Formation Process of the Integrated Core(Fe-6.5wt.%Si)@Shell(SiO2) Structure Obtained via Fluidized Bed Chemical Vapor Deposition
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
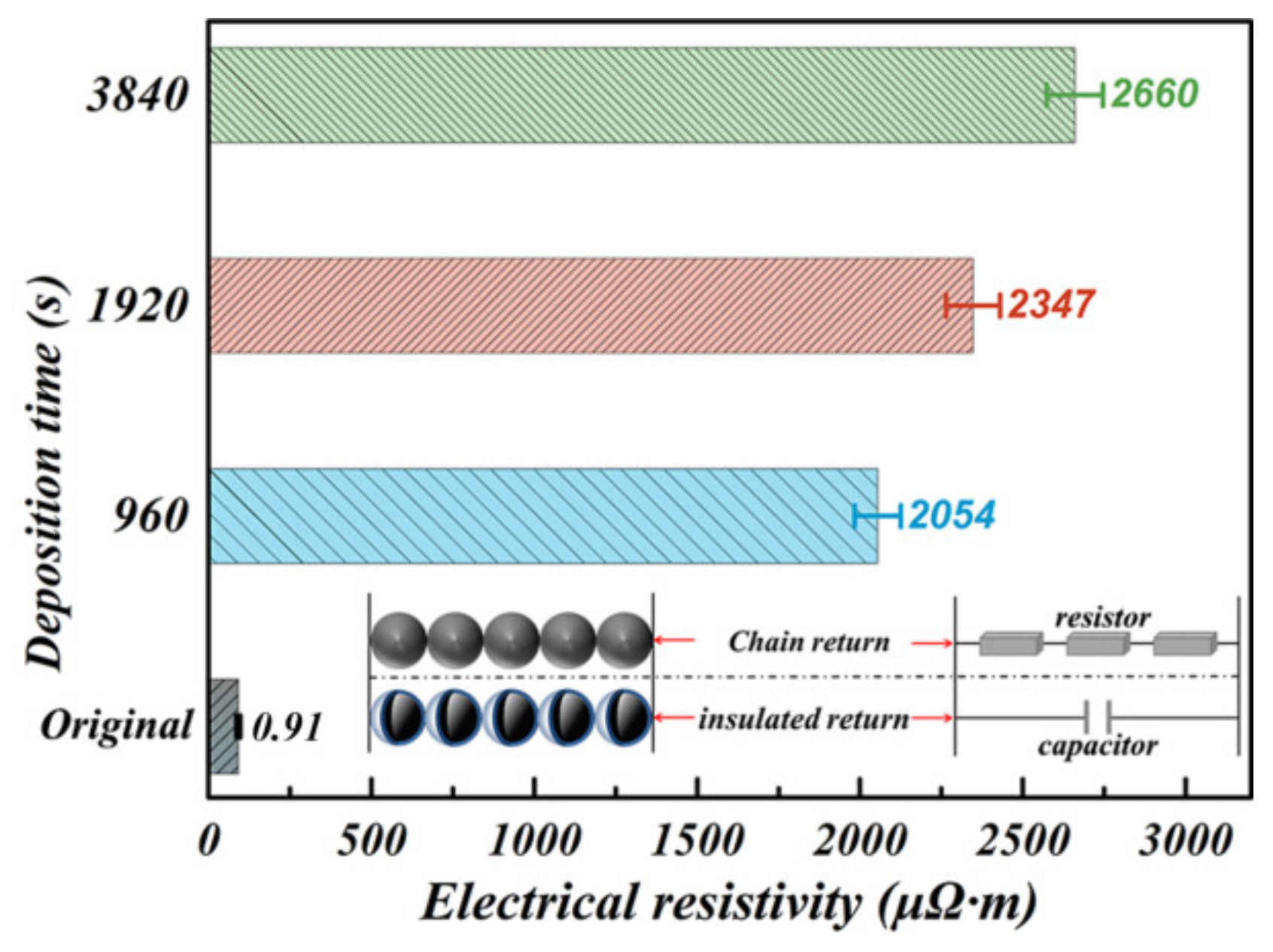
3.1. Synthesis Process of Fe-6.5wt.%Si/SiO2 C-S Structure
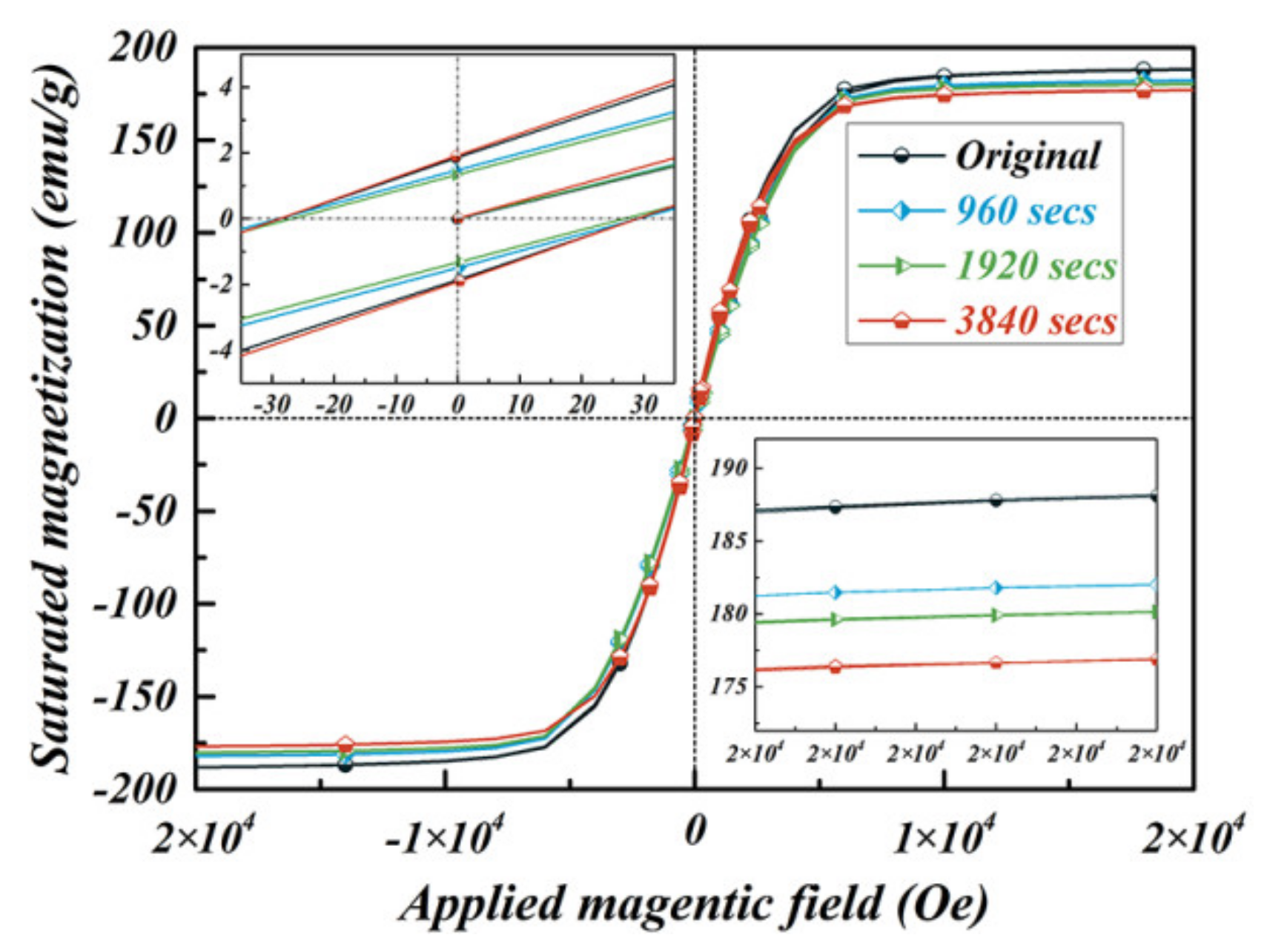
3.2. Static Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shokrollahi, H.; Janghorban, K. Soft magnetic composite materials (smcs). J. Mater. Process. Technol. 2007, 189, 1–12. [Google Scholar] [CrossRef]
- Périgo, E.; Weidenfeller, B.; Kollár, P.; Füzer, J. Past, present, and future of soft magnetic composites. Appl. Phys. Rev. 2018, 5, 031301. [Google Scholar] [CrossRef]
- Shokrollahi, H. The magnetic and structural properties of the most important alloys of iron produced by mechanical alloying. Mater. Des. 2009, 30, 3374–3387. [Google Scholar] [CrossRef]
- Liu, D.; Wu, C.; Yan, M.; Wang, J. Correlating the microstructure, growth mechanism and magnetic properties of fesial soft magnetic composites fabricated via hno3 oxidation. Acta Mater. 2018, 146, 294–303. [Google Scholar] [CrossRef]
- Liu, L.; Yue, Q.; Li, G.; Xu, K.; Wang, J.; Wu, Z.; Fan, X. Influence of sio2 insulation layers thickness distribution on magnetic behaviors of fe-si@ sio2 soft magnetic composites. J. Phys. Chem. Solids 2019, 132, 76–82. [Google Scholar] [CrossRef]
- King, D.M.; Liang, X.; Weimer, A.W. Functionalization of fine particles using atomic and molecular layer deposition. Powder Technol. 2012, 221, 13–25. [Google Scholar] [CrossRef]
- Ferguson, J.D.; Weimer, A.W.; George, S.M. Atomic layer deposition of ultrathin and conformal al2o3 films on bn particles. Thin Solid Films 2000, 371, 95–104. [Google Scholar] [CrossRef]
- King, D.M.; Spencer, J.A.; Liang, X.; Hakim, L.F.; Weimer, A.W. Atomic layer deposition on particles using a fluidized bed reactor with in situ mass spectrometry. Surf. Coat. Technol. 2007, 201, 9163–9171. [Google Scholar] [CrossRef]
- Rauwel, E.; Galeckas, A.; Rauwel, P.; Nilsen, O.; Walmsley, J.; Rytter, E.; Fjellvåg, H. Ald applied to conformal coating of nanoporous γ-alumina: Spinel formation and luminescence induced by europium doping. J. Electrochem. Soc. 2012, 159, 45–49. [Google Scholar] [CrossRef]
- Fan, X.; Wu, Z.; Li, G.; Wang, J.; Xiang, Z.; Gan, Z. High resistivity and low core loss of intergranular insulated fe–6.5 wt.% si/sio2 composite compacts. Mater. Des. 2016, 89, 1251–1258. [Google Scholar] [CrossRef]
- Luo, F.; Fan, X.A.; Luo, Z.; Hu, W.; Li, G.; Li, Y.; Liu, X.; Wang, J. Ultra-low inter-particle eddy current loss of fe3si/al2o3 soft magnetic composites evolved from fesial/fe3o4 core-shell particles. J. Magn. Magn. Mater. 2019, 484, 218–224. [Google Scholar] [CrossRef]
- Nazir, S.; Jiang, S.; Cheng, J.; Yang, K. Enhanced interfacial perpendicular magnetic anisotropy in fe/mgo heterostructure via interfacial engineering. Appl. Phys. Lett 2019, 114, 072407. [Google Scholar] [CrossRef]
- Rauwel, E.; Nilsen, O.; Rauwel, P.; Walmsley, J.C.; Frogner, H.B.; Rytter, E.; Fjellv?g, H. Oxide coating of alumina nanoporous structure using ald to produce highly porous spinel. Chem. Vap. Depos. 2012, 18, 315–325. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, C.; Luo, D.; Yan, M. Soft magnetic composites based on the fe elemental, binary and ternary alloy systems fabricated by surface nitridation. J. Magn. Magn. Mater. 2019, 481, 140–149. [Google Scholar] [CrossRef]
- Liu, D.; Wu, C.; Yan, M. Investigation on sol–gel Al2O3 and hybrid phosphate-alumina insulation coatings for fesial soft magnetic composites. J. Mater. Sci. 2015, 50, 6559–6566. [Google Scholar] [CrossRef]
- Wu, C.; Chen, H.; Lv, H.; Yan, M. Interplay of crystallization, stress relaxation and magnetic properties for fecunbsib soft magnetic composites. J. Alloys Compd. 2016, 673, 278–282. [Google Scholar] [CrossRef]
- Pooladi, M.; Shokrollahi, H.; Lavasani, S.; Yang, H. Investigation of the structural, magnetic and dielectric properties of mn-doped bi2fe4o9 produced by reverse chemical co-precipitation. Mater. Chem. Phys. 2019, 229, 39–48. [Google Scholar] [CrossRef]
- Shokrollahi, H. Structure, synthetic methods, magnetic properties and biomedical applications of ferrofluids. Mater. Sci. Eng. C 2013, 33, 2476–2487. [Google Scholar]
- Fan, X.; Wang, J.; Wu, Z.; Li, G. Core–shell structured FeSiAl/SiO2 particles and Fe3Si/Al2O3 soft magnetic composite cores with tunable insulating layer thicknesses. Mater. Sci. Eng. B 2015, 201, 79–86. [Google Scholar] [CrossRef]
- Geng, K.; Xie, Y.; Xu, L.; Yan, B. Structure and magnetic properties of ZrO2-coated Fe powders and Fe/ZrO2 soft magnetic composites. Adv. Powder Technol. 2017, 28, 2015–2022. [Google Scholar] [CrossRef]
- Hsiang, H.I.; Fan, L.F.; Hung, J.J. Phosphoric acid addition effect on the microstructure and magnetic properties of iron-based soft magnetic composites. J. Magn. Magn. Mater. 2018, 447, 1–8. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, Z.; Fan, X.; Zhou, L.; Wang, W.; Xu, K. Facile synthesis of Fe-6.5 wt% Si/SiO2 soft magnetic composites as an efficient soft magnetic composite material at medium and high frequencies. J. Alloys Compd. 2018, 742, 90–98. [Google Scholar] [CrossRef]
- Wu, S.L.; Chen, C.M.; Kuo, J.H.; Wey, M.Y. Synthesis of carbon nanotubes with controllable diameter by chemical vapor deposition of methane using Fe@Al2O3 core–shell nanocomposites. Chem. Eng. Sci. 2020, 217, 115541. [Google Scholar] [CrossRef]
- Turnbull, A.; Horner, D.A.; Connolly, B.J. Challenges in modelling the evolution of stress corrosion cracks from pits. Eng. Fract. Mech. 2008, 76, 633–640. [Google Scholar] [CrossRef]
- Serp, P.; Feurer, R.; Kalck, P.; Kihn, Y.; Faria, J.; Figueiredo, J. A chemical vapour deposition process for the production of carbon nanospheres. Carbon 2001, 39, 621–626. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Wang, M.; Wang, X.X.; Huang, W.D. Analysis of heterogeneous nucleation on rough surfaces based on wenzel model. Acta Phys. Sin. 2011, 6, 066402–066405. [Google Scholar]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Seel, S.C.; Thompson, C.V. Tensile stress generation during island coalescence for variable island-substrate contact angle. J. Appl. Phys. 2003, 93, 9038–9042. [Google Scholar] [CrossRef]
- Woodward, J.; Gwin, H.; Schwartz, D. Contact angles on surfaces with mesoscopic chemical heterogeneity. Langmuir 2000, 16, 2957–2961. [Google Scholar] [CrossRef]
- Baskaran, A.; Smereka, P. Mechanisms of stranski-krastanov growth. J. Appl. Phys. 2012, 111, 044321. [Google Scholar] [CrossRef]
- Castro, M.; Domınguez-Adame, F.; Sánchez, A.; Rodrıguez, T. Model for crystallization kinetics: Deviations from kolmogorov–johnson–mehl–avrami kinetics. Appl. Phys. Lett. 1999, 75, 2205–2207. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Luo, B.; Sun, C.; Li, Y.; Han, Y. Influence of bromine modification on collecting property of lauric acid. Miner. Eng. 2015, 79, 24–30. [Google Scholar] [CrossRef]
- Buchwalter, L.P. Chromium and tantalum adhesion to plasma-deposited silicon dioxide and silicon nitride. J. Adhes. Sci. Technol. 1995, 9, 97–116. [Google Scholar] [CrossRef]
- Temuujin, J.; Okada, K.; MacKenzie, K.J.D.; Jadambaa, T. Characterization of porous silica prepared from mechanically amorphized kaolinite by selective leaching. Powder Technol. 2001, 121, 259–262. [Google Scholar] [CrossRef]
- Kachurovskaya, N.A.; Zhidomirov, G.M.; Aristov, Y.I. On the problem of differentiation of acetone adsorption species on the silica gel: Molecular models of adsorption complexes. J. Mol. Catal. A Chem. 2000, 158, 281–285. [Google Scholar] [CrossRef]
- Coltrin, M.E.; Ho, P.; Moffat, H.K.; Buss, R.J. Chemical kinetics in chemical vapor deposition: Growth of silicon dioxide from tetraethoxysilane (teos). Thin Solid Films 2000, 365, 251–263. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Liu, L. Thermal stability of poss/methylsilicone nanocomposites. Compos. Sci. Technol. 2007, 67, 2864–2876. [Google Scholar] [CrossRef]
- He, S.; Sun, G.; Cheng, X.; Dai, H.; Chen, X. Nanoporous SiO2 grafted aramid fibers with low thermal conductivity. Compos. Sci. Technol. 2017, 146, 91–98. [Google Scholar] [CrossRef]
- Nanophase Materials: Synthesis-Properties-Applications; Hadjipanayis, G.C.; Siegel, R.W. (Eds.) Springer Science & Business Media: Berlin, Germany, 2012; Volume 260. [Google Scholar]
- Geng, K.; Xie, Y.; Yan, L.; Yan, B. Fe-si/zro2 composites with core-shell structure and excellent magnetic properties prepared by mechanical milling and spark plasma sintering. J. Alloys Compd. 2017, 718, 53–62. [Google Scholar] [CrossRef]
- Feng, Y.; Li, W.; Wang, J.; Yin, J.; Fei, W. Core–shell structured batio 3@ carbon hybrid particles for polymer composites with enhanced dielectric performance. J. Mater. Chem. A 2015, 3, 20313–20321. [Google Scholar] [CrossRef]
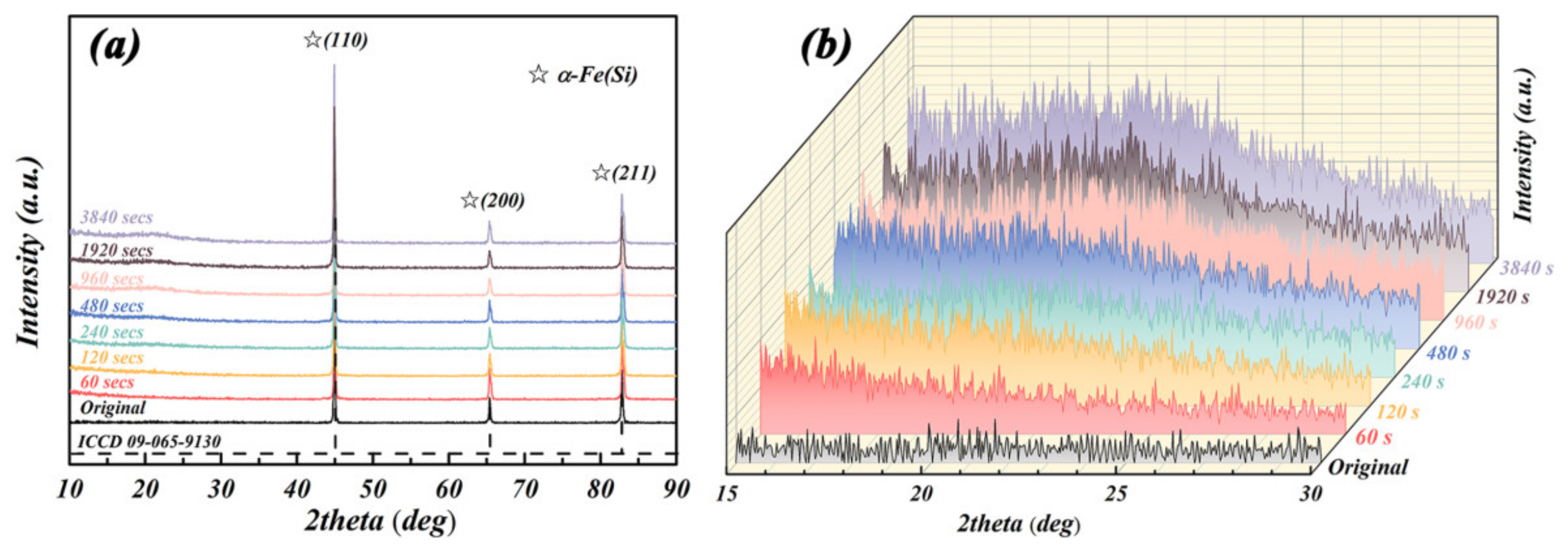


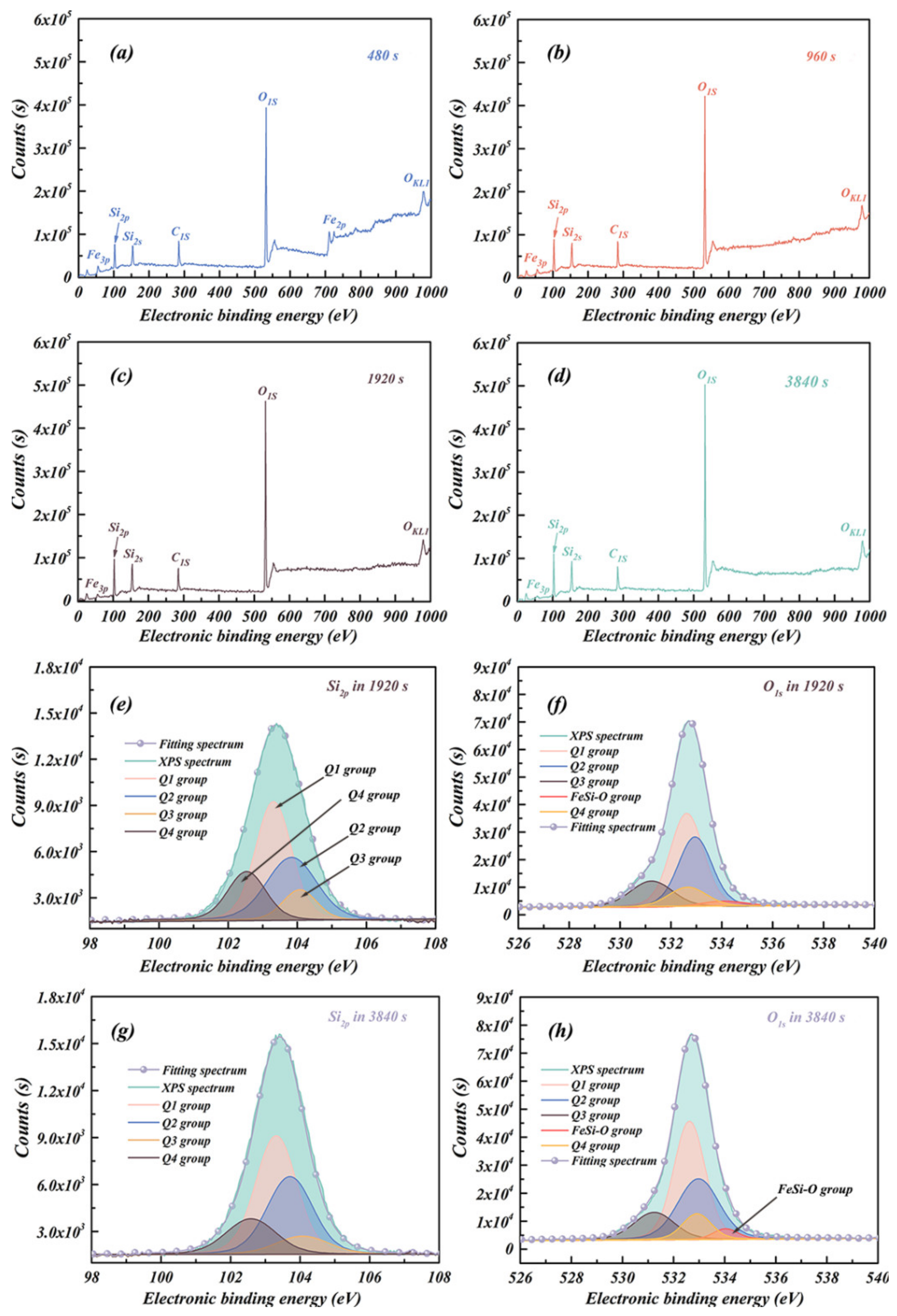
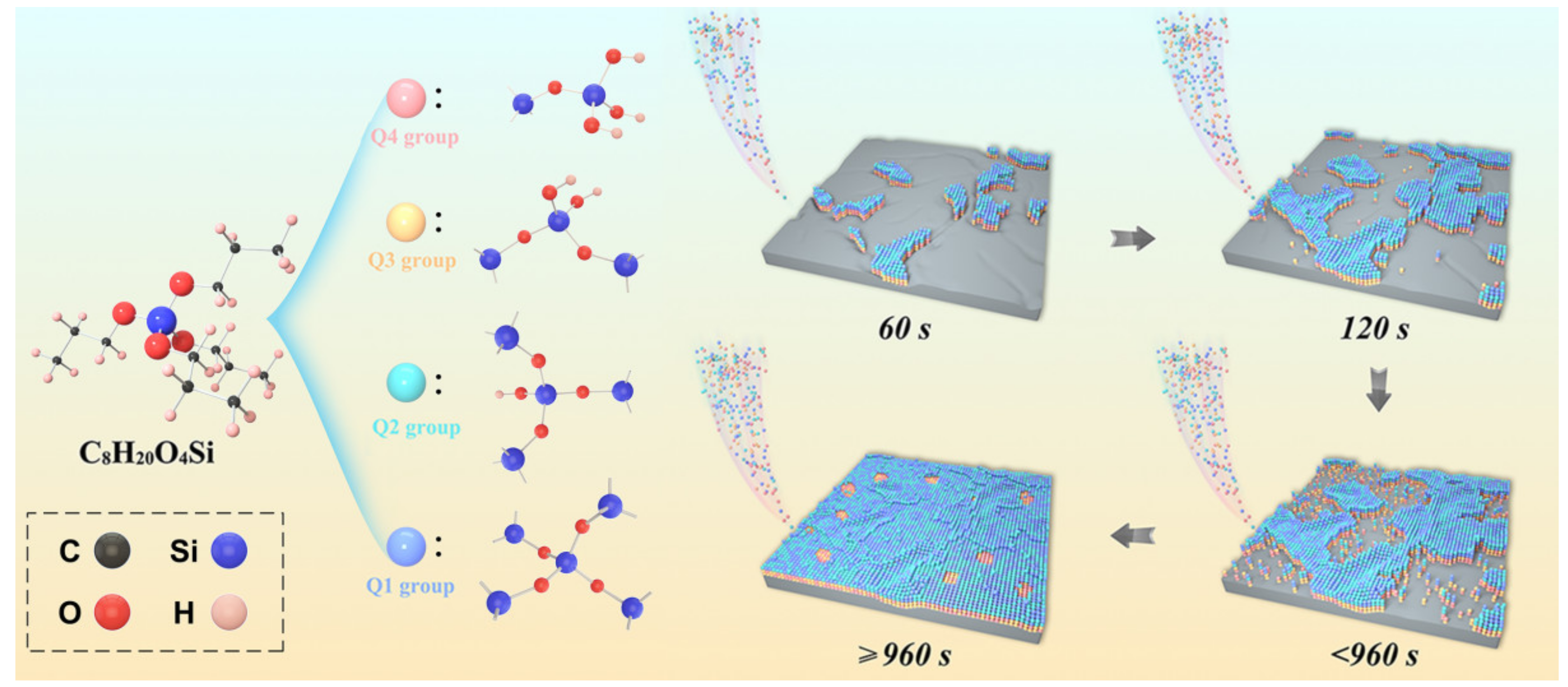
| Samples | Crystal Size (nm) | Micro-Strain (%) | Lattice Constant (Å) | |
|---|---|---|---|---|
| Original Fe-6.5wt.%Si particles | 55.1 ± 0.10 | 0.126 ± 0.0016 | 2.8508 ± 0.006 | |
| Particle samples after deposition for 60 to 3840 s | 60 s | 53.7 ± 0.06 | 0.131 ± 0.0013 | 2.8509 ± 0.004 |
| 120 s | 57.3 ± 0.08 | 0.122 ± 0.0017 | 2.8511 ± 0.003 | |
| 240 s | 56.1 ± 0.09 | 0.128 ± 0.0017 | 2.8510 ± 0.004 | |
| 480 s | 54.6 ± 0.09 | 0.123 ± 0.0019 | 2.8509 ± 0.005 | |
| 960 s | 58.7 ± 0.09 | 0.128 ± 0.0018 | 2.8510 ± 0.002 | |
| 1920 s | 58.8 ± 0.11 | 0.127 ± 0.0020 | 2.8511 ± 0.004 | |
| 3840 s | 54.1 ± 0.13 | 0.126 ± 0.0021 | 2.8513 ± 0.006 | |
| Deposition Time | Peaks | Relative Sensitivity Factors | Position | Full Width Half Maximum | Area | Atomic Ratio |
|---|---|---|---|---|---|---|
| 1920 s | Si2p | 1 | 102.54 eV | 1.40 | 4610.1 | 12.81% |
| Si2p | 1 | 103.31 eV | 1.31 | 10,896.3 | 42.56% | |
| Si2p | 1 | 103.84 eV | 1.53 | 7956.0 | 31.08% | |
| Si2p | 1 | 104.07 eV | 1.16 | 2138.9 | 8.35% | |
| O1s | 1 | 531.24 eV | 1.98 | 21,095.4 | 14.07% | |
| O1s | 1 | 532.62 eV | 1.53 | 65,018.5 | 43.87% | |
| O1s | 1 | 532.81 eV | 1.80 | 16,911.4 | 11.33% | |
| O1s | 1 | 532.97 eV | 1.87 | 43,835.3 | 29.24% | |
| O1s | 1 | 534.03 eV | 2.61 | 2978.7 | 1.99% | |
| 3840 s | Si2p | 1 | 102.54 eV | 1.70 | 4343.8 | 15.95% |
| Si2p | 1 | 103.31 eV | 1.39 | 11,862.6 | 43.55% | |
| Si2p | 1 | 103.87 eV | 1.55 | 8693.9 | 31.92% | |
| Si2p | 1 | 104.07 eV | 1.80 | 2337.9 | 8.58% | |
| O1s | 1 | 531.24 eV | 1.87 | 20,603.5 | 13.28% | |
| O1s | 1 | 532.62 eV | 1.45 | 69,023.3 | 44.49% | |
| O1s | 1 | 532.81 eV | 1.33 | 13,883.4 | 8.95% | |
| O1s | 1 | 532.97 eV | 1.91 | 46,698.8 | 30.10% | |
| O1s | 1 | 534.03 eV | 1.16 | 4922.8 | 3.17% |
| Samples | Theoretical Value (emu/g) | Measured Value (emu/g) | |
|---|---|---|---|
| original Fe-6.5wt.%Si particles | 188.1 | 188.1 | |
| Fe-6.5wt.%Si/SiO2 C-S particles | 960 s | 182.0 | 182.9 |
| 1920 s | 180.3 | 181.0 | |
| 3840 s | 176.9 | 178.4 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Xian, C.; Jia, J.; Liao, X.; Kong, H.; Xu, K. Formation Process of the Integrated Core(Fe-6.5wt.%Si)@Shell(SiO2) Structure Obtained via Fluidized Bed Chemical Vapor Deposition. Metals 2020, 10, 520. https://doi.org/10.3390/met10040520
Wu Z, Xian C, Jia J, Liao X, Kong H, Xu K. Formation Process of the Integrated Core(Fe-6.5wt.%Si)@Shell(SiO2) Structure Obtained via Fluidized Bed Chemical Vapor Deposition. Metals. 2020; 10(4):520. https://doi.org/10.3390/met10040520
Chicago/Turabian StyleWu, Zhaoyang, Chen Xian, Jixiang Jia, Xiangwei Liao, Hui Kong, and Kun Xu. 2020. "Formation Process of the Integrated Core(Fe-6.5wt.%Si)@Shell(SiO2) Structure Obtained via Fluidized Bed Chemical Vapor Deposition" Metals 10, no. 4: 520. https://doi.org/10.3390/met10040520





