Structural Evolution of Nano-sized Oxide Particles Formed in Mechanically Alloyed Fe-10Cr-5Y2O3 Powders
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
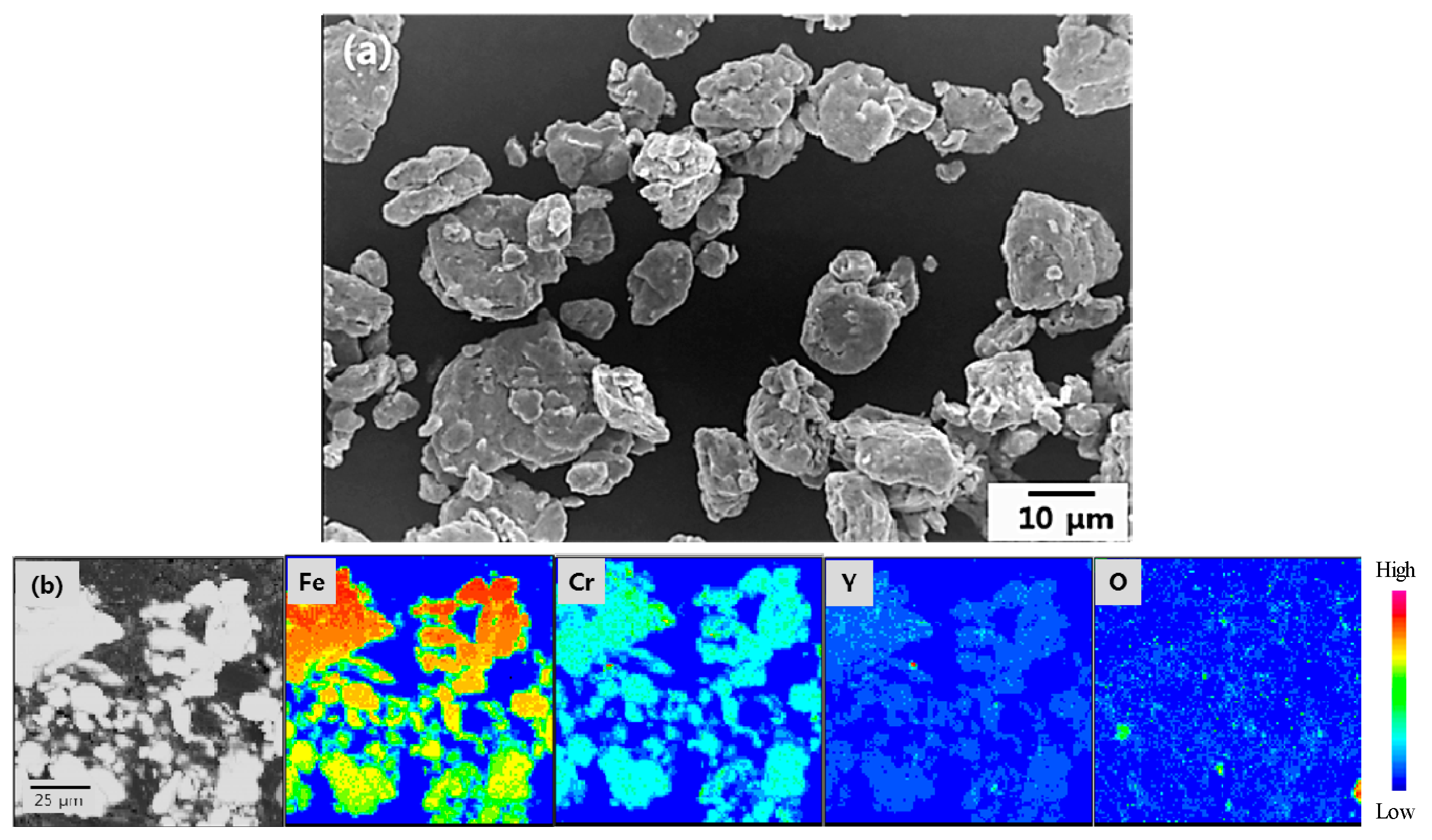
3.1. Microstructure of Mechanically Alloyed Powders
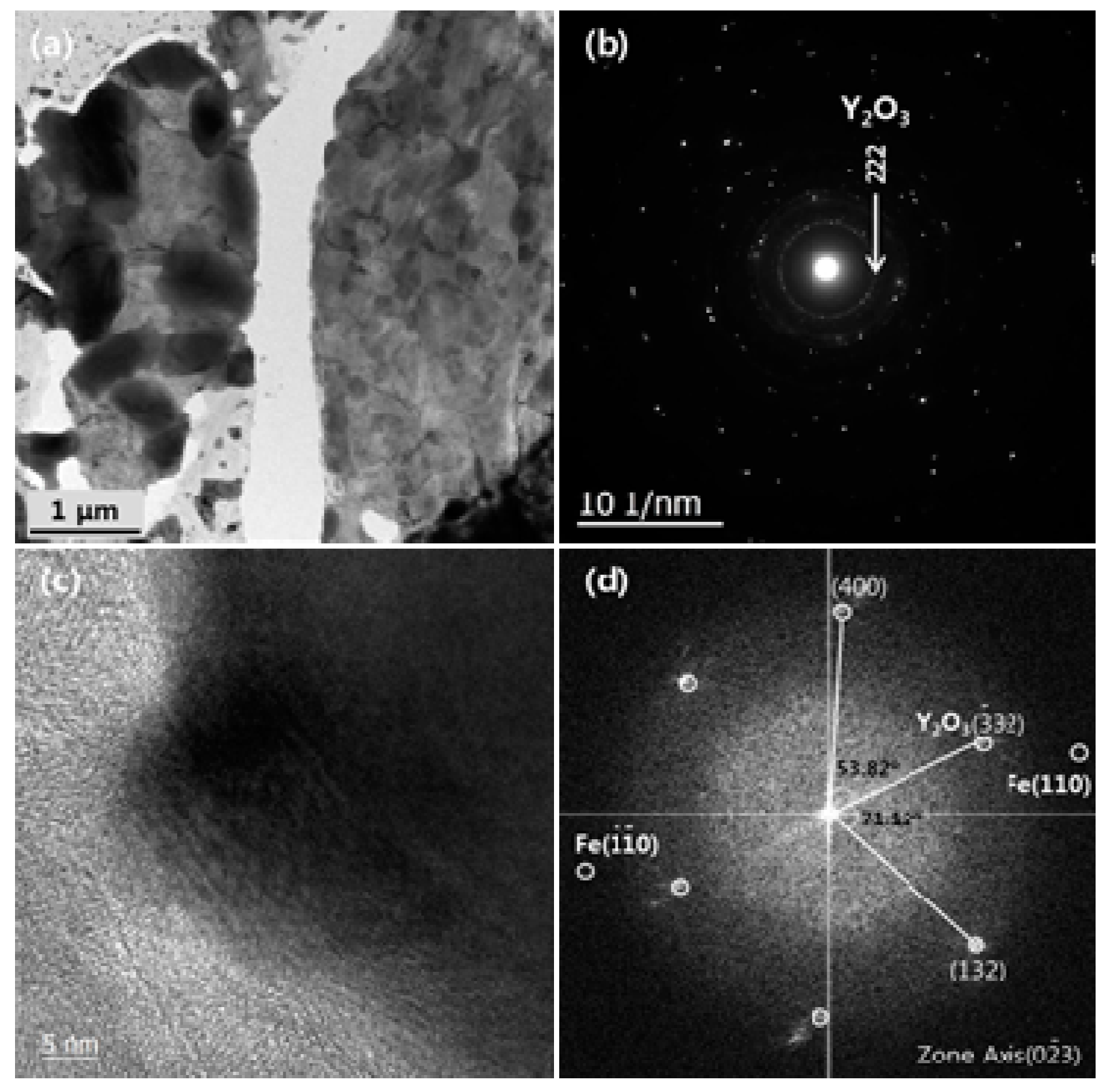
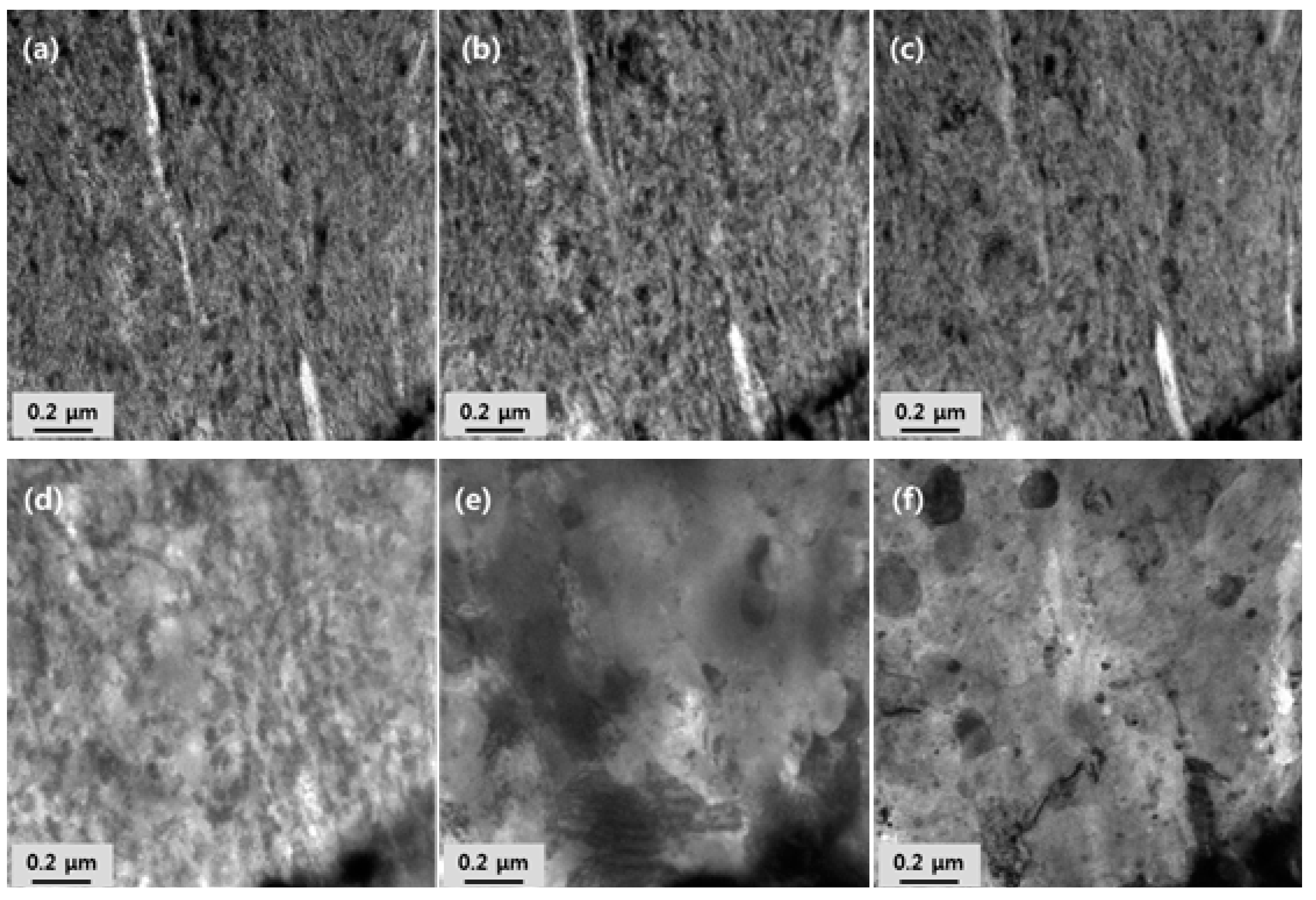
3.2. Microstructural Evolution of Oxide Particles
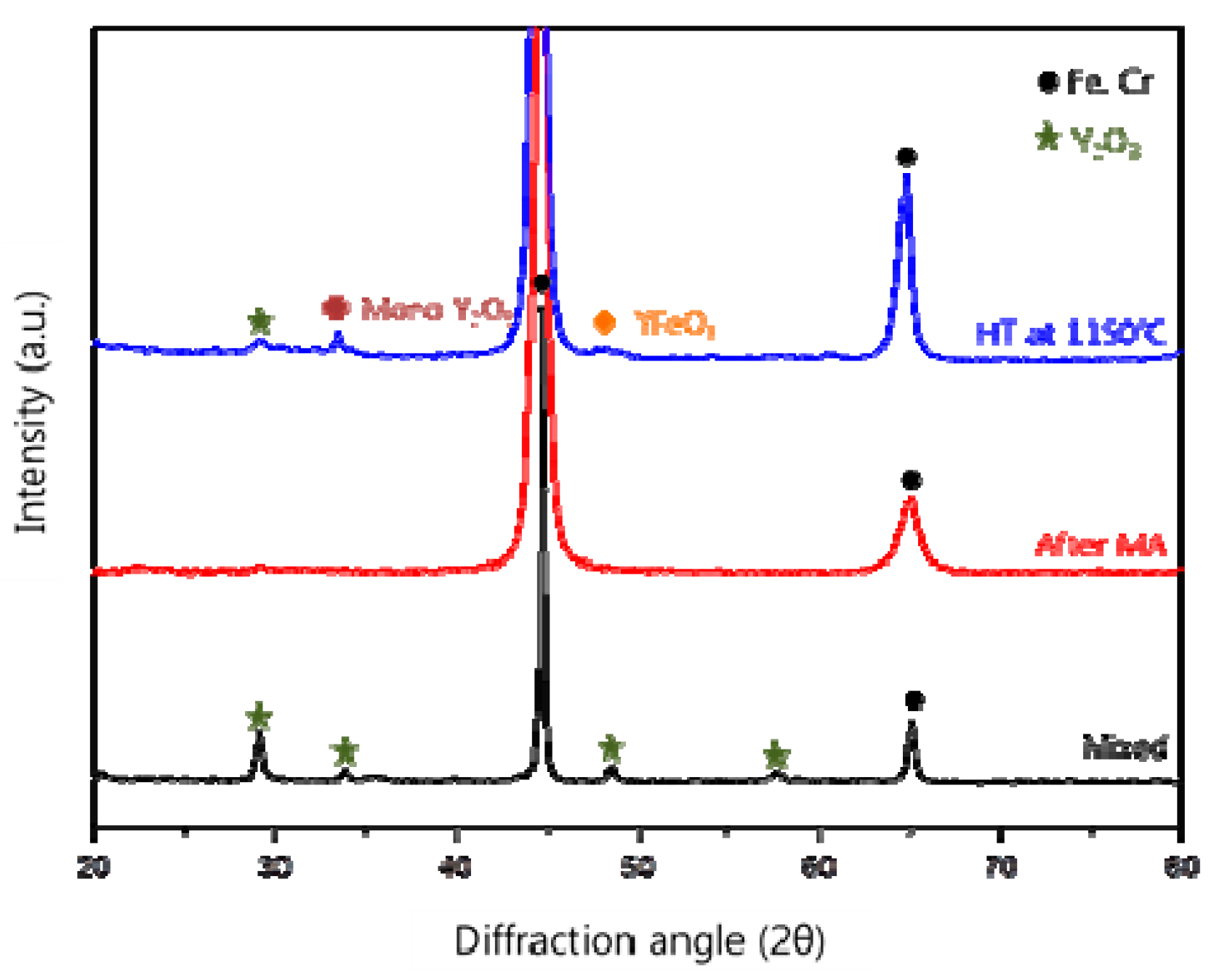
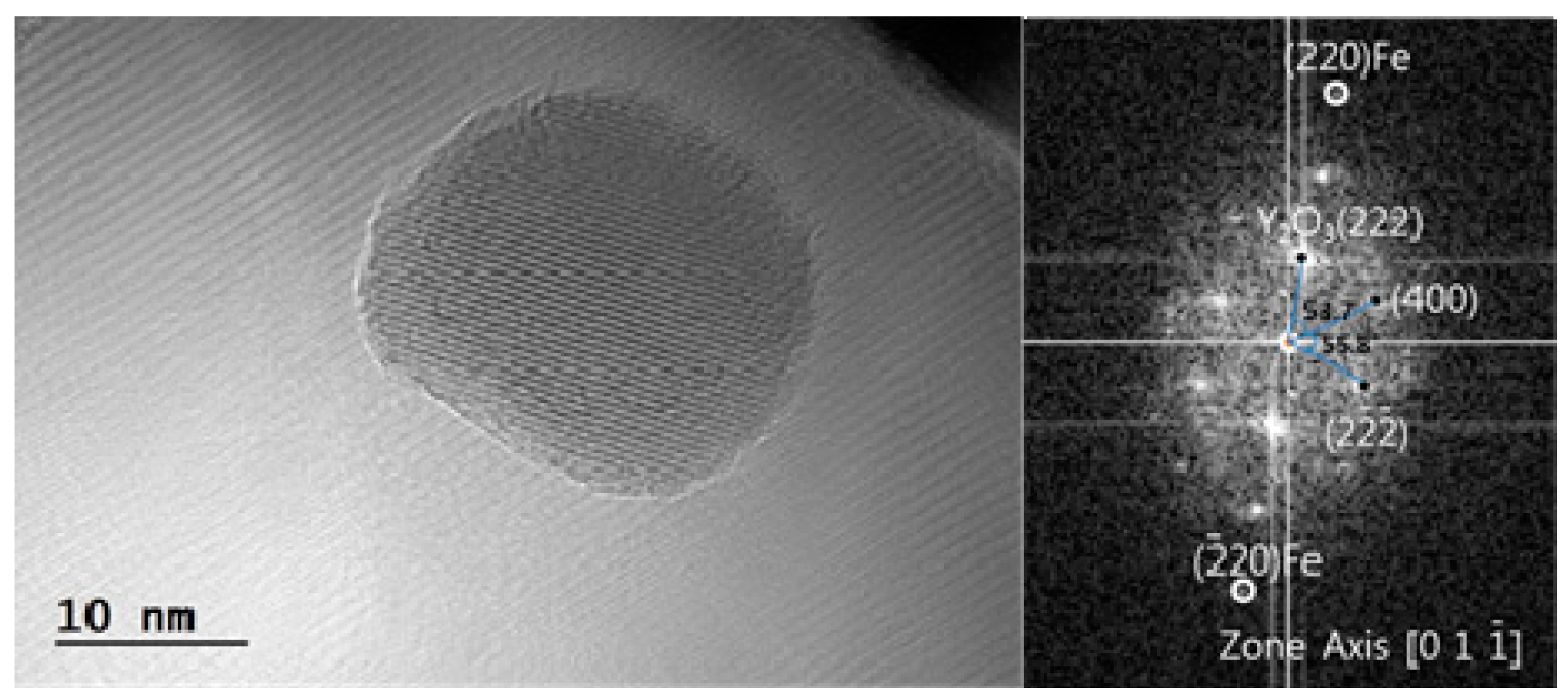
3.3. Crystal Structure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, T.K.; Noh, S.; Kang, S.H.; Park, J.J.; Jin, H.J.; Lee, M.K.; Jang, J.; Rhee, C.K. Current Status and Future Prospective of Advanced Radiation Resistant Oxide Dispersion Strengthened Steel (ARROS) Development for Nuclear Reactor System Applications. Nucl. Eng. Technol. 2016, 48, 572–594. [Google Scholar] [CrossRef]
- Jin, H.J.; Kim, T.K. Neutron irradiation performance of Zircaloy-4 under research reactor operating conditions. Ann. Nucl. Energy 2015, 75, 309–315. [Google Scholar] [CrossRef]
- Kim, T.K.; Noh, S.; Kang, S.H.; Jin, H.J.; Kim, G.E. Development of advanced radiation resistant ODS steel for fast reactor systems applications. World J. Eng. Technol. 2016, 3, 125–128. [Google Scholar] [CrossRef]
- Kim, T.K.; Han, C.H.; Kang, S.H.; Noh, S.; Jang, J. Effects of oxygen concentration on the size distribution of oxide particles in ODS steel. Curr. Nanosci. 2014, 10, 94–96. [Google Scholar] [CrossRef]
- Kim, T.K.; Bae, C.S.; Kim, D.H.; Jang, J.; Kim, S.H.; Lee, C.B.; Hahn, D. Microstructural observation and tensile isotropy of an austenitic ODS steel. Nucl. Eng. Technol. 2008, 40, 1–6. [Google Scholar] [CrossRef]
- Noh, S.; Choi, B.K.; Kang, S.H.; Kim, T.K. Influence of mechanical alloying atmospheres on the microstructures and mechanically properties of 15Cr ODS steels. Nucl. Eng. Technol. 2014, 46, 857–862. [Google Scholar] [CrossRef]
- Mao, X.; Oh, K.H.; Kang, S.H.; Kim, T.K.; Jang, J. On the coherency of Y2Ti2O7 particles with austenitic matrix of oxide dispersion strengthened steel. Acta Mater. 2015, 89, 141–152. [Google Scholar] [CrossRef]
- Susila, P.; Sturm, D.; Heilmaier, M.; Murty, B.S.; Subramanya, V. Effect of yttria particle size on the microstructure and compression creep properties of nanostructured oxide dispersion strengthened ferritic (Fe-12Cr-2W-0.5Y2O3) alloy. Mater. Sci. Eng. A 2011, 528, 4579–4584. [Google Scholar] [CrossRef]
- Srinivasan, D.; Corderman, R.; Subramanian, P.R. Strengthening mechanisms (via hardness analysis) in nanocrystalline NiCr with nanoscaled Y2O3 and Al2O3 dispersoids. Mater. Sci. Eng. A 2006, 416, 211–218. [Google Scholar] [CrossRef]
- Sakasegawa, H.; Tamura, M.; Ohtsuka, S.; Ukai, S.; Tanigawa, H.; Kohyama, A.; Fujiwara, M. Precipitation behavior of oxide particles in mechanically alloyed powder of oxide-dispersion-strengthened steel. J. Alloys Compd. 2008, 452, 2–6. [Google Scholar] [CrossRef]
- Kim, S.W.; Shobu, T.; Ohtsuka, S.; Kaito, T.; Inoue, M.; Ohuma, M. Kinetic approach for growth and coalescence of nano-size oxide particles in 9Cr-ODS steel using high-energy synchrotron radiation X-rays in SPring-8. Mater. Trans. 1993, 204, 65–73. [Google Scholar] [CrossRef]
- Lescoat, M.L.; Ribs, J.; Chen, Y.; Marquis, E.A.; Bordas, E.; Trocellier, P.; Serruys, Y.; Gentils, A.; Kaitasov, O.; Carlan, Y.; et al. Radiation-induced ostwald ripening in oxide dispersion strengthened ferritic steels irradiated at high ion dose. Acta Mater. 2014, 78, 328–340. [Google Scholar] [CrossRef]
- Brocq, M.; Radiguet, B.; Le Breton, J.M.; Cuvilly, F.; Pareige, P.; Legendre, F. Nanoscale characterization and clustering mechanism in an Fe-Y2O3 model ODS alloy processed by reactive ball milling and annealing. Acta Mater. 2010, 58, 1806–1814. [Google Scholar] [CrossRef]
- London, A.J.; Santra, S.; Amirthapandian, S.; Panigrahi, B.K.; Sarguna, R.M.; Balaji, S.; Vijay, R.; Sundar, C.S.; Lozano-perez, S.; Grovenor, C.R.M. Effect of Ti and Cr on dispersion, structure and composition of oxide nano-particles in model ODS alloys. Acta Mater. 2015, 97, 223–233. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.E.; Kim, T.K.; Noh, S. Structural Evolution of Nano-sized Oxide Particles Formed in Mechanically Alloyed Fe-10Cr-5Y2O3 Powders. Metals 2020, 10, 310. https://doi.org/10.3390/met10030310
Kim GE, Kim TK, Noh S. Structural Evolution of Nano-sized Oxide Particles Formed in Mechanically Alloyed Fe-10Cr-5Y2O3 Powders. Metals. 2020; 10(3):310. https://doi.org/10.3390/met10030310
Chicago/Turabian StyleKim, Ga Eon, Tae Kyu Kim, and Sanghoon Noh. 2020. "Structural Evolution of Nano-sized Oxide Particles Formed in Mechanically Alloyed Fe-10Cr-5Y2O3 Powders" Metals 10, no. 3: 310. https://doi.org/10.3390/met10030310
APA StyleKim, G. E., Kim, T. K., & Noh, S. (2020). Structural Evolution of Nano-sized Oxide Particles Formed in Mechanically Alloyed Fe-10Cr-5Y2O3 Powders. Metals, 10(3), 310. https://doi.org/10.3390/met10030310



