Mechanism of Melt Separation in Preparation of Low-Oxygen High Titanium Ferroalloy Prepared by Multistage and Deep Reduction
Abstract
:1. Introduction
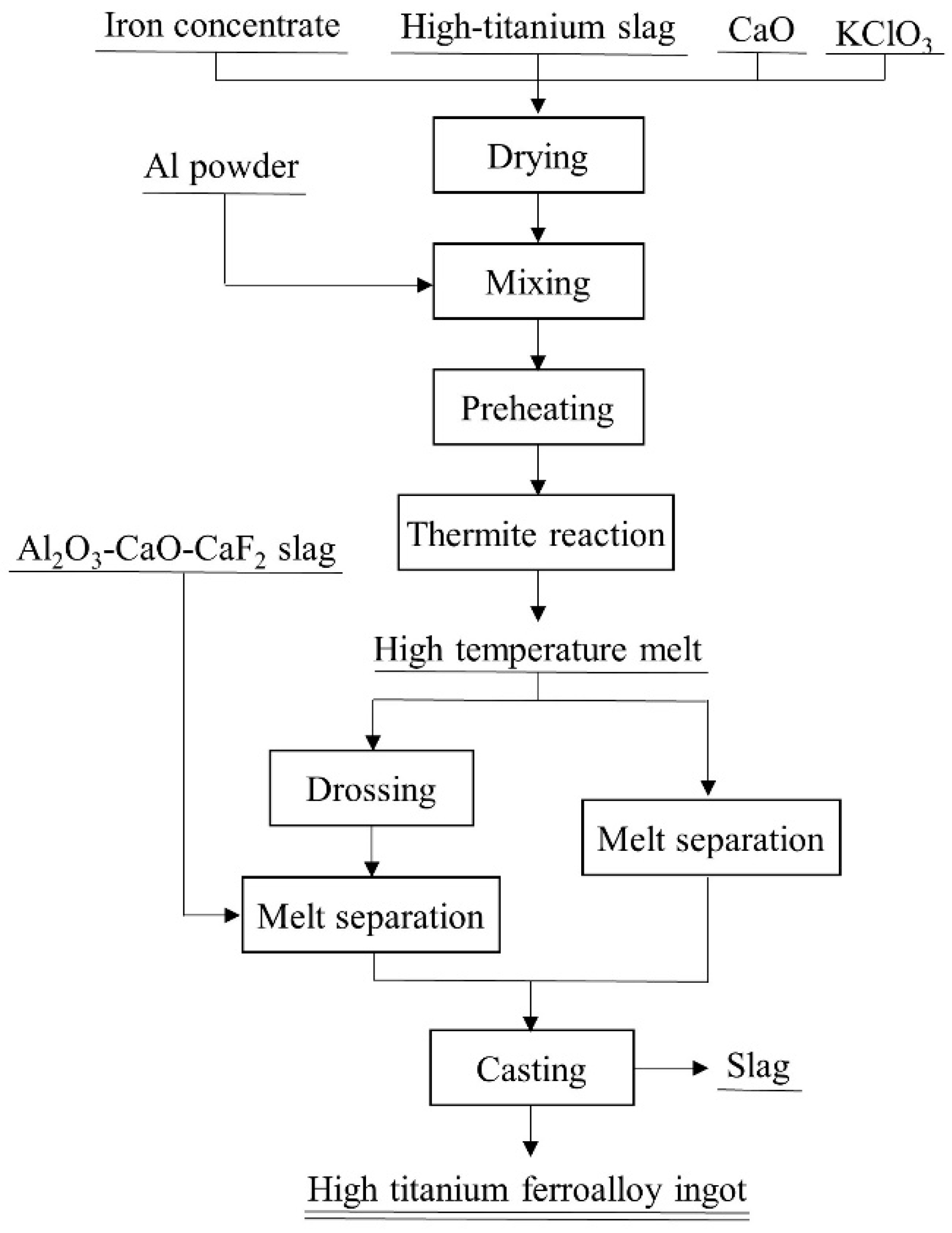
2. Experimental Part
3. Results and Discussion
3.1. Characteristics of Slags
3.2. SEM Images and EDS Analysis
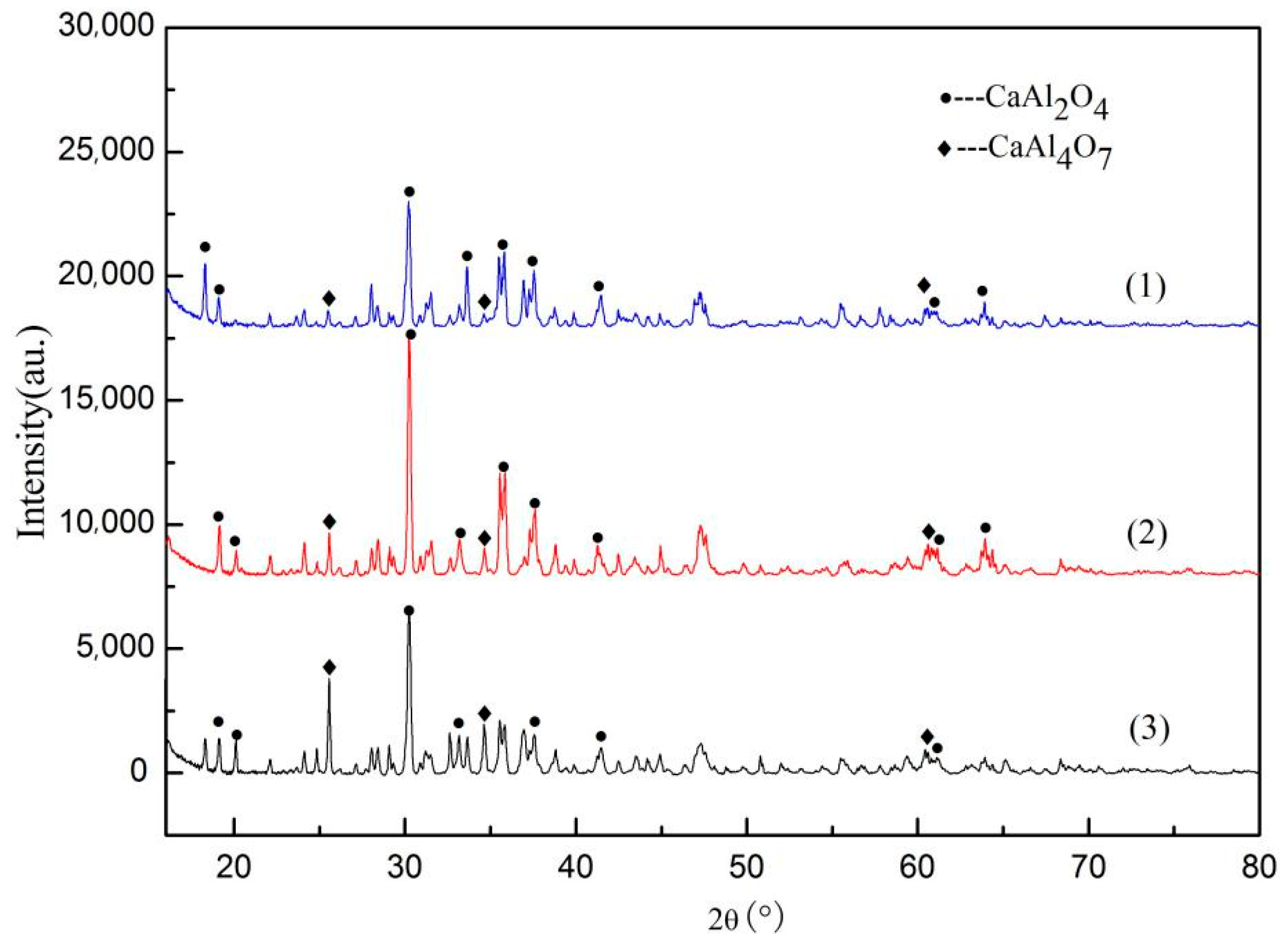
3.3. Phase of Alloys
3.4. Oxygen Content and Chemical Composition Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dou, Z.H.; Fan, S.G.; Cheng, C.; Zhang, T.A.; Shi, G.Y. Research Advance and Application of High Titanniumferrous with Low Oxygen. Metall. Eng. 2015, 2, 107–121. [Google Scholar] [CrossRef]
- Shah, S.J.; Henein, H.; Ivey, D.G. Microstructural Characterization of Ferrotitanium and Ferroniobium. Mater. Charact. 2013, 78, 96–107. [Google Scholar] [CrossRef]
- Qi, C.C.; Hua, X.Y.; Chen, K.H.; Jie, Y.F.; Zhou, Z.R.; Ru, J.J.; Xiong, L.; Gong, K. Preparation of Ferrotitanium Alloy from Ilmenite by Electrochemical Reduction in Chloride Molten Salts. JOM 2015, 68, 1–7. [Google Scholar] [CrossRef]
- Yang, X.; Hu, M.L.; Bai, C.G.; Lv, X.W. Formation Behavior of CaTiO3 during Electrochemical Deoxidation of Ilmenite Concentrate to Prepare Fe-Ti Alloy. Rare Met. 2016, 35, 275–281. [Google Scholar]
- Shi, R.; Bai, C.; Hu, C.; Liu, X.; Du, J. Experimental Investigation on the Formation Mechanism of the TiFe Alloy by the Molten-salt Electrolytic Ilmenite Concentrate. Int. J. Miner. Metall. Mater. 2011, 47, 99–104. [Google Scholar] [CrossRef]
- Du, J.H.; Xi, Z.P.; Li, Q.Y.; Li, Z.X.; Tang, Y. Effect of TiO2 Cathode Performance on Preparation of Ti by Electro-deoxidation. Trans. Nonferrous Met. Soc. China 2007, 17, 514–521. [Google Scholar]
- Cheng, C.; Zhang, T.A.; Dou, Z.H. Formation Mechanism and Distribution of Al and O in the Ferrotitanium with Different Ti Contents Prepared by Thermite Method. JOM 2019, 71, 3584–3589. [Google Scholar] [CrossRef]
- Liu, X.Y.; Hu, M.L.; Bai, C.G.; Lv, X.W.; Du, J.H. The Deoxidizing Process of the Preparation FeTi Alloy by Electrolysis Ilmenite Concentrate in Molten Salt. J. Funct. Mater. 2011, 42, 2042–2046. [Google Scholar]
- Pourabdoli, M.; Raygan, S.; Abdizadeh, H.; Hanaei, K. A New Process for the Production of Ferrotitanium from Titanium Slag. Can. Metall. Q. 2007, 46, 17–23. [Google Scholar] [CrossRef]
- Dou, Z.H.; Zhang, T.A.; Zhang, H.B.; Zhang, Z.Q.; Niu, L.P.; He, J.C. Basic Research on Preparation of High Titanium Ferroalloy with Low Oxygen Content by Thermit Reduction. J. Process Eng. 2010, 10, 1119–1125. [Google Scholar]
- Tan, S.; Örs, T.; Aydnol, M.K.; Öztürk, T.; Karakaya, İ. Synthesis of FeTi from Mixed Oxide Precursors. J. Alloys Compd. 2009, 475, 368–372. [Google Scholar] [CrossRef]
- Hu, M.; Bai, C.; Liu, X.; Lv, X.I.; Du, J. Deoxidization Mechanism of Preparation FeTi Alloy using Ilmenite Concentrate. Int. J. Miner. Metall. Mater. 2011, 47, 193–198. [Google Scholar] [CrossRef]
- Pazdnikov, I.P.; Dubrovskii, A.Y.; Zelyanskii, A.V. Problem of Optimizing the Production of High-Quality Ferrotitanium. Titan. Ind. 2005, 2, 21–22. [Google Scholar]
- Chumarev, V.M.; Dubrovskii, A.Y.; Pazdnikov, I.P.; Shurygin, Y.Y.; Sel’Menskikh, N.I. Technological Possibilities of Manufacturing High-grade Ferrotitanium from Crude Ore. Russ. Metall. 2008, 6, 459–463. [Google Scholar] [CrossRef]
- Dou, Z.H.; Zhang, T.A.; Zhang, H.B.; Zhang, Z.Q.; Niu, L.P.; He, J.C. Research on Preparation of High Titanium Ferroalloy with Low Oxygen through Vacuum Secondary Reduction Refining. Rare Met. Mater. Eng. 2012, 41, 420–425. [Google Scholar]
- Dou, Z.H.; Zhang, T.A.; Yao, J.M.; Jiang, X.L.; Niu, L.P.; He, J.C. Research on Properties of Al2O3-CaO Slag. J. Process Eng. 2009, 9, 247–249. [Google Scholar]
- Shi, G.Y.; Zhang, T.A.; Niu, L.P.; Dou, Z.H. Research on Properties of Low Fluorine CaF2-CaO-Al2O3-MgO-SiO2 Refining Slag. J. Process Eng. 2011, 11, 695–697. [Google Scholar]
- Dou, Z.H.; Zhang, T.A.; Yao, J.M.; Niu, L.P. Viscosities of Slags in CaO-Al2O3-CaF2-SiO2 System. J. Northeast. Univ. Nat. Sci. 2008, 29, 1000–1003. [Google Scholar]
- Dou, Z.H.; Zhang, T.A.; Zhang, H.B.; Zhang, Z.Q.; Niu, L.P.; Yao, Y.L.; He, J.C. Preparation of High Titanium Ferrous with Low Oxygen Content by Thermite Reduction-SHS. J. Cent. South Univ. Technol. 2012, 41, 899–904. [Google Scholar]
- Cheng, C.; Dou, Z.H.; Zhang, T.A.; Su, J.M.; Zhang, H.J.; Liu, Y.; Niu, L.P. Oxygen Content of High Ferrotitanium Prepared by Thermite Method with Different Melt Separation Temperatures. Rare Met. 2019, 38, 892–898. [Google Scholar] [CrossRef]
- Han, Z.J.; Liu, L.; Lind, M.; Holappa, L. Mechanism and Kinetics of Transformation of Alumina Inclusions by Calcium Treatment. Acta Metall. Sin. 2006, 19, 1–8. [Google Scholar] [CrossRef]
- Jerebtsov, D.A.; Mikhailov, G.G. Phase Diagram of CaO-Al2O3 System. Ceram. Int. 2001, 27, 25–28. [Google Scholar] [CrossRef]
- Zhao, H.M.; Wang, X.H.; Xie, B. Effect of Components on Desulfurization of an Al2O3-CaO Base Premolten Slag Containing SrO. J. Univ. Sci. Technol. Beijing 2005, 12, 225–230. [Google Scholar]
- Foadian, F.; Soltanieh, M.; Adeli, M.; Etminanbakhsh, M. A Study on the Formation of Intermetallics during the Heat Treatment of Explosively Welded Al-Ti Multilayers. Metall. Mater. Trans. A 2014, 45, 1823–1832. [Google Scholar] [CrossRef]
- He, S.P.; Chen, G.J.; Guo, Y.T.; Shen, B.Y.; Wang, Q. Morphology Control for Al2O3 Inclusion without Ca Treatment in High-aluminum Steel. Metall. Mater. Trans. B 2015, 46, 585–594. [Google Scholar] [CrossRef] [Green Version]




| Raw Material | TiO2 | TFe | Al2O3 | SiO2 | CaO | MgO | Others |
|---|---|---|---|---|---|---|---|
| High-Titanium Slag | 86.03 | 2.40 | 3.30 | 5.60 | 0.17 | 0.98 | Bal. |
| Iron Concentrate | - | 63.07 | 2.71 | 3.40 | 0.54 | 0.45 | Bal. |
| Number | Separation Time | Al2O3 | CaO | TiO2 | CaF2 | SiO2 | MgO | Fe2O3 | Others |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 min | 12.01 | 0.64 | 67.03 | - | 4.54 | 0.03 | 13.39 | Bal. |
| 2 | 60 min | 12.56 | 0.14 | 67.41 | - | 3.96 | 0.01 | 13.57 | Bal. |
| 3 | 90 min | 12.94 | 0.56 | 65.97 | - | 4.04 | 0.07 | 14.38 | Bal. |
| 4 | 30 min | 49.76 | 33.31 | 7.69 | 4.32 | 0.94 | 0.54 | 0.33 | Bal. |
| 5 | 60 min | 52.09 | 31.06 | 5.82 | 5.5 | 0.81 | 0.50 | 0.2 | Bal. |
| 6 | 90 min | 53.17 | 28.15 | 6.81 | 5.48 | 1.24 | 0.86 | 0.27 | Bal. |
| Region | Ti | Fe | Al | Si | O |
|---|---|---|---|---|---|
| 1 | 81.69 | 2.14 | 0.78 | - | 15.39 |
| 2 | - | - | 59.5 | - | 40.5 |
| 3 | 59.88 | 20.74 | 7.94 | 1.73 | 9.71 |
| 4 | 53.24 | 32.52 | 3.47 | 2.59 | 8.18 |
| 5 | 84.5 | - | - | - | 15.5 |
| 6 | - | - | 58.3 | - | 41.7 |
| 7 | 55.42 | 28.49 | 5.66 | 2.89 | 7.54 |
| 8 | 82.15 | - | - | - | 17.85 |
| 9 | - | - | 61.88 | - | 38.12 |
| 10 | 42.59 | 40.25 | 11.64 | 3.18 | 2.34 |
| 11 | 54.88 | 27.43 | 5.71 | 5.38 | 6.6 |
| 12 | 97.28 | - | - | - | 2.72 |
| 13 | 43.31 | 40.72 | 11.2 | 3.37 | 1.4 |
| 14 | 55.61 | 24.7 | 7.89 | 5.43 | 6.37 |
| 15 | 98.34 | - | - | - | 1.66 |
| 16 | 44.55 | 43.12 | 8.48 | 2.73 | 1.12 |
| 17 | 79.42 | 7.67 | 4.26 | 6.65 | 2 |
| Number | Melt Separation Time | Ti | Al | Si | O | Fe |
|---|---|---|---|---|---|---|
| 1 | - | 51.04 | 10.38 | 5.65 | 9.36 | Bal. |
| 2 | 30 min | 61.20 | 7.24 | 5.26 | 5.89 | Bal. |
| 3 | 60 min | 62.06 | 6.68 | 5.47 | 4.65 | Bal. |
| 4 | 90 min | 62.12 | 6.52 | 5.36 | 4.54 | Bal. |
| 5 | 30 min | 66.72 | 4.56 | 5.08 | 1.75 | Bal. |
| 6 | 60 min | 67.76 | 4.26 | 4.64 | 1.55 | Bal. |
| 7 | 90 min | 68.24 | 4.24 | 4.86 | 1.56 | Bal. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.; Dou, Z.; Zhang, T. Mechanism of Melt Separation in Preparation of Low-Oxygen High Titanium Ferroalloy Prepared by Multistage and Deep Reduction. Metals 2020, 10, 309. https://doi.org/10.3390/met10030309
Cheng C, Dou Z, Zhang T. Mechanism of Melt Separation in Preparation of Low-Oxygen High Titanium Ferroalloy Prepared by Multistage and Deep Reduction. Metals. 2020; 10(3):309. https://doi.org/10.3390/met10030309
Chicago/Turabian StyleCheng, Chu, Zhihe Dou, and Tingan Zhang. 2020. "Mechanism of Melt Separation in Preparation of Low-Oxygen High Titanium Ferroalloy Prepared by Multistage and Deep Reduction" Metals 10, no. 3: 309. https://doi.org/10.3390/met10030309





