Digital Holographic Study of pH Effects on Anodic Dissolution of Copper in Aqueous Chloride Electrolytes
Abstract
:1. Introduction
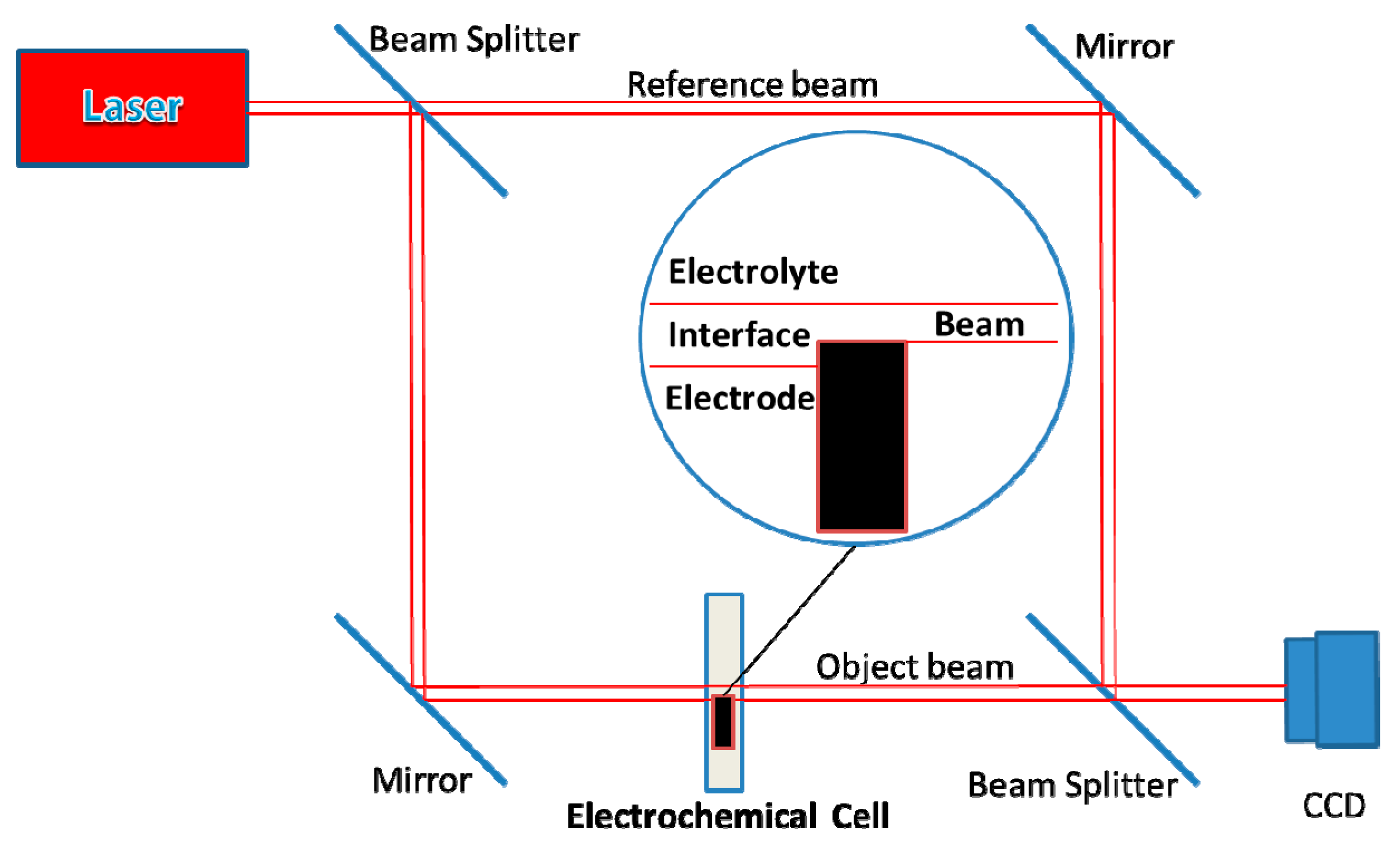
2. Materials and Methods
3. Results and Discussion
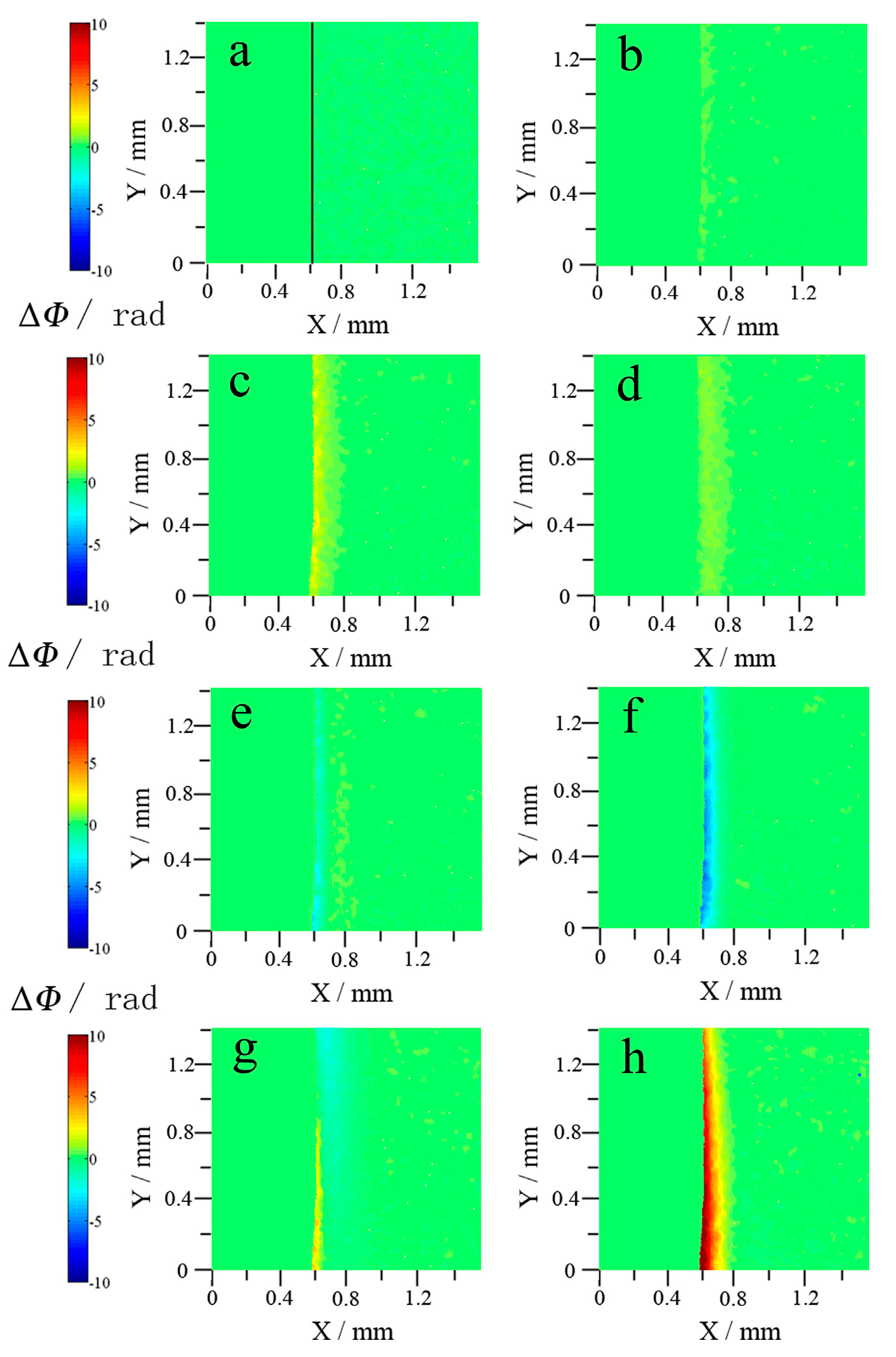
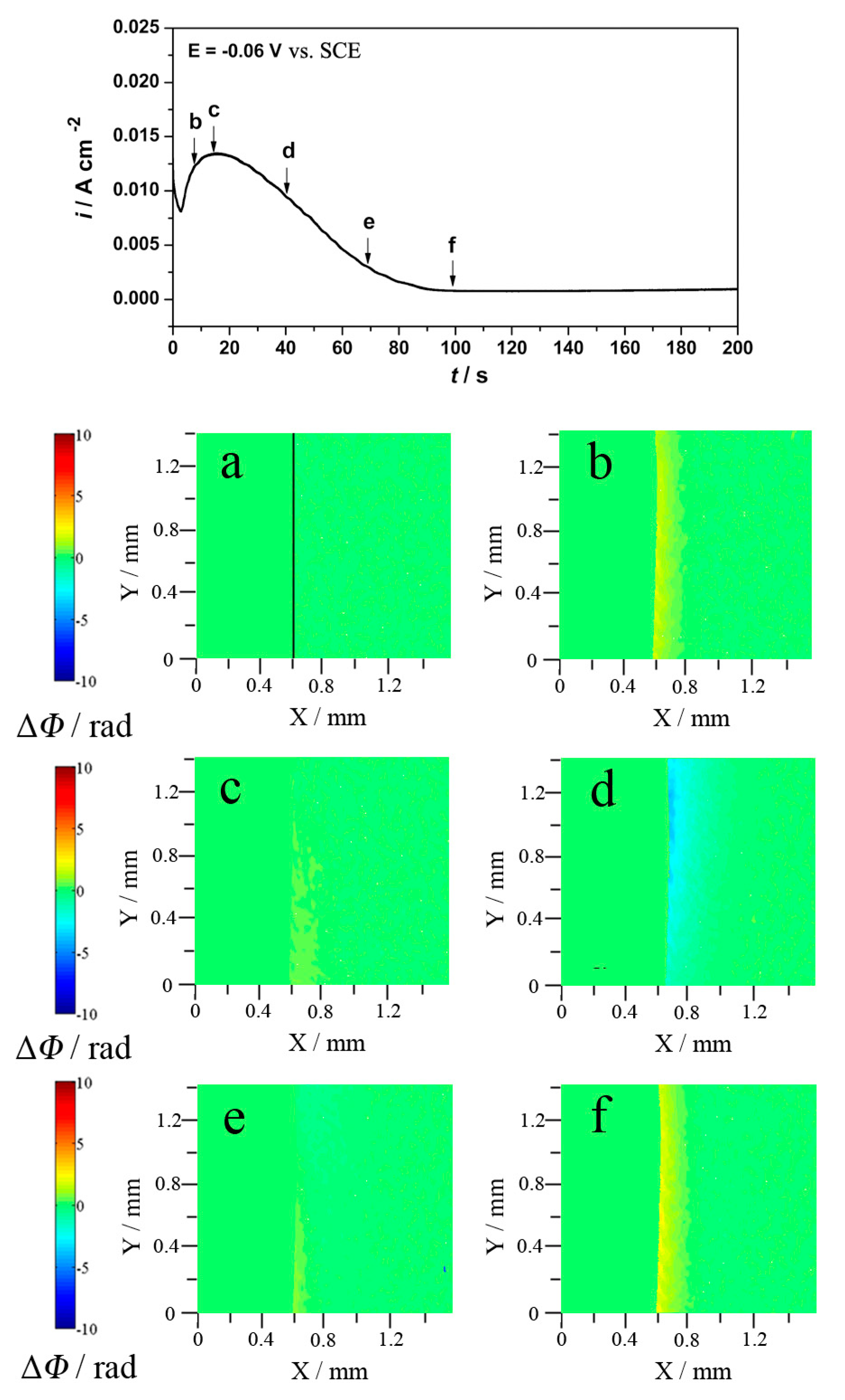
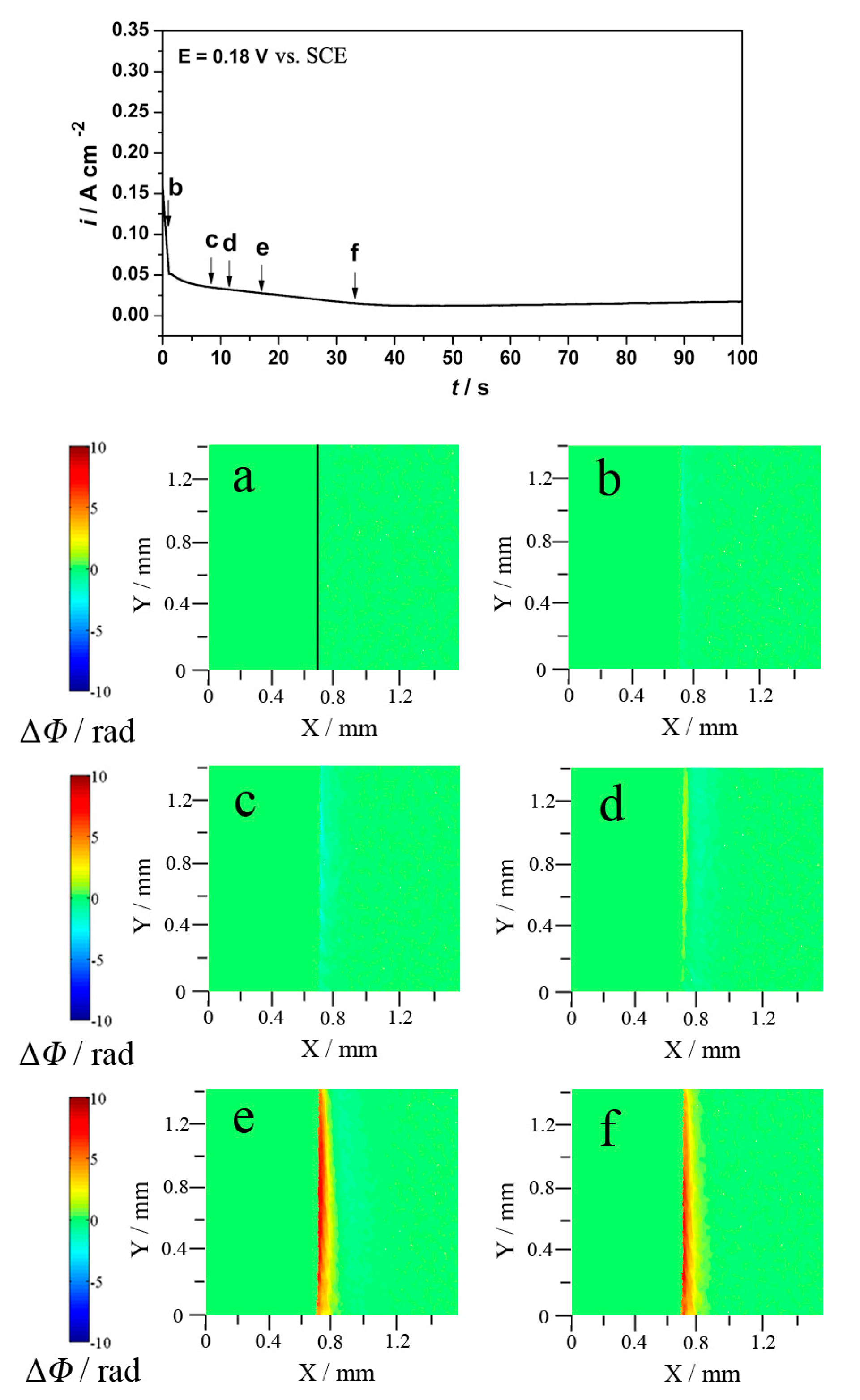
3.1. Neutral and Acid Chloride Electrolytes
3.1.1. Potentiodynamic and Holographic Results
3.1.2. Potentiostatic and Holographic Results
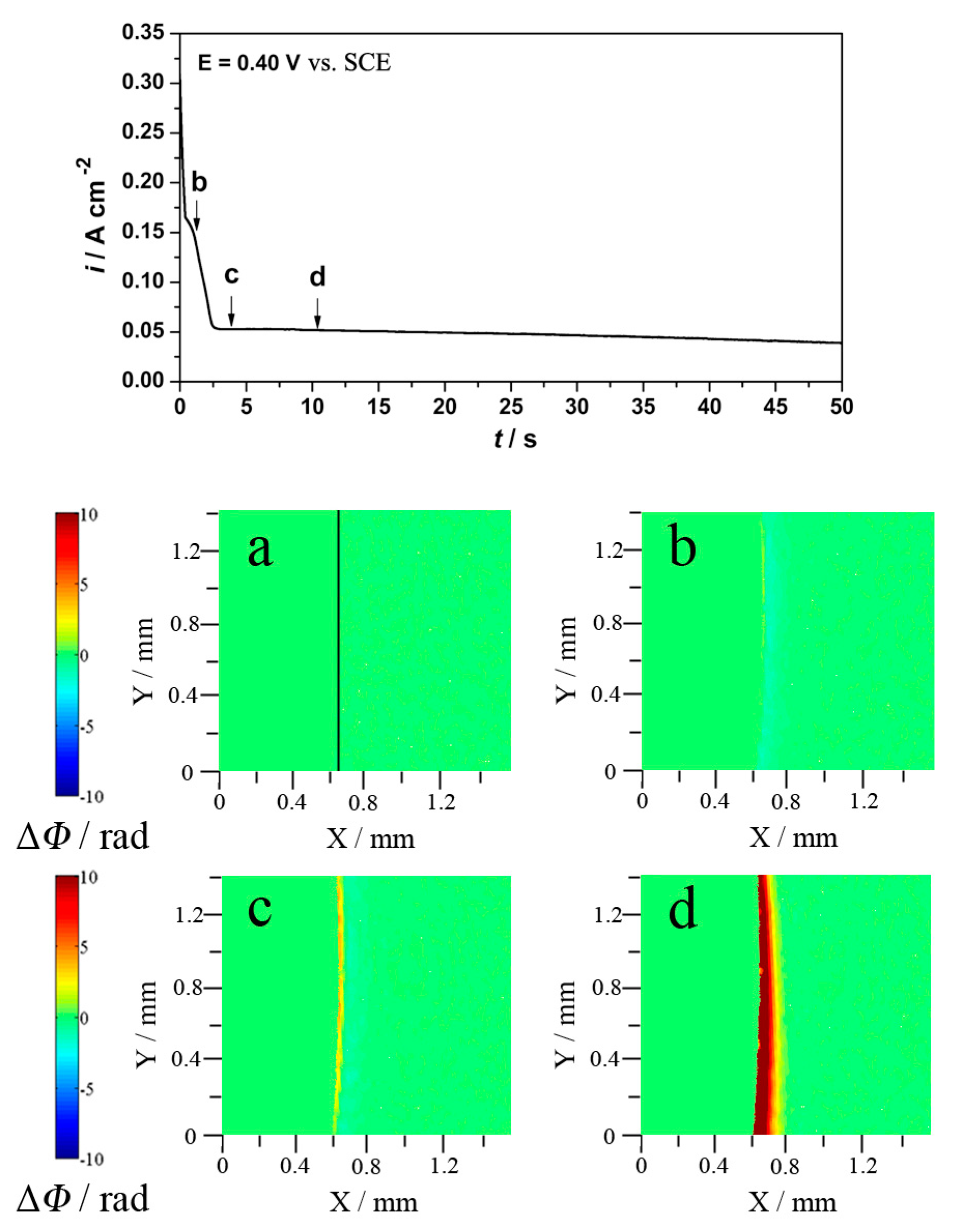
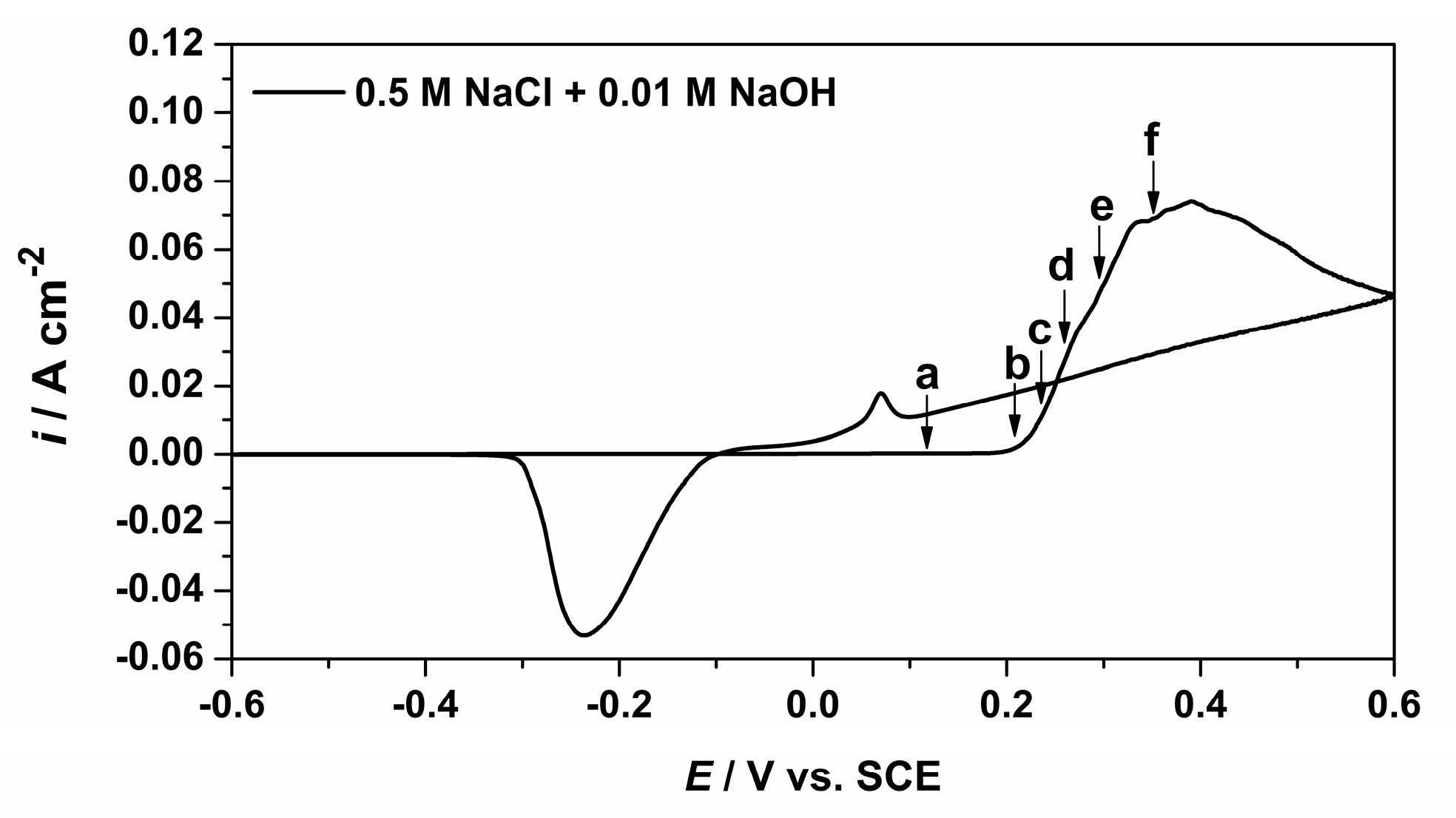
3.2. Alkaline Chloride Electrolyte
3.3. Effects of pH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kear, G.; Barker, B.D.; Walsh, F.C. Electrochemical corrosion of unalloyed copper in chloride media—A critical review. Corros. Sci. 2004, 46, 109–135. [Google Scholar] [CrossRef]
- El Warraky, A.; El Shayeb, H.A.; Sherif, E.S.M. Pitting corrosion of copper in chloride solutions. Anti-Corros. Method Mater. 2004, 51, 52–61. [Google Scholar] [CrossRef]
- Milic, S.M.; Antonijevic, M.M. Some aspects of copper corrosion in presence of benzotriazole and chloride ions. Corros. Sci. 2009, 51, 28–34. [Google Scholar] [CrossRef]
- Antonijevic, M.M.; Alagic, S.C.; Petrovic, M.B.; Radovanovic, M.B.; Stamenkovic, A.T. The influence of pH on electrochemical behavior of copper in presence of chloride ions. Int. J. Electrochem. Sci. 2009, 4, 516–524. [Google Scholar]
- Milan, M.M.; Milić, S.M.; Petrović, M.B. Films formed on copper surface in chloride media in the presence of azoles. Corros. Sci. 2009, 51, 1228–1237. [Google Scholar]
- Yuan, B.; Wang, C.; Li, L.; Chen, S. Real time observation of the anodic dissolution of copper in NaCl solution with the digital holography. Electrochem. Commun. 2009, 11, 1373–1376. [Google Scholar] [CrossRef]
- Antonijevic, M.M.; Milic, S.M.; Radovanovic, M.B.; Petrovic, M.B.; Stamenkovic, A.T. Influence of pH and chlorides on electrochemical behavior of brass in presence of benzotriazole. Int. J. Electrochem. Sci. 2009, 4, 1719–1734. [Google Scholar]
- Liao, X.; Cao, F.; Zheng, L.; Liu, W.; Chen, A.; Zhang, J.; Cao, C. Corrosion behaviour of copper under chloride-containing thin electrolyte layer. Corros. Sci. 2011, 53, 3289–3298. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, C.; Li, L.; Chen, S. Investigation of the effects of the magnetic field on the anodic dissolution of copper in NaCl solutions with holography. Corros. Sci. 2012, 58, 69–78. [Google Scholar] [CrossRef]
- Martinez-Lombardia, E.; Gonzalez-Garcia, Y.; Lapeire, L.; De Graeve, I.; Verbeken, K.; Kestens, L.; Mol, J.M.C.; Terryn, H. Scanning electrochemical microscopy to study the effect of crystallographic orientation on the electrochemical activity of pure copper. Electrochim. Acta 2014, 116, 89–96. [Google Scholar] [CrossRef]
- Wang, D.; Xiang, B.; Liang, Y.; Song, S.; Liu, C. Corrosion control of copper in 3.5 wt. % NaCl solution by Domperidone: Experimental and theoretical study. Corros. Sci. 2014, 85, 77–86. [Google Scholar] [CrossRef]
- Ogata, S.; Kobayashi, N.; Kitagawa, T.; Shima, S.; Fukunaga, A.; Takatoh, C.; Fukuma, T. Nanoscale corrosion behavior of polycrystalline copper fine wires indilute NaCl solution investigated by in-situ atomic force microscopy. Corros. Sci. 2016, 105, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, J.; Fernández-Pérez, B.M.; Eifert, A.; Souto, R.M.; Kranz, C. Simultaneous atomic force-scanning electrochemical microscopy (AFM-SECM) imaging of copper dissolution. Electrochim. Acta 2016, 201, 320–332. [Google Scholar] [CrossRef]
- Ramirez-Cano, J.A.; Veleva, L.; Souto, R.M.; Fernandez-Perez, B.M. SECM study of the pH distribution over Cu samples treated with 2-mercaptobenzothiazole in NaCl solution. Electrochem. Commun. 2017, 78, 60–63. [Google Scholar] [CrossRef]
- Ravichandran, R.; Rajendran, N. Influence of benzotriazole derivatives on the dezincification of 65–35 brass in sodium chloride. Appl. Surf. Sci. 2005, 239, 182–192. [Google Scholar] [CrossRef]
- El-Sayed, E.M.; Park, S.M. Effects of 2-amino-5-(ethylthio)-1,3,4-thiadiazole on copper corrosion as a corrosion inhibitor in 3% NaCl solutions. Appl. Surf. Sci. 2006, 252, 8615–8623. [Google Scholar]
- Larabi, L.; Benali, O.; Mekelleche, S.M.; Harek, Y. 2-Mercapto-1-methylimidazole as corrosion inhibitor for copper in hydrochloric acid. Appl. Surf. Sci. 2006, 253, 1371–1378. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; Erasmus, R.M.; Comins, J.D. Inhibition of copper corrosion in acidic chloride pickling solutions by 5-(3-aminophenyl)-tetrazole as a corrosion inhibitor. Corros. Sci. 2008, 50, 3439–3445. [Google Scholar] [CrossRef]
- Curkovic, H.O.; Stupnisek-Lisac, E.; Takenouti, H. The influence of pH value on the efficiency of imidazole based corrosion inhibitors of copper. Corros. Sci. 2010, 52, 398–405. [Google Scholar] [CrossRef]
- Liao, Q.Q.; Yue, Z.W.; Yang, D.; Wang, Z.H.; Li, Z.H.; Ge, H.H.; Li, Y.J. Inhibition of copper corrosion in sodium chloride solution by the self-assembled monolayer of sodium diethyldithiocarbamate. Corros. Sci. 2011, 53, 1999–2005. [Google Scholar] [CrossRef]
- Khiati, Z.; Othman, A.A.; Sanchez-Moreno, M.; Bernard, M.-C.; Joiret, S.; Sutter, E.M.M.; Vivier, V. Corrosion inhibition of copper in neutral chloride media by a novel derivative of 1,2,4-triazole. Corros. Sci. 2011, 53, 3092–3099. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.-Q.; Xie, B.; Gao, L.-X.; Joo, H.G.; Lee, K.Y. Inhibition of copper corrosion in acidic chloride solution by methionine combined with cetrimonium bromide/cetylpyridinium bromide. J. Appl. Electrochem. 2011, 41, 491–498. [Google Scholar] [CrossRef]
- Hong, S.; Chen, W.; Zhang, Y.; Luo, H.Q.; Li, M.; Li, N.B. Investigation of the inhibition effect of trithiocyanuric acid on corrosion of copper in 3.0 wt. % NaCl. Corros. Sci. 2013, 66, 308–314. [Google Scholar] [CrossRef]
- Neodo, S.; Carugo, D.; Wharton, J.A.; Stokes, K.R. Electrochemical behaviour of nickel–aluminium bronze in chloride media: Influence of pH and benzotriazole. J. Electroanal. Chem. 2013, 695, 38–46. [Google Scholar] [CrossRef]
- Betova, I.; Bojinov, M.; Lilja, C. Influence of chloride on the long-term interaction of copper with deoxygenated neutral aqueous solutions. Corros. Sci. 2013, 76, 192–205. [Google Scholar] [CrossRef]
- Tansug, G.; Tüken, T.; Giray, E.S.; Fındıkkıran, G.; Sıg˘ırcık, G.; Demirkol, O.; Erbil, M. A new corrosion inhibitor for copper protection. Corros. Sci. 2014, 84, 21–29. [Google Scholar] [CrossRef]
- Badawy, W.A.; El-Rabiei, M.M.; Nady, H. Synergistic effects of alloying elements in Cu-ternary alloys inchloride solutions. Electrochim. Acta 2014, 120, 39–45. [Google Scholar] [CrossRef]
- Wu, Z.; Cheng, Y.F.; Liu, L.; Lv, W.; Hu, W. Effect of heat treatment on microstructure evolution and erosion–corrosion behavior of a nickel–aluminum bronze alloy in chloride solution. Corros. Sci. 2015, 98, 260–270. [Google Scholar] [CrossRef]
- Zhang, X.H.; Liao, Q.Q.; Nie, K.B.; Zhao, L.L.; Yang, D.; Yue, Z.W.; Ge, H.H.; Li, Y.J. Self-assembled monolayers formed by ammonium pyrrolidine dithiocarbamate on copper surfaces in sodium chloride solution. Corros. Sci. 2015, 93, 201–210. [Google Scholar] [CrossRef]
- Yu, Y.Z.; Zhang, D.Q.; Zeng, H.J.; Xie, B.; Gao, L.X.; Lin, T. Synergistic effects of sodium lauroyl sarcosinate and glutamic acid in inhibition assembly against copper corrosion in acidic solution. Appl. Surf. Sci. 2015, 355, 1229–1237. [Google Scholar] [CrossRef]
- Yu, Y.Z.; Yang, D.; Zhang, D.Q.; Wang, Y.Z.; Gao, L.X. Anti-corrosion film formed on HAl77-2 copper alloy surface by aliphatic polyamine in 3 wt. % NaCl solution. Appl. Surf. Sci. 2017, 392, 768–776. [Google Scholar] [CrossRef]
- Denpo, K.; Okumura, T.; Fukunaka, Y.; Kondo, Y. Measurement of concentration profiles of Cu2+ ion and H+ ion near a plane vertical cathode by two-wavelength holographic interferometry. J. Electrochem. Soc. 1985, 132, 1145–1150. [Google Scholar] [CrossRef]
- Chao, C.W.; Lin, S.P.; Wang, Y.Y.; Wan, C.C.; Yang, J.T. Continuous monitoring of acid stratification during charge/discharge by holographic laser interferometry. J. Power Sources 1995, 55, 243–246. [Google Scholar] [CrossRef]
- Habib, K. In-situ monitoring of pitting corrosion of copper alloys by holographic interferometry. Corros. Sci. 1998, 40, 1435–1440. [Google Scholar] [CrossRef]
- Konishi, Y.; Nakamura, Y.; Fukunaka, Y.; Tsukada, K.; Hanasaki, K. Anodic dissolution phenomena accompanying supersaturation of copper sulfate along a vertical plane copper anode. Electrochim. Acta 2003, 48, 2615–2624. [Google Scholar] [CrossRef]
- Yang, X.; Chen, S.; Wang, C.; Li, L. In-line digital holography for the study of dynamic processes of electrochemical reaction. Electrochem. Commun. 2004, 6, 643–647. [Google Scholar] [CrossRef]
- Nishikawa, K.; Fukunaka, Y.; Sakka, T.; Ogata, Y.H.; Selman, J.R. Ionic mass transfer during electrochemical dissolution of Li metal in PC electrolyte solution. J. Electroanal. Chem. 2005, 584, 63–69. [Google Scholar] [CrossRef]
- Nishikawa, K.; Fukunaka, Y.; Sakka, T.; Ogata, Y.; Selman, J.R. Robert Selman, in situ measurement of lithium mass transfer during charging and discharging of a Ni–Sn alloy electrode. J. Power Sources 2007, 174, 668–672. [Google Scholar] [CrossRef]
- Yuan, B.; Chen, S.; Yang, X.; Wang, C.; Li, L. Mapping the transient concentration field within the diffusion layer by use of the digital holographic reconstruction. Electrochem. Commun. 2008, 10, 392–396. [Google Scholar] [CrossRef]
- Yang, X.; Eckert, K.; Heinze, A.; Uhlemann, M. The concentration field during transient natural convection between vertical electrodes in a small-aspect-ratio cell. J. Electroanal. Chem. 2008, 613, 97–107. [Google Scholar] [CrossRef]
- Tada, E.; Kaneko, H. Optical visualization of concentration field of Zn2+ during galvanic corrosion of a Zn/steel couple. Corros. Sci. 2010, 52, 3421–3427. [Google Scholar] [CrossRef]
- Klages, P.E.; Rotermund, M.K.; Rotermund, H.H. Simultaneous holographic, ellipsometric, and optical imaging of pitting corrosion on SS 316LVM stainless steel. Corros. Sci. 2012, 65, 128–135. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Di, J.; Rauf, A.; Hao, J. Dynamically measuring unstable reaction–diffusion process by using digital holographic interferometry. Opt. Lasers Eng. 2014, 57, 1–5. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Yuan, B.; Li, L.; Wang, C. Effects of the magnetic field on the corrosion dissolution of the 304SSFeCl3 system. Electrochim. Acta 2016, 222, 619–626. [Google Scholar] [CrossRef]
- Nimdeo, Y.M.; Joshi, Y.M.; Muralidhar, K. Diffusion of charged nano-disks in aqueous media: Influence of competing inter-particle interactions and thermal effects. Chem. Eng. Sci. 2017, 164, 71–80. [Google Scholar] [CrossRef]
- Cong, H.; Michels, H.T.; Scully, J.R. Passivity and pit stability behavior of copper as a function of selected water chemistry variables. J. Electrochem. Soc. 2009, 156, C16–C27. [Google Scholar] [CrossRef]
- Cong, H.; Scully, J.R. Use of coupled multielectrode arrays to elucidate the pH dependence of copper pitting in potable water. J. Electrochem. Soc. 2010, 157, C36–C46. [Google Scholar] [CrossRef]
- Ni, Q.; Xia, X.; Zhang, J.; Dai, N.; Fan, Y. Electrochemical and SVET studies on the typical polarity reversal of Cu–304 stainless steel galvanic couple in cl--containing solution with different pH. Electrochim. Acta 2017, 247, 207–215. [Google Scholar] [CrossRef]
- Bianchi, G.; Longhi, P. Copper in sea-water, potential-pH diagrams. Corros. Sci. 1973, 13, 853–864. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, C.; Yuan, B.; Li, Z.; Li, L.; Wang, C. Digital Holographic Study of pH Effects on Anodic Dissolution of Copper in Aqueous Chloride Electrolytes. Metals 2020, 10, 487. https://doi.org/10.3390/met10040487
Yan C, Yuan B, Li Z, Li L, Wang C. Digital Holographic Study of pH Effects on Anodic Dissolution of Copper in Aqueous Chloride Electrolytes. Metals. 2020; 10(4):487. https://doi.org/10.3390/met10040487
Chicago/Turabian StyleYan, Changqi, Boyu Yuan, Zhenhui Li, Liang Li, and Chao Wang. 2020. "Digital Holographic Study of pH Effects on Anodic Dissolution of Copper in Aqueous Chloride Electrolytes" Metals 10, no. 4: 487. https://doi.org/10.3390/met10040487






