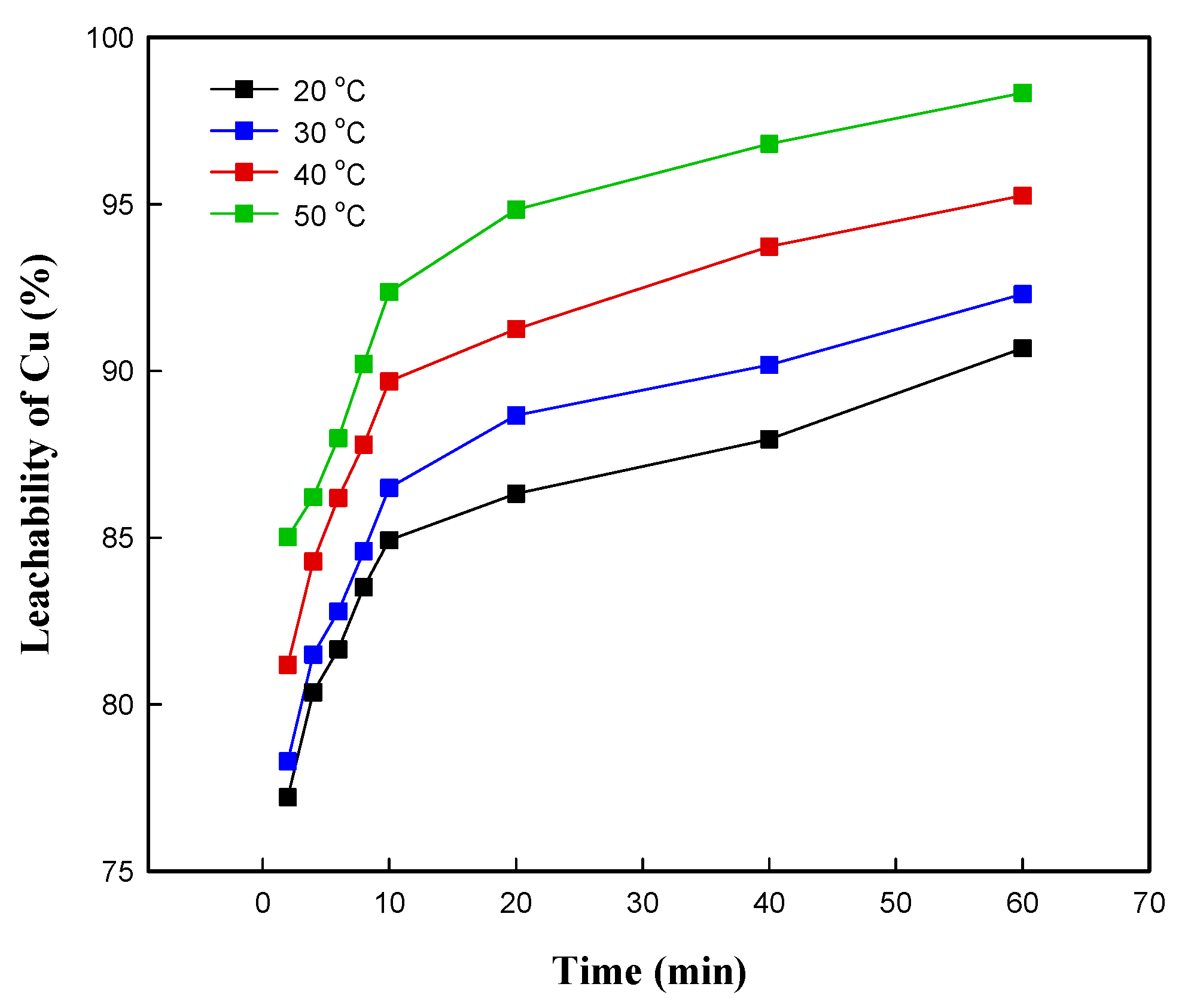
3.3.2. Effect of Leaching Temperature and Time
3.4. Kinetics and Mechanism of Copper Leaching in Sulfuric Acid
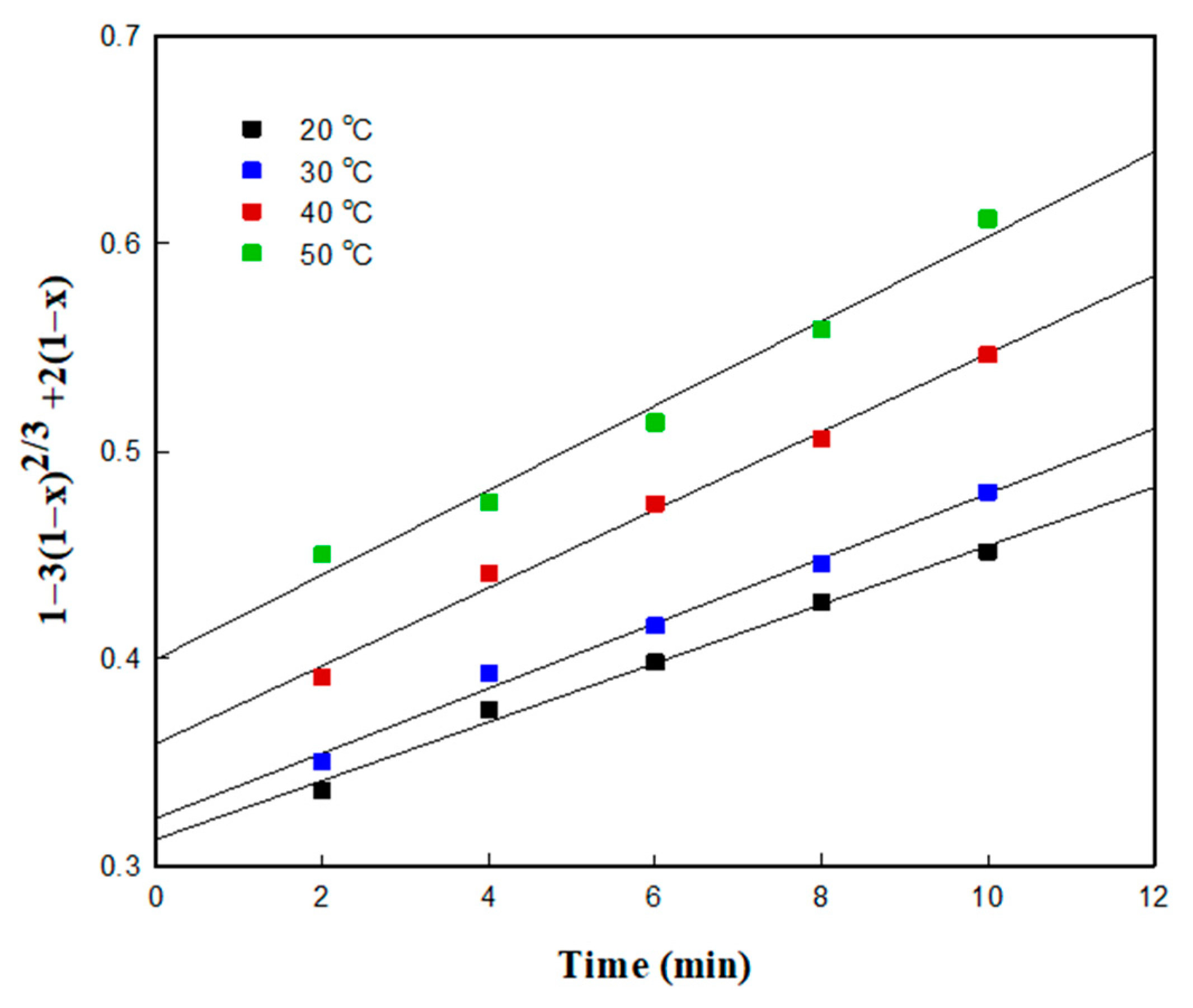
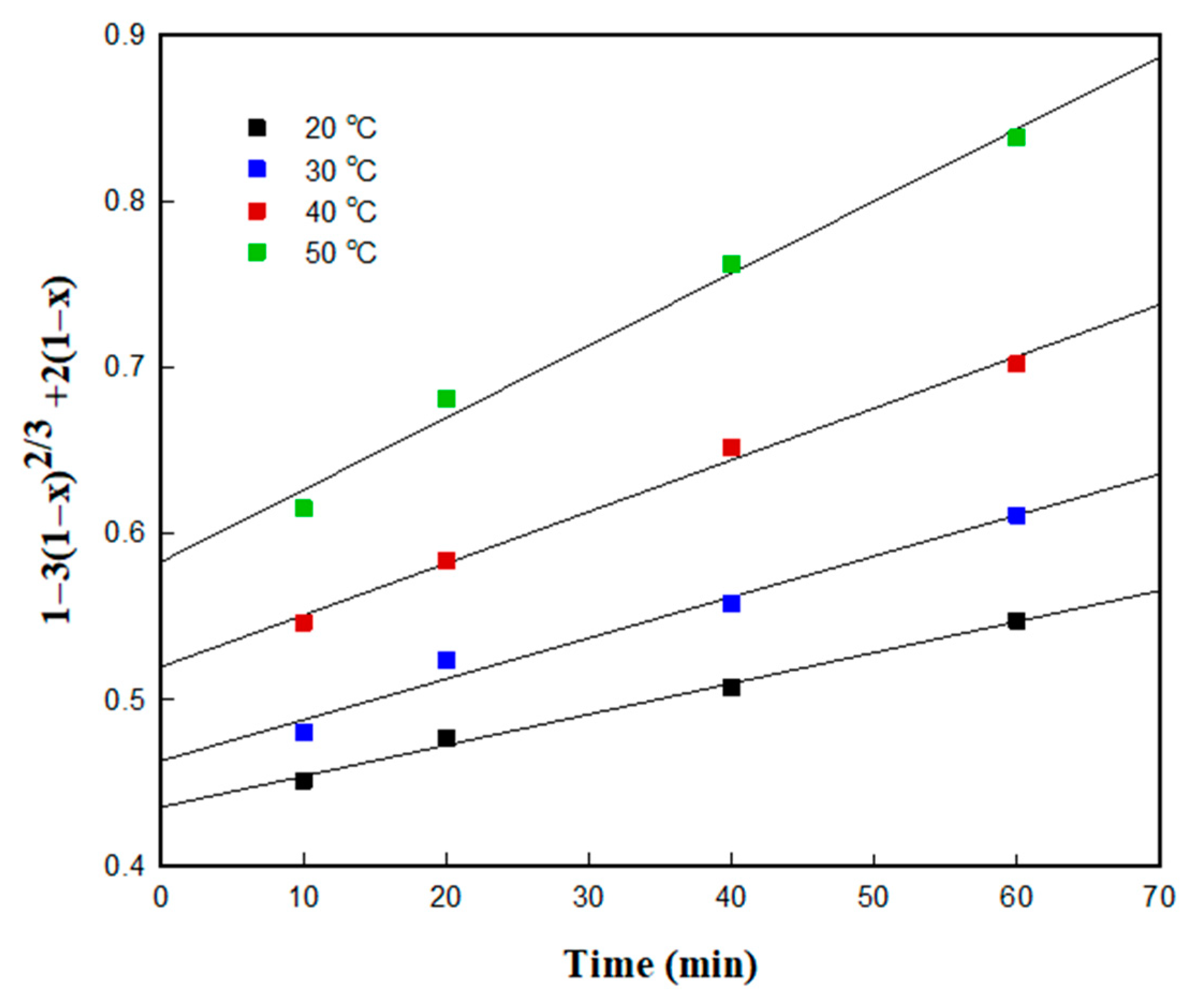
3.4.1. Kinetic Studies Based on the Shrinking Core Mode (SCM)
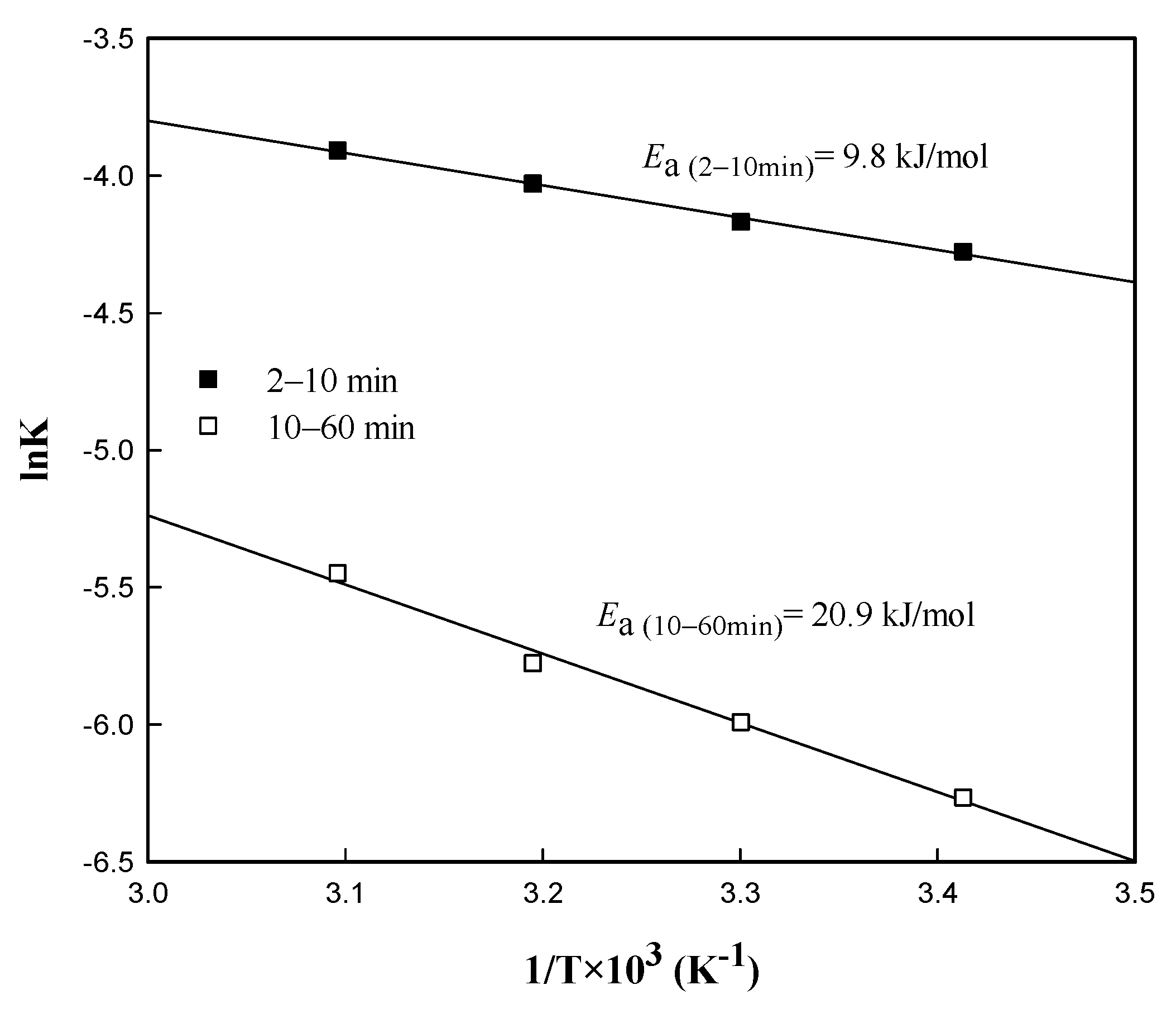
3.4.2. Determination of the Kinetic Model
3.4.3. Mechanism of Cu Leaching from Waste Sludge in Sulfuric Acid
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tang, Y.; Lee, P.H.; Shih, K. Copper Sludge from Printed Circuit Board Production/Recycling for Ceramic Materials: A Quantitative Analysis of Copper Transformation and Immobilization. Environ. Sci. Technol. 2013, 47, 8609–8615. [Google Scholar] [CrossRef] [PubMed]
- Printed Circuit Board Recycling Methods, Handout 10, Workshop Materials on WEEE Management in Taiwan, United States Environmental Protection Agency. Available online: https://www.epa.gov/sites/production/files/2014-05/documents/handout-10-circuitboards.pdf (accessed on 9 January 2020).
- Cho, B.G.; Cho, Y.J.; Lee, J.C.; Yoo, K. Korea’s metal resources recycling research project—Valuable recycling. Geosyst. Eng. 2019, 22, 48–58. [Google Scholar] [CrossRef]
- Xie, F.; Cai, T.; Yang, M.; Li, H.; Li, C.; Huang, Z.; Yuan, G. Recovery of Cu and Fe from Printed Circuit Board waste sludge by ultrasound: Evaluation of industrial application. J. Clean. Prod. 2009, 17, 1494–1498. [Google Scholar] [CrossRef]
- Wu, C.H.; Kuo, C.Y.; Lo, S.L. Removal of Metals from Industrial Sludge by Extraction with Different Acids. J. Environ. Sci. Health. A Tox Hazard. Subst. Environ. Eng. 2004, 39, 2205–2219. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Wu, C.H.; Lo, S.L. Leaching efficiency of copper from industrial sludge with traditional and microwave acid extraction. J. Hazard. Mater. B 2005, 120, 249–256. [Google Scholar] [CrossRef]
- Wu, C.H.; Kuo, C.Y.; Lo, S.L. Recovery of heavy metals from industrial sludge using various acid extraction approaches. Water Sci. Technol. 2009, 59, 289–293. [Google Scholar] [CrossRef]
- Thawornchaisit, U.; Juthaisong, K.; Parsongjeen, K.; Phoengchan, P. Optimizing acid leaching of copper from the wastewater treatment sludge of a printed circuit board industry using factorial experimental design. J. Mater. Cycles Waste Manag. 2019, 21, 1291–1299. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, F.; Ma, Y. Ultrasonic recovery of copper and iron through simultaneous utilization of Printed Circuit Boards (PCB) spent acid etching solution and PCB waste sludge. J. Hazard. Mater. 2011, 185, 155–156. [Google Scholar] [CrossRef]
- Hu, S.H.; Tsai, M.S.; Yen, F.S.; Onlin, T. Recovery of copper-contaminated sludge in a two-stage leaching process. Environ. Prog. 2006, 25, 72–78. [Google Scholar] [CrossRef]
- Hu, S.H.; Wu, J.Y.; Hsiao, T.C.; Yen, F.S.; Tsai, M.S. A Study of Copper Recovery from Copper-Contaminated Sludge with Ferrite and Selective Leaching Processes. Environ. Prog. 2007, 26, 104–112. [Google Scholar] [CrossRef]
- Tu, Y.J.; Chang, C.K.; You, C.F.; Lou, J.C. Recycling of Cu powder from industrial sludge by combined acid leaching, chemical exchange and ferrite process. J. Hazard. Mater. 2010, 181, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.H.; Hu, S.C.; Fu, Y.P. Resource Recovery of Copper Contaminated Sludge with Jarosite Process and Selective Precipitation. Environ. Prog. Sustain. Energy 2012, 31, 379–385. [Google Scholar] [CrossRef]
- Shen, Y.G.; Huang, Q.Y. Heavy metals recovery from printed circuitboard industry wastewater sludge bythermophilic bioleaching process. J. Chem. Technol. Biotechnol. 2014, 89, 158–164. [Google Scholar]
- Hassanzadeh, A. A new statistical view to modeling of particle residence time distribution in full-scale overflow ball mill operating in closed-circuit. Geosyst. Eng. 2018, 21, 199–209. [Google Scholar] [CrossRef]
- Au-Yeung, A.C.F.; Denes, G.; Greedan, J.E.; Eaton, D.R.; Birchall, T. A novel synthetic route to “iron trihydroxide, Fe(OH)3”: Characterization and magnetic properties. Inorg. Chem. 1984, 23, 1513–1517. [Google Scholar] [CrossRef]
- Ohtsuka, K.; Suda, M.; Ono, M. Preparation and Properties of Iron(II) Hydroxide-(Sodium Fluoride Tetrasilicic Mica) Intercalation Complexes. Bull. Chem. Soc. Jpn. 1988, 61, 815–820. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Yin, Y.; Hou, H.; Fan, N.; Yuan, F.; Shi, Y.; Meng, A. Preparation and characterization of Cu (OH)2 and CuO nanowires by the coupling route of microemulsion with homogenous precipitation. Solid State Commun. 2010, 150, 585–589. [Google Scholar] [CrossRef]
- Cudennec, Y.; Lecerf, A. The transformation of Cu(OH)2 into CuO, revisited. Solid State Sci. 2003, 5, 1471–1474. [Google Scholar] [CrossRef] [Green Version]
- Pramanik, N.C.; Bhuiyan, T.I.; Nakanishi, M.; Fujii, T.; Takada, J.; Seok, S.I. Synthesis and characterization of cerium substituted hematite by sol-gel method. Mater. Lett. 2005, 59, 3783–3787. [Google Scholar] [CrossRef]
- Monhemius, J. Precipitation diagrams for metal hydroxides, sulfides, arsenates and phosphates. Trans. Inst. Min. Metall. 1977, 86, 202–206. [Google Scholar]
- Stefánsson, A. Iron(III) Hydrolysis and Solubility at 25 °C. Environ. Sci. Technol. 2007, 41, 6117–6123. [Google Scholar] [CrossRef] [PubMed]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1999; pp. 570–580. [Google Scholar]
- Habashi, F. Extractive Metallurgy; Gordon and Breach, Science Publishers Inc.: New York, NY, USA, 1969; Volume I, pp. 114–115, 143–144. [Google Scholar]
- Razavizadeh, H.; Afshar, M.R. Leaching of Sarcheshmeh copper oxide ore in sulfuric acid solution. Min. Metall. Explor. 2008, 25, 85–90. [Google Scholar] [CrossRef]
- Azizi, A.; Bayati, B.; Karamoozian, M. A comprehensive study of the leaching behavior and dissolution kinetics of copper oxide ore in sulfuric acid lixiviant. Scientia Iranica 2018, 25, 1412–1422. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.H.; Wang, Y.J.; Chern, J.M. Extraction kinetics of heavy metal-containing sludge. J. Hazard. Mater. 2005, 123, 112–119. [Google Scholar] [CrossRef]
- Yazici, E.Y.; Deveci, H. Ferric sulphate leaching of metals from waste printed circuit boards. Int. J. Min. Process. 2014, 133, 39–45. [Google Scholar] [CrossRef]
- Kim, E.-y.; Kim, M.-s.; Lee, J.-c.; Jeong, J.; Pandey, B.D. Leaching kinetics of copper from waste printed circuit by electro-generated chlorine in HCl solution. Hydrometallurgy 2011, 107, 124–132. [Google Scholar] [CrossRef]




| Metals | UOM | Composition |
|---|---|---|
| Cu | % | 18.34 |
| Fe | 29.35 | |
| Ca | 6.24 | |
| Pb | 2.06 | |
| Sn | 1.72 | |
| Mg | ppm | 7134 |
| Al | 2277 | |
| Zn | 1165 | |
| Na | 1053 | |
| Si | 0 |
| Kinetic Model | Equation | Fitting Results | |
|---|---|---|---|
| Average Value of Regression Coefficient R2 | Active Energy Ea(kJ/mol) and Regression Coefficient R2 | ||
| Stage 1: 2–10 min | |||
| Film diffusion control | Not fit | Not fit | |
| Ash diffusion control | 0.98 | 9.8 kJ/mol / R2 = 0.99 | |
| Chemical control | 0.98 | 9.7 kJ/mol / R2 = 0.97 | |
| Film diffusion control | 0.97 | 5.2 kJ/mol / R2 = 0.87 | |
| Stage 2: 10–60 min | |||
| Film diffusion control | Not fit | Not fit | |
| Ash diffusion control | 0.98 | 20.9 kJ/mol / R2 = 0.99 | |
| Chemical control | 0.97 | 25.47 kJ/mol / R2 = 0.97 | |
| Film diffusion control | 0.96 | 19.6 kJ/mol / R2 = 0.93 | |
| Temperature (°C) | |
|---|---|
| Stage 1: 2–10 min | |
| 20 | 0.0139 |
| 30 | 0.0155 |
| 40 | 0.0188 |
| 50 | 0.0201 |
| Stage 2: 10–60 min | |
| 20 | 0.0019 |
| 30 | 0.0025 |
| 40 | 0.0031 |
| 50 | 0.0043 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinh, H.B.; Kim, S.; Lee, J. Selective Copper Recovery by Acid Leaching from Printed Circuit Board Waste Sludge. Metals 2020, 10, 293. https://doi.org/10.3390/met10020293
Trinh HB, Kim S, Lee J. Selective Copper Recovery by Acid Leaching from Printed Circuit Board Waste Sludge. Metals. 2020; 10(2):293. https://doi.org/10.3390/met10020293
Chicago/Turabian StyleTrinh, Ha Bich, Seunghyun Kim, and Jaeryeong Lee. 2020. "Selective Copper Recovery by Acid Leaching from Printed Circuit Board Waste Sludge" Metals 10, no. 2: 293. https://doi.org/10.3390/met10020293





